Abstract
Mycobacterium tuberculosis is a concern in patients with human immunodeficiency virus (HIV) infection. Rifampin (RIF), an agent used against M. tuberculosis, is contraindicated with most HIV protease inhibitors. Atazanavir (ATV) has clinical efficacy comparable to a standard of care regimen in naive patients and, when dosed with low-dose ritonavir (RTV), also in treatment-experienced patients. We evaluated here the safety and pharmacokinetics of ATV, resulting from three regimens of ATV, RTV, and RIF in 71 healthy subjects. The pharmacokinetics for ATV and RTV were assessed after 6 and 10 days of dosing with ATV 400 mg (n = 53) and with ATV-RTV at 300 and 100 mg (ATV/RTV 300/100; n = 52), respectively. Steady-state pharmacokinetics for ATV, RTV, RIF, and desacetyl-rifampin (des-RIF) were measured after 10 days of dosing of ATV/RTV/RIF 300/100/600 (n = 17), ATV/RTV/RIF 300/200/600 (n = 17), or ATV/RTV/RIF 400/200/600 (n = 14). An RIF 600-alone arm was enrolled as a control group (n = 18). With ATV/RTV/RIF 400/200/600, ATV area under the concentration-time curve values were comparable, but the Cmin values were lower relative to ATV 400 alone. ATV exposures were substantially reduced for the other RIF-containing regimens relative to ATV 400 alone and for all regimens relative to ATV/RTV 300/100 alone. RIF and des-RIF exposures were 1.6- to 2.5-fold higher than with RIF 600 alone. The incidence of grade 3/4 alanine aminotransferase/aspartate aminotransferase values was limited to 1 subject each in both the ATV/RTV/RIF 300/200/600 and the ATV/RTV/RIF 400/200/600 treatments. Coadministration of ATV with RIF was safe and generally well tolerated. Since ATV exposures were reduced in all regimens, ATV and RIF should not be coadministered at the dosing regimens studied.
Mycobacterium tuberculosis infection is a bacterial infection spread via the respiratory contact and is a substantial cause of morbidity and mortality in humans. This infection is of particular concern in individuals who are also infected with human immunodeficiency virus (HIV). Coinfection may contribute to a worsening of the course of HIV or M. tuberculosis infection and an acceleration of mortality (4). Treatment regimens containing rifampin (RIF) are particularly important in developing countries, since they are highly effective and generally less expensive than alternative regimens.
HIV protease inhibitors (PIs) are commonly included as a key component of highly active antiretroviral therapy, often in combination with two nucleoside reverse transcriptase inhibitors for the treatment of HIV. The coadministration of RIF is contraindicated with all currently marketed HIV PIs except for full-strength and schedule ritonavir (RTV) at 600 mg twice daily (BID). This dose and schedule of RTV is seldom used due to intolerability issues, but RTV is frequently used at a low dose (100 mg once daily [QD] or BID) to enhance plasma concentrations of other HIV PIs (10, 13, 17). In addition, the package insert of RTV recommends that alternate antimycobacterial agents such as rifabutin should be considered.
RIF is a potent inducer of both intestinal and hepatic CYP3A4 (3). All HIV PIs are substrates for CYP3A4 (for a review, see reference 2). As a nonspecific inducer, RIF has also been reported to induce the phase I metabolic enzymes CYP1A2 and CYP2C9, the phase II metabolic enzymes UDP-glucuronyltransferases and sulfotransferases (3), and the efflux pump system P-glycoprotein (7), which have all been implicated in PI disposition.
The PI atazanavir (ATV) is also a CYP3A4 substrate (5, 14), so a substantial decrease in ATV exposures can be anticipated with coadministration with RIF. To overcome the inductive effect of RIF on CYP450-mediated metabolism, coadministration of RTV is an option with a potential clinical application. Other studies have shown that the addition of RTV to a RIF-based combination was able to compensate for the inductive effects on saquinavir (19), nelfinavir (1), and lopinavir (11).
The primary objective of the present study was to assess the pharmacokinetics of ATV resulting from three regimens of ATV/RTV/RIF relative to those of ATV with or without RTV in healthy subjects. Secondary objectives were to study the pharmacokinetics of RTV and RIF and to assess the safety of the various drug combinations.
(This study was presented in part at the 12th Conference on Retroviruses and Opportunistic Infections, Boston, Mass., 22 to 25 February 2005.)
MATERIALS AND METHODS
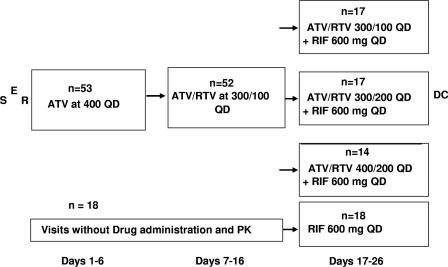
Study design.
This was an open-label, multiple-dose, randomized, drug interaction study in healthy volunteers. Subjects had to give informed consent, and the study protocol was approved by the IRB Institutional Review Board, Wychen, The Netherlands. Subjects underwent screening evaluations to determine eligibility within 21 days prior to study enrollment. Subjects were admitted to the clinical facility the evening prior to the initial dose of ATV (day −1). Seventy-one subjects were randomized to one of four treatment sequences on day 1 (Fig. 1). Fifty-three subjects received ATV 400 mg QD on days 1 through 6, followed by ATV and RTV at 300 and 100 mg, respectively (ATV/RTV 300/100), QD on days 7 through 16 (n = 52). These subjects then received one of three treatments from days 17 to 26 as randomized on day 1: ATV/RTV/RIF 300/100/600 (n = 17), ATV/RTV/RIF 300/200/600 (n = 17), or ATV/RTV/RIF 400/200/600 (n = 14). An additional 18 subjects underwent procedures identical to those who received study drug on days 1 to 16, except that they did not receive study drug or submit to a pharmacokinetic evaluation on days 1 through 16. This group received RIF 600 alone on days 17 to 26 and underwent both trough and serial (day 26) pharmacokinetic sampling during this period. All study drugs were administered within 5 min after completing a light meal (∼300 kcal, 20% fat). Subjects participated as outpatients receiving directly observed therapy except for the days of full pharmacokinetic sampling and contiguous days, when they were housed in the clinic. The subjects entered the clinic the evening prior to full pharmacokinetic sampling (days 5, 15, and 25) and remained in the clinic until the sampling period was completed (the morning of days 7 and 17 and discharge on day 27). Blood samples were collected for pharmacokinetic analysis up to 24 h postdose on days 6, 16, and 26 and on additional days for trough levels. Safety assessments were performed at selected times throughout the study. Subjects were closely monitored for adverse events (AEs) throughout the study.
FIG. 1.
Study design. S, screening; E, enrollment; R, randomization; DC, discharge.
Study subjects.
Healthy subjects as determined by medical history, physical examination, 12-lead electrocardiogram, and clinical laboratory evaluations were eligible to participate in the study. Negative results from a Mantoux tuberculin test and, if necessary, a chest X-ray were utilized to rule out tuberculosis.
Subjects met all of the following criteria for inclusion in the study: a signed informed consent form; no clinically significant deviations from normal in medical history, physical examination, electrocardiogram exam (ECG), or clinical laboratory determinations; a body mass index (i.e., weight in kilograms/[height in meters]2) of 18 to 30 kg/m2, inclusive; body weight of ≥60 kg for males and ≥50 kg for females; and ages 18 to 50, inclusive.
Exclusion criteria included the following: any sound medical, psychiatric, and/or social reason as determined by the investigator; evidence of organ dysfunction or any clinically significant deviation from normal in physical examination, vital signs, ECG, or clinical laboratory determinations; positive urine screen for drugs of abuse either at screening or before dosing; positive blood screen for hepatitis B surface antigen and/or hepatitis C antibody; positive blood screen for HIV type 1 or 2 antibody; positive serum or urine for β-HCG; Mantoux test of >15 mm or >0 to 15 mm with chest X-ray positive for tuberculosis; creatinine clearance of <80 ml/min; liver enzymes (total bilirubin, alkaline phosphatase, aspartate aminotransferase [AST], alanine aminotransferase [ALT]) above the upper limit of normal; use of any agent, within 8 weeks of dosing, known to induce or inhibit drug-metabolizing enzymes (e.g., cimetidine); use of any prescription drugs or over-the-counter acid controllers within 4 weeks prior to enrollment; use of any other drugs, including over-the-counter medications or herbal preparations, within 1 week prior to enrollment (an exception was made for investigator-approved use of ibuprofen); use of an oral, injectable, or implantable hormonal contraceptive agent within 1 month of enrollment; use of St. John's Wort (hypericum) within 4 weeks prior to study enrollment or throughout the study; and the consumption of grapefruit- or Seville orange-containing products within 1 week of study entry and throughout the study.
Safety.
Safety assessments were based on medical review of AE reports and the results of vital sign measurements, ECGs, physical examinations, and clinical laboratory tests. The incidence of AEs was tabulated and reviewed for potential significance and clinical importance. AE data were obtained by volunteering of information by the subjects and constant monitoring and daily questioning of the subjects by the study staff, as well as by the investigator review of vital signs, ECGs, laboratory, and other data.
Pharmacokinetics.
Blood samples were collected for intensive pharmacokinetic evaluation on day 6 (ATV 400), day 16 (ATV/RTV 300/100), and day 26 (ATV/RTV/RIF/des-RIF) prior to dosing and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 12, 16, and 24 h after dosing. In addition, trough blood samples were collected on selected days during study drug administration. Blood samples, 3 ml for RIF/des-RIF and 4 ml for ATV/RTV analysis, were collected into tripotassium EDTA tubes. The tubes were inverted gently a few times and were centrifuged for 10 min at approximately 1,000 × g in a refrigerated centrifuge (at ca. 5°C) to separate the cellular elements from plasma. The plasma samples were then assayed by validated liquid chromatography-tandem mass spectrometry methods. The range of the standard curve was 5 to 5,000 ng/ml for ATV and RTV and 50 to 35,000 ng/ml for RIF and des-RIF. The between-run variability and within-run variability expressed as the coefficient of variation (CV) was no greater than 3.7 and 18.9% for ATV, 4.9 and 12.1% for RTV, 3.3 and 6.6% for RIF, and 4.7 and 7.6% for des-RIF, respectively.
The plasma concentration-time data were analyzed by a noncompartmental method using Kinetica version 4.2 (Thermo Corp., Philadelphia, PA). The peak plasma concentration (Cmax) and the time to reach Cmax (Tmax) were recorded directly from experimental observations for each treatment period. The slope of the terminal phase of the plasma profile, K, was determined by log-linear regression of at least three datum points, which yielded a minimum mean square error using no weighting factor. The absolute value of K was used to estimate the apparent terminal half-life (T-half) by the following calculation: T-half = ln2/K. The area under the plasma concentration-time curve over a dosing interval [AUC(TAU)] was calculated by trapezoidal and log-trapezoidal summations, where TAU = 24 h.
Statistical methods.
To estimate the effects of RIF and RTV on the pharmacokinetics of ATV, general linear model analyses were performed on the log(Cmax), the log[AUC(TAU)], and the log(Cmin) of ATV. The factors in the analyses were treatment as a fixed effect and subject as a repeated-measure effect. Point estimates and 90% confidence intervals (90% CI) were calculated to compare the treatments ATV/RTV 300/100, ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, and ATV/RTV/RIF 400/200/600 versus ATV 400 and the treatments ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, and ATV/RTV/RIF 400/200/600 versus ATV/RTV 300/100. Similar analyses were also performed for RTV. The effects of ATV and RTV on the pharmacokinetics of RIF and des-RIF were also evaluated by analyses of variance on the log(Cmax) and log[AUC(TAU)]. Point estimates and 90% CI values were calculated to compare treatments ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, and ATV/RTV/RIF 400/200/600 versus treatment RIF 600. The estimated differences on the log scale were converted back to the original scale to obtain ratios of geometric means on the original scale. Geometric means estimated from the general linear model are also referred as adjusted geometric means (adjusted by the factors included in the model). No adjustments were made for multiplicity. For summary statistics and analysis of the Cmin data, BLQ (for below limit of quantification) values were set to half of the lower limit of quantification (LLQ) for the analytical method used in the present study. All statistical analyses were performed by using SAS/STAT version 8.2.
Although the sample size was not based on statistical power considerations, 14 subjects in each of the treatment groups provided 82% confidence that the estimated ratios of the ATV AUC geometric means when ATV is administered alone or with RTV versus when ATV is administered with RTV and RIF is within 20% of the true population value. In order to allow for dropouts, 18 subjects were enrolled in each treatment sequence.
RESULTS
Subject accrual and demographics.
A total of 71 subjects were enrolled into the present study. Of the 71 subjects randomized to treatment in the present study, 65 (92%) completed treatment and 6 (8%) discontinued from the study early after receiving study medication. Four subjects discontinued the study due to AEs (hypotension, exanthema/pruritis, dizziness, and vomiting; n = 1 for each). The other two subjects discontinued due to poor compliance with the study protocol (antisocial behavior; n = 1) and withdrawal of consent to accept an employment opportunity (n = 1).
Forty-seven (66%) of the subjects were male, and twenty-four (34%) were female. The mean (standard deviation [SD]) age of all subjects was 31 (SD = 10) years. Sixty-three (89%) of the subjects were Caucasian, four (6%) were black, and four (6%) were classified as “other.” “Other” races included mixed black/Asian, mixed Caucasian/black, and American Indian/Alaskan Native (two subjects). The mean body mass index of all subjects was 24 (SD = 3) kg/m2. The mean body weight for all subjects was 79 (SD = 11) kg.
Pharmacokinetics.
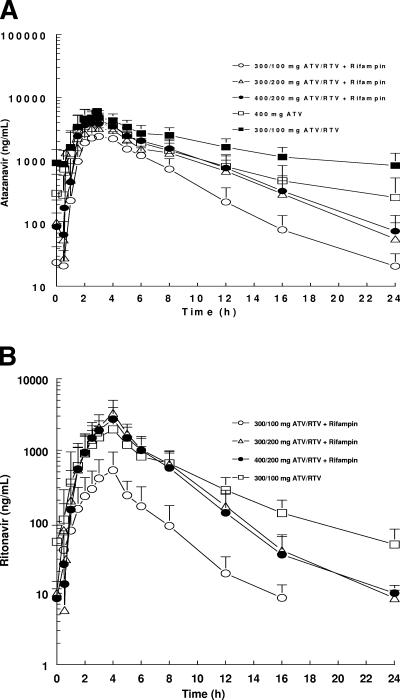
The mean plasma concentration-time profiles of ATV in the five treatment groups are presented in Fig. 2A (see also the supplemental material). Summary statistics for ATV pharmacokinetic parameters are listed in. Table 1 Finally, the adjusted geometric means, ratios of adjusted geometric means, and 90% CI values for the ratios of AUC(TAU), Cmax, and Cmin are summarized in Table 2.
FIG. 2.
(A) Steady-state mean plasma concentration-time profiles for ATV. (B) Steady-state mean plasma concentration-time profiles for RTV. The SD values are indicated.
TABLE 1.
Summary statistics for ATV pharmacokinetic parametersa
| Pharmacokinetic parameter | Result with regimen:
|
||||
|---|---|---|---|---|---|
| ATV 400 (day 6; n = 52) | ATV/RTV 300/100 (day 16; n = 48) | ATV/RTV/RIF 300/100/600 (day 26; n = 16) | ATV/RTV/RIF 300/200/600 (day 26; n = 17) | ATV/RTV/RIF 400/200/600 (day 26; n = 14) | |
| Geometric mean AUC(TAU) (ng · h/ml) (CV%) | 28,204.3 (38) | 44,517.1 (33) | 12,661.2 (33) | 20,691.2 (34) | 24,041.9 (35) |
| Geometric mean Cmax (ng/ml) (CV%) | 5,337.1 (24) | 5,135.7 (31) | 2,611.0 (33) | 3,342.2 (33) | 3,906.4 (30) |
| Geometric mean Cmin (ng/ml)b (CV%) | 186.9 (104) | 707.3 (58) | 18.1 (58) | 42.7 (81) | 52.9 (78) |
| Median Tmax (h) (min, max) | 2.0 (1, 4) | 2.5 (2, 4) | 2.8 (2, 4) | 2.5 (2, 4) | 2.5 (2, 4) |
| Mean T-half (h) (SD) | 6.3 (2.4) | NEc | 3.4 (0.7) | 3.2 (0.6) | 3.5 (0.8) |
BLQ values were replaced by 1/2LLQ = 2.5 ng/ml. Day 6, ATV 400; day 16, ATV/RTV 300/100; day 26, ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, or ATV/RTV/RIF 400/200/600. n = number of subjects.
24 h postdose.
NE, not evaluated.
TABLE 2.
Summary of statistical analyses of ATV pharmacokinetic parameters relative to ATV 400 and to ATV/RTV 300/100a
| Pharmacokinetic parameter | Adjusted geometric mean for regimen:
|
Ratio of adjusted geometric mean point estimate (90% CI)
|
|||
|---|---|---|---|---|---|
| ATV/RTV/RIF 300/100/600 (day 26; n = 16) | ATV 400 (day 6; n = 52) | ATV/RTV 300/100 (day 16; n = 48) | Day 26 vs day 6 | Day 26 vs day 16 | |
| AUC(TAU) (ng · h/ml) | 11,626.9 | 27,169.4 | 41,725.9 | 0.43 (0.37, 0.49) | 0.28 (0.25, 0.32) |
| Cmax (ng/ml) | 2,330.5 | 5,272.6 | 5,010.8 | 0.44 (0.39, 0.50) | 0.47 (0.41, 0.53) |
| Cmin (ng/ml) | 15.9 | 181.1 | 685.6 | 0.09 (0.07, 0.11) | 0.02 (0.02, 0.03) |
| ATV/RTV/RIF 300/200/600 (day 26; n = 17) | ATV 400 (day 6; n = 52) | ATV/RTV 300/100 (day 16; n = 48) | |||
| AUC(TAU) (ng · h/ml) | 18,863.6 | 27,169.4 | 41,725.9 | 0.69 (0.62, 0.78) | 0.45 (0.41, 0.50) |
| Cmax (ng/ml) | 3,171.1 | 5,272.6 | 5,010.8 | 0.60 (0.54, 0.67) | 0.63 (0.57, 0.70) |
| Cmin (ng/ml) | 40.6 | 181.1 | 685.6 | 0.22 (0.18, 0.28) | 0.06 (0.05, 0.07) |
| ATV/RTV/RIF 400/200/600 (day 26; n = 14) | ATV 400 (day 6; n = 52) | ATV/RTV 300/100 (day 16; n = 48) | |||
| AUC(TAU) (ng · h/ml) | 29,890.8 | 27,169.4 | 41,725.9 | 1.10 (0.90, 1.35) | 0.72 (0.60, 0.85) |
| Cmax (ng/ml) | 4,313.8 | 5,272.6 | 5,010.8 | 0.82 (0.67, 1.00) | 0.86 (0.71, 1.04) |
| Cmin (ng/ml) | 74.4 | 181.1 | 685.6 | 0.41 (0.29, 0.58) | 0.11 (0.08, 0.15) |
n = number of subjects.
The coadministration of RIF reduced the exposures to ATV considerably. Relative to ATV 400 or ATV/RTV 300/100 alone, in all treatments with the exception of ATV/RTV/RIF 400/200/600, the Cmax, AUC, and Cmin for ATV were statistically significantly lower (Table 2).
Relative to ATV 400, the ATV AUC and Cmax values were reduced ca. 30 to 60% with a greater decrease in Cmin values. With ATV/RTV/RIF 400/200/600, the ATV AUC and Cmax values were comparable to those observed when ATV was administered alone at 400 mg. However, ATV Cmin values were still significantly reduced by ca. 60%.
Relative to ATV/RTV 300/100, ATV/RTV/RIF 300/100/600 and ATV/RTV/RIF 300/200/600 resulted in a reduction of ca. 40 to 70% in ATV AUC and Cmax, with a >90% decrease in Cmin values. With ATV/RTV/RIF 400/200/600, AUC and Cmax were only moderately decreased by ca. 29 and 13%, respectively, but with again a 90% decrease in Cmin values.
The AUC and Cmin values of ATV when coadministered with RTV at a dose of ATV/RTV 300/100 were 1.5- and 3.8-fold higher than the corresponding values at ATV 400 alone, respectively. However, the Cmax values for the two groups were similar. Trough values for ATV suggested that subjects were at steady state for ATV by the end of each treatment period.
The effect of RIF on RTV pharmacokinetics was almost identical to the effect observed for ATV (Fig. 2B). RTV exposures (Cmax and AUC) were decreased by ca. 80% with ATV/RTV/RIF 300/100/600 compared to RTV exposures noted with the ATV/RTV 300/100 regimen. Specifically, adjusted geometric means for Cmax were reduced from 1,877 to 390 ng/ml and for AUC from 10,213 to 1,516 ng · h/ml. Conversely, RTV AUC values with ATV/RTV/RIF 300/200/600 mg were comparable to the values noted with ATV/RTV 300/100 administration. The adjusted geometric means for RTV AUC were 11,313 to 11,995 ng · h/ml compared to 10,213 ng · h/ml. Cmax values were ca. 60% higher than those observed with ATV/RTV 300/100 administration. Specifically, the adjusted geometric means for RTV Cmax were 3,005 to 3,147 ng/ml compared to 1,877 ng/ml. All treatments with RIF showed a 50% decrease in T-half and a substantial decrease (>80%) in Cmin values for RTV.
Even though RTV trough concentrations were below the level of quantification for most subjects receiving RIF with ATV/RTV 300/100 and for some subjects receiving ATV/RTV/RIF 300/200/600 and ATV/RTV/RIF 400/200/600, it is expected that, considering the pharmacokinetics of RTV, the steady state would have been achieved with 10 days of dosing.
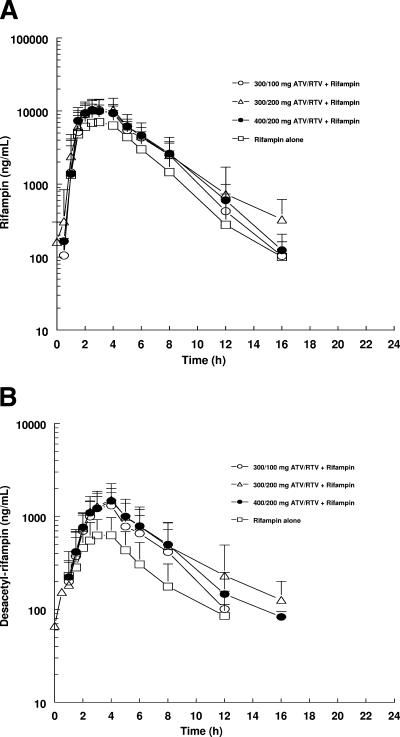
Because we included a separate group of subjects who took RIF 600 QD alone, it was possible to estimate the effect of adding ATV/RTV on RIF AUC and Cmax. The mean plasma concentration-time profiles of RIF in the four groups are presented in Fig. 3A. Summary statistics for RIF pharmacokinetic parameters are listed in Table 3. The adjusted geometric means, ratios of adjusted geometric means, and 90% CI values for the ratios of AUC(TAU) and Cmax are summarized in Table 4 (Cmin of RIF is below the detection limit). RIF exposures were increased on coadministration of all ATV/RTV/RIF treatments relative to RIF 600 alone. The increase in AUC of RIF ranged from ca. 49 to 64% with ATV/RTV/RIF treatments compared to RIF 600 alone. The increase in RIF Cmax ranged from ca. 32 to 39% after ATV/RTV/RIF treatments relative to RIF 600 alone. All increases in exposures were statistically significant. The T-half values of RIF were comparable across all treatment groups and thus did not appear to be affected by the coadministration of ATV and RTV.
FIG. 3.
(A) Steady-state mean plasma concentration-time profiles for RIF in groups. (B) Steady-state mean plasma concentration-time profiles for des-RIF. The SD values are indicated.
TABLE 3.
Summary statistics for RIF pharmacokinetic parametersa
| Pharmacokinetic parameter | Result with regimen:
|
|||
|---|---|---|---|---|
| ATV/RTV/RIF 300/100/600 (day 26; n = 16) | ATV/RTV/RIF 300/200/600 (day 26; n = 17) | ATV/RTV/RIF 400/200/600 (day 26; n = 14) | RIF 600 (day 26; n = 14) | |
| Geometric mean AUC(TAU) (ng · h/ml) (CV%) | 46,571.8 (36) | 49,483.9 (47) | 51,257.0 (21) | 31,267.7 (46) |
| Geometric mean Cmax (ng/ml) (CV%) | 10,613.1 (35) | 11,222.6 (37) | 10,983.3 (18) | 8,057.6 (45) |
| Geometric mean Cmin (ng/ml)b (CV%) | 25.0 (0) | 25.0 (0) | 25.0 (0) | 25.0 (0) |
| Median Tmax (h) (min, max) | 2.5 (2, 4) | 2.5 (2, 4) | 2.5 (2, 4) | 2.5 (2, 4) |
| Mean T-half (h) (SD) | 1.6 (0.2) | 1.7 (0.5) | 1.7 (0.4) | 1.5 (0.2) |
BLQ values were replaced by 1/2LLQ = 25 ng/ml. Day 26, ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, or ATV/RTV/RIF 400/200/600; day 26, RIF 600. n = number of subjects.
24 h postdose.
TABLE 4.
Summary of statistical analyses of RIF pharmacokinetic parameters relative to RIF 600a
| Pharmacokinetic parameter | Adjusted geometric mean for regimen:
|
Ratio of adjusted geometric mean point estimate (90% CI) (day 26 vs day 6) | |
|---|---|---|---|
| ATV/RTV/RIF 300/100/600 (day 26; n = 16) | RIF 600 mg (day 26; n = 14) | ||
| AUC(TAU) (ng · h/ml) | 46,571.8 | 31,267.7 | 1.49 (1.20, 1.85) |
| Cmax (ng/ml) | 10,613.1 | 8,057.6 | 1.32 (1.09, 1.59) |
| ATV/RTV/RIF 300/200/600 (day 26; n = 17) | RIF 600 mg (day 26; n = 14) | ||
| AUC(TAU) (ng · h/ml) | 49,483.9 | 31,267.7 | 1.58 (1.28, 1.96) |
| Cmax (ng/ml) | 11,222.6 | 8,057.6 | 1.39 (1.15, 1.68) |
| ATV/RTV/RIF 400/200/600 (day 26; n = 14) | RIF 600 mg (day 26; n = 14) | ||
| AUC(TAU) (ng · h/ml) | 51,257.0 | 31,267.7 | 1.64 (1.31, 2.05) |
| Cmax (ng/ml) | 10,983.3 | 8,057.6 | 1.36 (1.12, 1.66) |
n = number of subjects.
Finally, the effect of ATV/RTV on des-RIF pharmacokinetics was similar to the effect observed for RIF (Fig. 3B). The coadministration of ATV/RTV/RIF in all treatment groups resulted in increased des-RIF exposures of 2.2- to 2.7-fold for AUC and 2-fold for Cmax compared to the exposures produced on administration of RIF alone. Specifically, the increases in adjusted geometric mean AUC were 5,535 to 6,640 ng · h/ml versus 2,493 ng · h/ml and for adjusted geometric mean Cmax were 1,285 to 1,390 ng/ml versus 674 ng/ml. Similar to RIF, the Cmin for des-RIF was found to be below the detection limit.
Safety.
There were no deaths or serious AEs in the study (Table 5). The majority of AEs (89%) were mild in intensity and resolved prior to discharge. As noted above, four subject discontinuations were due to the AEs: hypotension, xanthema and pruritis, dizziness, and vomiting, all with onset during the ATV/RTV 300/100 treatment. The most frequently occurring AEs (>10% of subjects) were chromaturia, headache, fatigue, ocular icterus, dizziness, abdominal pain, urinary tract infection, jaundice, nausea, dyspepsia, vomiting, myalgia, and hyperhidrosis. AE occurrence (% of subjects) was comparable across treatments, with incidence of jaundice (icterus), headaches, fatigue, and dizziness being more prevalent in treatments ATV 400 and ATV/RTV 300/100 and chromaturia being more prevalent in RIF-containing regimens. Incidence of transaminase elevation was infrequent and not treatment related (Table 5).
TABLE 5.
Safety results
| Parameter | No. of subjects (%) for regimen:
|
|||||
|---|---|---|---|---|---|---|
| ATV 400 | ATV/RTV 300/100 | ATV/RTV/RIF 300/100/600 | ATV/RTV/RIF 300/200/600 | ATV/RTV/RIF 400/200/600 | RIF 600 | |
| No. of deaths | 0 | 0 | 0 | 0 | 0 | 0 |
| No. of discontinuations due to AEs | 0 | 4 (8) | 0 | 0 | 0 | |
| No. of serious AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Total no. of AEs | 39 (74) | 47 (90) | 16 (94) | 16 (94) | 14 (100) | 17 (94) |
| No. of subjects with: | ||||||
| Jaundice | 4 (8) | 6 (12) | 0 | 0 | 0 | 0 |
| Total bilirubin > grade 2 (>5 × ULN) | 21 (40) | 45 (87) | 5 (29) | 13 (76) | 9 (64) | 0 |
| ALT/AST grade 2 (2.6 to 5 × ULN) | 1 (2) | 0 | 0 | 1 (6) | 1 (6) | 1 (6) |
| ALT/AST grade 3/4 (>5 × ULN) | 0 | 0 | 0 | 1 (6) | 1 (6) | 0 |
DISCUSSION
The objective of this study was to evaluate the effect of RIF on the pharmacokinetics of ATV. ATV is a CYP3A4 substrate (5), (14), and RIF is a potent CYP3A4 inducer (3). Since both drugs may be used in combination, a substantial decrease in ATV concentrations was anticipated upon coadministration with RIF. To counteract the inductive effect of RIF, the addition of RTV, a potent CYP3A4 inhibitor, was studied as part of three alternate dosing strategies.
There were two control arms in the study, with the aim of obtaining ATV exposures comparable to ATV alone (ATV 400) and/or ATV with RTV (ATV/RTV 300/100) when ATV, RTV, and RIF were administered together. In the ATV/RTV/RIF 300/100/600 treatment regimen, the combination of 300/100 ATV/RTV was studied with 600 mg of RIF. The ATV/RTV 300/100 regimen is currently being used in treatment-experienced HIV-positive patients. This regimen is also used in patients receiving efavirenz, since ATV with RTV was observed to sufficiently counteract the inductive effect of efavirenz, a moderately potent CYP3A4 inducer (18).
Since RIF is considered to be a more potent inducer of CYP3A4 than efavirenz (8), two other regimens, ATV/RTV/RIF 300/200/600 and ATV/RTV/RIF 400/200/600, were also studied. In these regimens, the dose of RTV was increased to 200 mg in anticipation of the ability of RTV to decrease the clearance and thereby increase the Cmin values of ATV with only a modest effect on ATV Cmax.
It has been reported that 400 mg of ATV and 200 mg of RTV increases the exposures (AUC) and Cmin values of ATV by approximately 2.3- and 10-fold, respectively, relative to the 400-mg regimen alone (15). Therefore, the ATV/RTV/RIF 400/200/600 regiment was selected with the expectation of providing the most robust exposures to ATV compared to the ATV/RTV/RIF 300/100/600 and ATV/RTV/RIF 300/200/600 regimens.
The results showed that relative to ATV at 400 mg alone, ATV exposures (Cmax, AUC, and Cmin) were decreased considerably with ATV/RTV/RIF 300/100/600 or ATV/RTV/RIF 300/200/600. Conversely, with ATV/RTV/RIF 400/200/600, the AUC of ATV was increased by 10%. However, the Cmin of ATV was still decreased by 59% relative to that observed with 400 mg alone. Because it is generally accepted that Cmin is the most relevant pharmacokinetic parameter predicting antiviral activity (2) for HIV PIs and Cmin in all of the tested regimens was significantly reduced, none of these adjusted regimens of ATV/RTV can be recommended together with RIF.
Relative to ATV/RTV 300/100, ATV exposures (Cmax, AUC, and Cmin) were substantially decreased in all treatment regimens with coadministration of RIF and therefore, as noted above, none of the adjusted regimens of ATV/RTV can be recommended with RIF.
When dosed with RTV, examination of ATV profiles showed that the concentrations noted with ATV/RTV/RIF 300/200/600 and ATV/RTV/RIF 400/200/600 appeared to be superimposable with ATV concentrations observed with administration of ATV at 400 mg alone until approximately 12 h (Fig. 2A). After the 12-h time point, the concentrations declined steeply. This observation suggests that an additional dose of ATV/RTV at approximately 12 h may alleviate the reduction in ATV concentrations seen with the coadministration of RIF.
RTV exposures (AUC) when dosed at 200 mg with ATV and RIF were generally comparable to RTV exposures observed with ATV 300/100 ATV/RTV alone. In contrast, RTV exposures when dosed at 100 mg with ATV and RIF were very low relative to RTV exposures observed with 300/100 ATV/RTV. The Cmin values for RTV were decreased substantially in all treatment regimens upon coadministration of RIF compared to those observed with ATV/RTV 300/100 alone.
Interestingly, exposures (AUC) to RIF (50 to 60%) and des-RIF (two- to threefold) were higher when RIF was coadministered with ATV/RTV compared to administration of RIF 600 alone. The mechanism for the increase in RIF and des-RIF concentrations when coadministered with ATV and RTV is unknown. ATV is a CYP3A4 substrate and inhibitor. It is also a weak inhibitor of P-gp with a 50% inhibitory concentration of 29 μM in vitro (16). Since RIF and des-RIF are not CYP3A4 substrates, the increased exposures to RIF and des-RIF were unexpected (3). However, this observation of increased exposures for RIF was also noted with indinavir and RIF where RIF AUC was increased 73% (9). From previous reports, RTV has not been noted to increase RIF exposures (12). Therefore, it is possible that other mechanisms, such as competition between ATV and RIF for biliary secretion or transporters other than P-gp, may play a role in the increased RIF exposures observed with coadministration of ATV.
The observation of elevated liver transaminase levels in several previously reported studies in which RIF was coadministered with the PIs saquinavir or lopinavir/ritonavir (6, 11) has raised interest in the relative contributions of PIs and RIF in the induction of increased transaminase levels. As such, the design of the present study addressed this concern by introducing a parallel cohort that followed study procedures similar to those of other cohorts but did not receive any drug on days 1 to 16 and received RIF only on days 17 to 26. In the present study, the incidences of transaminase elevation were infrequent and not treatment related. Most reported transaminase increases were grade 1 in severity. Only a slight increase in the frequency of grade 1 to 3 severity elevations in ALT levels was noted in treatment groups where RIF was dosed with 200 mg of RTV (Table 5). AE occurrence was comparable across treatments, with the incidence of jaundice (icterus), headaches, fatigue, and dizziness being more prevalent in treatments ATV 400 and ATV/RTV 300/100 and chromaturia being more prevalent in RIF-containing regimens, as expected.
The most frequently observed laboratory abnormality in the ATV-containing regimens was elevated total bilirubin. Almost all subjects were noted to have elevated bilirubin levels while receiving the ATV 400 or ATV/RTV 300/100 regimens, including many of grade 3/4 severity. This observation was not unexpected since these subjects were exposed to undiminished levels of ATV in these two treatment groups and to higher ATV exposures over a longer dosing period for treatment ATV/RTV 300/100. Although ATV exposures were decreased with RIF coadministration in treatments ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, and ATV/RTV/RIF 400/200/600, the frequency of sample collection and lack of a washout period after the ATV/RTV 300/100 treatment ensured that bilirubin elevations would still be observed in the RIF-containing regimens. Bilirubin levels were shown to return to within normal limits with follow-up. This reversal is consistent with that seen after the withdrawal of ATV in other healthy-population studies. ATV is known to bind, in a predominantly competitive manner, the bilirubin glucuronidating isozyme, UGT 1A1. There is no indication from preclinical and clinical data collected to date that the increases in total bilirubin represent a signal for a hepatotoxic process.
As expected, bilirubin levels were decreased to within the normal range with the addition of RIF. It is difficult to estimate, however, what proportion of the decrease is due to decreased ATV exposures versus that due to the reported inductive effect of RIF on UGT1A1 (7).
In summary, the treatment regimens ATV/RTV/RIF 300/100/600, ATV/RTV/RIF 300/200/600, and ATV/RTV/RIF 400/200/600 were unable to fully counteract the inductive properties of RIF. The increases in RIF and des-RIF concentrations were unexpected with ATV and RTV coadministration and require further investigation. Nonetheless, RIF should not be coadministered with ATV and RTV using the studied regimens in HIV-infected patients due to the potential for reduced ATV exposures and the possibility for subsequent decreased viral susceptibility to ATV. However, the results of the present study allow for further exploration of alternate dosing strategies of ATV and RTV with RIF.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Bergshoeff, A. S., T. F. Wolfs, S. P. Geelen, and D. M. Burger. 2003. Ritonavir-enhanced pharmacokinetics of nelfinavir/M8 during rifampin use. Ann. Pharmacother. 37:521-525. [DOI] [PubMed] [Google Scholar]
- 2.Boffito, M., E. Acosta, D. Burger, C. V. Fletcher, C. Flexner, R. Garaffo, G. Gatti, M. Kurowski, C. F. Perno, G. Peytavin, M. Regazzi, and D. Back. 2005. Therapeutic drug monitoring and drug-drug interactions involving antiretroviral drugs. Antivir. Ther. 10:469-477. [PubMed] [Google Scholar]
- 3.Burman, W. J., K. Gallicano, and C. Peloquin. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327-341. [DOI] [PubMed] [Google Scholar]
- 4.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith, D., and C. Perry. 2003. Atazanavir. Drugs 63:1679-1693. [DOI] [PubMed] [Google Scholar]
- 6.Grange, S., M. Schutz, C. Schmitt, M. Riek, and E. Gaudel-Ehrhart. 2005. Unexpected hepatotoxicity observed in a healthy volunteer study on the effects of multiple dose rifampicin on the steady-state pharmacokinetics of ritonavir-boosted saquinavir and vice versa, abstr. 35. Sixth International Workshop on Clinical Pharmacology of HIV Therapy, Montreal, Quebec, Canada, 28 to 30 April 2005.
- 7.Greiner, B., M. Eichelbaum, P. Fritz, H. P. Kreichgauer, O. von Richter, J. Zundler, and H. K. Kroemer. 1999. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J. Clin. Investig. 104:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariparsad, N., S. C. Nallani, R. S. Sane, D. J. Buckley, A. R. Buckley, and P. B. Desai. 2004. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J. Clin. Pharmacol. 44:1273-1281. [DOI] [PubMed] [Google Scholar]
- 9.Jaruraatanasirikul, S., and S. Sriwiriyajan. Effect of indinavir on the pharmacokinetics of rifampicin in HIV-infected patients. J. Pharm. Pharmacol. 53:409-412. [DOI] [PubMed]
- 10.King, J. R., H. Wynn, R. Brundage, and E. P. Acosta. 2004. Pharmacokinetic enhancement of protease inhibitor therapy. Clin. Pharmacokinet. 43:291-310. [DOI] [PubMed] [Google Scholar]
- 11.La Porte, C. J., E. P. Colbers, R. Bertz, D. S. Voncken, K. Wikstrom, M. J. Boeree, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2004. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob. Agents Chemother. 48:1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno, S., D. Podzamczer, R. Blazquez, J. A. Iribarren, E. Ferrer, J. Reparaz, J. M. Pena, E. Cabrero, and L. Usan. 2001. Treatment of tuberculosis in HIV-infected patients: safety and antiretroviral efficacy of the concomitant use of ritonavir and rifampin. AIDS 15:1185-1187. [DOI] [PubMed] [Google Scholar]
- 13.Moyle, G. J., and D. Back. 2001. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med. 2:105-113. [DOI] [PubMed] [Google Scholar]
- 14.Musial, B. L., J. K. Chojnacki, and C. I. Coleman. 2004. Atazanavir: a new protease inhibitor to treat HIV infection. Am. J. Health Syst. Pharm. 61:1365-1374. [DOI] [PubMed] [Google Scholar]
- 15.O'Mara, E., V. Mummaneni, M. Bifano, D. Randall, H. Uderman, L. Knox, and M. Geraldes. 2001. Steady-state pharmacokinetic interaction study between BMS-232632 and ritonavir in healthy subjects, abstr. 740. Eighth Conference on Retrovirus and Opportunistic Infections, Chicago, Ill., 4 to 8 February 2001.
- 16.Perloff, E. S., S. X. Duan, P. R. Skolnik, D. J. Greenblatt, and L. L. von Moltke. 2005. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab. Dispos. 33:764-770. [DOI] [PubMed] [Google Scholar]
- 17.Scott, J. D. 2005. Simplifying the treatment of HIV infection with ritonavir-boosted protease inhibitors in antiretroviral-experienced patients. Am. J. Health Syst. Pharm. 62:809-815. [DOI] [PubMed] [Google Scholar]
- 18.Tacket, D., M. Child, S. Agarwala, M. Geraldes, M. Geiger, B. Laura, and E. O'Mara. 2003. Atazanavir: a summary of two pharmacokinetic (PK) drug interaction studies in healthy subjects, abstr. 543. Tenth Conference on Retroviruses and Opportunistic Infections, Boston, Mass.
- 19.Veldkamp, A., R. M. W. Hoetelmans, J. H. Beijnen, J. W. Mulder, and P. L. Meenhorst. 1999. Ritonavir enables combined therapy with rifampin and saquinavir. Clin. Infect. Dis. 29:1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.