Abstract
Tenofovir (TFV) undergoes renal elimination by a combination of glomerular filtration and active tubular secretion. While transporter-mediated uptake of TFV from the blood into proximal-tubule cells has been well characterized, comparatively little is known about the efflux system responsible for transporting TFV into the lumen during active tubular secretion. Therefore, members of the ATP-binding cassette family of efflux pumps expressed at the apical side of proximal-tubule cells were studied for the ability to transport TFV. Studies in multiple independent in vitro systems show TFV not to be a substrate for P glycoprotein (Pgp) or multidrug resistance protein type 2 (MRP2). In contrast to Pgp and MRP2, TFV was observed to be a substrate for MRP4. TFV accumulated to fivefold lower levels in MRP4-overexpressing cells, and its accumulation could be increased by an MRP inhibitor. Furthermore, MRP4-overexpressing cells were found to be 2.0- to 2.5-fold less susceptible to cytotoxicity caused by TFV. ATP-dependent uptake of TFV was observed in membrane vesicles containing MRP4 but not in vesicles lacking the transporter. On the basis of these and previous results, the molecular transport pathway for the active tubular secretion of TFV through renal proximal-tubule cells involves uptake from the blood mediated by human organic anion transporters 1 and 3 and efflux into urine by MRP4. A detailed understanding of the molecular mechanism of TFV active tubular secretion will facilitate the assessment of potential renal drug-drug interactions with coadministered agents.
Tenofovir (TFV) disoproxil fumarate (TDF) is an oral prodrug of the nucleotide reverse transcriptase inhibitor TFV. Following oral administration, TDF undergoes rapid conversion to TFV in plasma. TFV is eliminated from systemic circulation renally through a combination of glomerular filtration and active tubular secretion. Active tubular secretion of TFV has been inferred from the observation that TFV renal clearance exceeds that of creatinine clearance in patients (1). Tubular secretion is mediated by specific uptake and efflux transporters that are localized to the basolateral and apical membranes of renal proximal tubules, respectively. They act in series and mediate the active transport of small soluble molecules from systemic circulation into urine. Renal drug-drug interactions may occur between therapeutics that are substrates for the same tubular transport pathways or that inhibit the pathway of a drug subject to renal excretion (3).
At the basolateral membrane of proximal tubules, TFV has been shown to be taken up by human organic anion transporter 1 (hOAT1) and hOAT3. The kinetics of TFV transport by hOAT1 and hOAT3 have been studied in cells stably expressing these transporters (6; T. Cihlar, K. Bleasby, A. Roy, and J. Prichard, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A448, 2004). In these model systems, TFV shows >20-fold higher affinity for hOAT1 than for hOAT3. However, hOAT3 expression in proximal tubules is higher than that of hOAT1 (20), suggesting that it may represent a parallel low-affinity, high-capacity transport pathway for TFV. In vitro studies also show that TFV is not a substrate for human organic cation transporter type 1 (hOCT1) or hOCT2 (Cihlar et al., 44th ICAAC). Taken together, these data suggest that hOAT1 is likely the primary basolateral uptake transporter involved in the tubular secretion of TFV, while hOAT3 represents a parallel pathway.
While transporter-mediated uptake of TFV from blood into proximal-tubule cells at the basolateral membrane has been well characterized, comparatively little is known about the apical efflux system(s) responsible for transporting TFV out of the intracellular space and into the tubular lumen. Recent publications have implicated the ATP-binding cassette (ABC) transporter subfamily member multidrug resistance-associated protein type 2 (MRP2) in the tubular efflux of TFV (14, 27, 36) (S. G. Louie, J. T. Lam, M. N. Neely, and P. Beringer, Abstr. 6th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 2.16, 2005). The proposal in these reports that TFV transport occurs via MRP2 is based on a sole in vitro study of intact teleost tubules demonstrating that cidofovir and adefovir, acyclic analogs structurally related to TFV, weakly inhibit the luminal accumulation of fluorescein-methotrexate (19), a putative substrate for MRP2 (18) and other MRPs (4, 16, 17). Hence, renal efflux of TFV by MRP2 has not been experimentally established and these hypotheses are based on multiple indirect assumptions from data generated in a system genetically distant from humans. In comparison to MRP2, another apically expressed renal efflux transporter, MRP4, might be a more likely candidate for the renal efflux of TFV on the basis of its interaction with adefovir, monophosphorylated nucleoside analogs, and cyclic nucleotides (15, 25, 26, 31). However, no experimental data demonstrating transport of TFV by MRP4 have been presented in the literature to date.
Similar to MRP2 and MRP4, the ABC transporter subfamily member P glycoprotein (Pgp) is also expressed on the apical membrane of proximal-tubule cells (10, 30, 33, 35). Pgp has been shown to transport a wide variety of xenobiotics. Hence, Pgp may potentially represent an alternative route for the tubular efflux of TFV. To better understand the molecular mechanism of active tubular secretion of TFV, experiments were performed with a broad range of in vitro systems to determine the role of the three major apical efflux transporters in the tubular secretion of TFV. Enhanced understanding of this process would facilitate assessment of the potential for renal drug-drug interactions that may occur between TFV and other coadministered agents.
(Work on MRP4 was presented in part as poster 91 at the 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, 13 to 16 November 2005, Dublin, Ireland [A. S. Ray, J. E. Vela, K. L. Robinson, T. Cihlar, and G. R. Rhodes, Abstr. 7th Int. Workshop Adverse Drug React. Lipodystrophy HIV, abstr. 91, p. L54, 2005], and as poster PE4.3/13 at the 10th European AIDS Conference (EACS), 17 to 20 November 2005, Dublin, Ireland [A. S. Ray, J. E. Vela, K. L. Robinson, T. Cihlar, and G. R. Rhodes, Abstr. 10th Eur. AIDS Conf., abstr. PE4.3/13, p. 50, 2005]).
MATERIALS AND METHODS
Reagents.
TFV and adefovir were provided by Gilead Sciences, Inc. (Foster City, CA). Probenecid, methotrexate, verapamil, cyclosporine A (CsA), calcein AM, and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO). MK571 was obtained from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA). [3H]estradiol-17-β-d-glucuronide was obtained from Perkin-Elmer (Wellesley, MA). [3H]TFV was obtained from Moravek Biochemicals (Brea, CA). Tissue culture reagents were obtained from Mediatech Inc. (Herndon, VA). The Nyosil M25 oil used to separate suspension cells from extracellular medium was obtained from TAI Lubricants, Inc. (Hockessin, DE). ATPase assays were done with membrane preparations from insect cells transfected with human Pgp or MRP2 obtained from BD Biosystems (Franklin Lakes, NJ). Vesicles from insect cells transfected with MRP2 or MRP4 were obtained from Solvo Biotechnology, Inc. (Budaors, Hungary; distributed by In Vitro Technologies, Baltimore, MD). All other reagents were the highest quality available from Sigma-Aldrich.
Cells.
Human ovarian carcinoma cell line 2008 transfected with human MRP2 and the Madin-Darby canine kidney cell line (MDCKII) transfected with human MRP2 or Pgp were obtained from Piet Borst at The Netherlands Cancer Institute. Transfected 2008 and MDCKII cells have been characterized in previous publications (8, 11, 13). Cells were grown under recommended conditions in Dulbecco's modified Eagle's medium-10% heat-inactivated fetal bovine serum-100 U penicillin G-100 μg streptomycin sulfate per ml. Cells were passaged twice a week and characterized for overexpression of transporters by Western blot analyses. Functional transporter expression was evaluated by determining a difference in calcein AM accumulation in parental and transfected cells (data not shown). The human T-leukemic lymphoblast cell line CEM-R1 overexpressing MRP4 was previously selected from parental CEM-SS cells by continuous passage in the presence of increasing concentrations of adefovir (26, 31). CEM-SS and -R1 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 U penicillin G-100 μg streptomycin sulfate per ml. These nonadherent cells were split 1/10 approximately twice a week. Continued overexpression of MRP4 was verified by a cytotoxicity assays with adefovir (see Table 2).
TABLE 2.
Cytotoxicities of adefovir, TFV, and TDF (oral prodrug of TFV) in parental (CEM-SS) and MRP4-overexpressing (CEM-R1) cells
| Compound | CC50 (μM)
|
Fold changea | |
|---|---|---|---|
| CEM-SS | CEM-R1 | ||
| Adefovir | 2,100 ± 283 | >20,000 | >9.5 |
| TFV | 5,933 ± 814 | 12,030 ± 1,060 | 2.0b |
| TDF | 40 ± 14 | 101 ± 11 | 2.5c |
Values represent the mean ± standard deviation of three (TFV) or four (TDF) independent experiments performed in triplicate.
Significant (P < 0.01) decrease in toxicity due to MRP4 expression based on Student's unpaired two-tailed t test assuming equal variance.
Significant (P < 0.001) decrease in toxicity due to MRP4 expression based on Student's unpaired two-tailed t test assuming equal variance.
Caco-2 model.
Validated bidirectional permeability assays were performed by Absorption Systems (Exton, PA) with the human colon carcinoma cell line Caco-2, which is known to apically express Pgp (12). Caco-2 monolayers were grown to confluence on collagen-coated, microporous polycarbonate membranes in 12-well Transwell plates. The permeability assay buffer for the donor chambers was Hanks balanced salt solution containing 10 mM HEPES and 15 mM glucose at pH 7.4. The same buffer was used in the receiver chamber with addition of 1% bovine serum albumin. Experiments were performed in the presence or absence of 10 μM CsA, a Pgp inhibitor. At 1 and 2 h after addition of the compounds under study, an aliquot was taken from the receiver chamber and replaced with fresh assay buffer. Cells were dosed on the apical side (for forward permeability) or the basolateral side (for reverse permeability) and incubated at 37°C with 5% CO2 in a humidified incubator. Each determination was performed in duplicate. The permeability of control standards atenolol, propranolol, and digoxin was determined to meet the acceptance criteria for each lot of cells. Samples were combined with acetonitrile containing 0.4% formic acid and analyzed by liquid chromatography-mass spectrometry. Lucifer yellow flux was measured for each monolayer after the assay to ensure that no damage was inflicted on the cell monolayers during the experiment. Apparent permeability (Papp) was calculated with the equation Papp = (dCr/dt) × Vr/(A × C0), where dCr/dt is the change in concentration in the receiver well over time expressed in micromolar per second, Vr is the volume in the receiver well, A is the area of the cell monolayer, and C0 is the initial concentration in the donor well.
Pgp and MRP2 ATPase assays.
The colorimetric assay for measuring transporter-associated ATPase activity was modified from those previously described (9, 29). Briefly, a 60-μl reaction mixture containing 20 μg membranes, 1 mM probenecid (positive control) or test drug, and 3 to 5 mM MgATP, in a buffer containing 50 mM Tris-morpholineethanesulfonic acid, 2 mM EGTA, 50 mM potassium chloride, 2 mM glutathione, 2 mM dithiothreitol, and 5 mM sodium azide, was incubated at 37°C for 40 min. An identical reaction mixture containing 400 μM sodium orthovanadate was assayed in parallel. Orthovanadate inhibits Pgp or MRP2 by trapping MgADP in the nucleotide-binding site. Thus, ATPase activity measured in the presence of orthovanadate represents non-MRP ATPase activity and can be subtracted from the activity generated without orthovanadate to yield vanadate-sensitive ATPase activity. The reaction was stopped by addition of 30 μl 10% sodium dodecyl sulfate, followed by addition of 200 μl of 35 mM ammonium molybdate in 15 mM zinc acetate-10% ascorbic acid (1:4) and incubation for an additional 20 min at 37°C. Liberation of inorganic phosphate was detected by absorbance at 800 nm and quantitated by comparing the absorbance to a phosphate standard curve.
Assays of uptake by MRP2- and MRP4-overexpressing cells.
The effect of overexpression of MRPs on TFV intracellular accumulation was assessed by doing continuous incubations of cells with TDF or TFV and determining the intracellular concentrations of TFV and its phosphorylated metabolites. Parental and MRP2-overexpressing 2008 or MDCKII cells were grown to confluence on 12-well tissue culture plates before the study. The experiments were started by addition of 1 μM TDF and incubation for 1 h. Incubations were stopped by washing cells with ice-cold phosphate-buffered saline to remove extracellular drug, and cells were harvested as previously described (24). CEM-SS and -R1 cells were seeded in T75 flasks at 106/ml and treated with 100 μM TFV or 1 μM TDF for 1 h. Incubations were stopped by separating the cells from the extracellular medium by spinning through oil as previously described (23). After isolation of cells from extracellular-drug-containing medium, extraction of intracellular metabolites was achieved by adding 70% methanol, followed by overnight incubation at −20°C. Cellular debris were then removed by centrifugation, and intracellular concentrations of TFV and its phosphorylated metabolites were determined by ion-pairing liquid chromatography coupled with tandem mass spectrometry essentially as described previously (21, 22, 24, 34).
Inhibition of calcein AM uptake in Pgp- and MRP2-overexpressing cells.
The fluorescent compound calcein has been shown to be a substrate for MRP1 and MRP2, and the nonfluorescent calcein ester prodrug calcein AM is a substrate for Pgp (11). Therefore, calcein and calcein AM serve as useful tools for the study of transport proteins. Confluent monolayers of parental or transfected cells in 24-well plates were incubated with 10 μM calcein AM for 1 h either alone or in the presence of various concentrations of test agents. Extracellular medium was removed, and the cells were washed twice with 1 ml ice-cold phosphate-buffered saline. Cells were then lysed by addition of 500 μl of 0.4% Triton X-100 and 20 mM Tris (pH 9.0). An aliquot of 200 μl of the cell lysate was then transferred to an opaque 96-well plate, and fluorescence was measured with an excitation λ of 494 nm and an emission λ of 517 nm.
TFV transport in MRP2 and MRP4 vesicles.
Membrane vesicles prepared from Sf9 insect cells expressing either MRP2 or MRP4 and control vesicles without transporters were used for studies of TFV transport in MRP2 and MRP4 vesicles. The vesicle transport assays were performed with a transport buffer (40 mM morpholinepropanesulfonic acid [MOPS]-Tris [pH 7.2], 50 mM KCl, 10 mM MgCl2) containing 5 mM ATP or AMP, 5 μM [3H]TFV (Moravek Biochemicals, Brea, CA) or [3H]estradiol-17-β-d-glucuronide (Perkin-Elmer, Boston, MA), and vesicles at a total protein concentration of 600 μg/ml. The total reaction volume was 250 μl. Following incubation at 37°C, 80-μl reaction mixture aliquots were collected at each time point, diluted into 1 ml ice-cold transport buffer, and vacuum filtered through GF/F glass filters (Whatman, Clifton, NJ). Filters were washed with 2 × 5 ml ice-cold transport buffer and dried, and radioactivity was counted. To assess the transporter-specific uptake of the substrates tested, MRP-containing vesicles were assayed side by side with the control vesicles. Accumulation of substrates in vesicles was expressed in picomoles per milligram of protein.
Assays of cytotoxicity in MRP2- and MRP4-overexpressing cells.
Cytotoxicities of TFV and the MRP2 substrate vinblastine in parental or MRP2-overexpressing MDCKII cells were determined in a 96-well format. Both cell types were seeded at 6,000 cells per well in 100 μl of medium. Test drugs were then serially diluted in triplicate and mixed with cells. Following a 3-day incubation, cell viability was determined by measuring the enzyme-dependent cleavage of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt (XTT; Sigma-Aldrich, St. Louis, MO) to a colored formazan dye. Formazan dye formation was monitored by measuring absorbance at 450 nm.
Cytotoxicity evaluation in MRP4-overexpressing CEM-R1 and control CEM-SS cells was performed in a 96-well format. Both cell lines were seeded in parallel at a concentration of 5,000 cells per well in 100 μl of fresh medium and mixed with an equal volume of serially diluted drugs. Each drug concentration was tested in triplicate. Following a 3-day incubation, cell viability was determined with a CellTiter Glo staining kit (Promega, Madison, WI). A 100-μl aliquot of medium was removed and replaced with the same volume of CellTiter Glo reagent. After mixing, the luminescence signal was measured and percent cell viability was calculated for each sample. Concentrations reducing cell viability by 50% (CC50s) were calculated by nonlinear regression with the Prizm program (GraphPad, San Diego, CA). Untreated cells and cells treated with 100 μM methotrexate served as 100% and 0% viability controls, respectively.
Data analysis.
Differences between experimental results were compared by a Student unpaired two-tailed t test assuming equal variance with Microsoft Excel. Results of accumulation and transport from at least two independent experiments and multiple individually dosed wells or flasks were compared for the statistical significance of differences observed. The means from at least three independently generated CC50s based on replicate treatment at eight different concentrations of each test compound were compared for the statistical significance of the differences observed.
RESULTS
Transport of TFV by Pgp.
The high expression of Pgp on the apical side of Caco-2 cells should result in a reduction of the forward permeability and increase in the reverse permeability for a Pgp substrate when tested in a transwell system. When combined, these changes should cause a Pgp substrate to have an efflux ratio (reverse permeability/forward permeability) of significantly greater than 1. The model Pgp substrate digoxin was found to have an efflux ratio of 9.9, and its flux was markedly affected by addition of the Pgp inhibitor CsA (Fig. 1A). In contrast, TFV showed an efflux ratio of close to 1 and addition of CsA did not markedly affect its forward or reverse permeability (Fig. 1B).
FIG. 1.
Forward and reverse permeability of 10 μM digoxin (A) or 50 μM TFV (B) in the presence or absence of CsA through Caco-2 monolayers. The Pgp substrate digoxin, when incubated alone, had a reverse permeability 9.9-fold higher than its forward permeability (***, P < 0.001 by Student's unpaired two-tailed t test assuming equal variance), and a marked change in permeability was observed upon addition of CsA. No significant difference in TFV forward and reverse permeability or effect of CsA was observed (all P values, >0.05). Similar results for TFV were obtained when it was incubated at 5 μM (data not shown). Values represent the mean ± standard deviation from three independent studies done in duplicate.
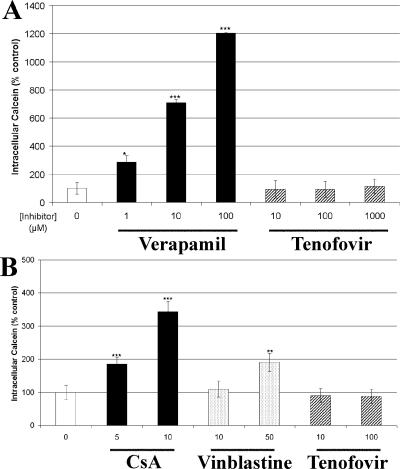
The accumulation of calcein after incubation with calcein AM in Pgp-overexpressing cells should be increased by the presence of a Pgp substrate or inhibitor because of competition or inhibition of Pgp-mediated calcein AM efflux. Incubations of calcein AM were done with parental or Pgp-transfected MDCKII cells to determine if TFV can interfere with the transport of a Pgp substrate. While the Pgp substrate verapamil was capable of significantly increasing the intracellular fluorescent signal from calcein in Pgp-transfected cells, TFV at concentrations of up to 1,000 μM showed no effect (Fig. 2A).
FIG. 2.
Effects of TFV and transport inhibitors on accumulation of the fluorescent substrate calcein after incubation of Pgp-transfected (A) or MRP2-transfected (B) MDCKII cells with calcein AM. The Pgp inhibitor verapamil and the MRP2 inhibitors CsA and vinblastine were used as positive controls. TFV, at all of the concentrations tested, showed no effect on the activity of either Pgp or MRP2. Values are the mean ± standard deviation from at least two independent experiments done in duplicate. Statistical significance was assessed by Student's unpaired two-tailed t test assuming equal variance (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
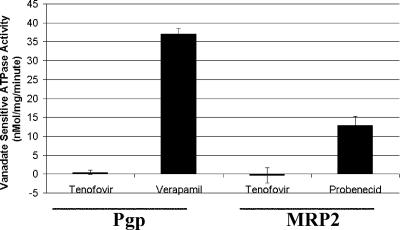
Transporters from the ABC family couple ATP hydrolysis with the transport of substrates. Therefore, in the presence of a substrate, the ATPase activity of Pgp should be stimulated. It was found that while the Pgp substrate verapamil could significantly stimulate ATPase activity, TFV was unable to elicit a marked effect on Pgp ATPase activity (Fig. 3).
FIG. 3.
Stimulation of vanadate-sensitive ATPase activity in isolated insect membranes overexpressing Pgp or MRP2 by known substrates and TFV. While verapamil was able to markedly increase the amount of vanadate-sensitive ATPase activity, TFV at a concentration of 1,000 μM was unable to stimulate ATP hydrolysis by Pgp-overexpressing membranes (A). While probenecid caused a marked increase in the amount of vanadate-sensitive ATPase activity, TFV at a concentration of 1,000 μM was unable to stimulate ATP hydrolysis by MRP2-overexpressing membranes (B). Values represent the mean ± standard deviation of at least two independent experiments done in duplicate.
Transport of TFV by MRP2.
Similar to studies done with Pgp-overexpressing cells, the effect of TFV on calcein accumulation in MRP2-overexpressing cells was determined. As shown in Fig. 2B, unlike the MRP2 substrates CsA and vinblastine, TFV was unable to alter calcein levels. It was also found that TFV did not stimulate the ATPase activity associated with MRP2 (Fig. 3).
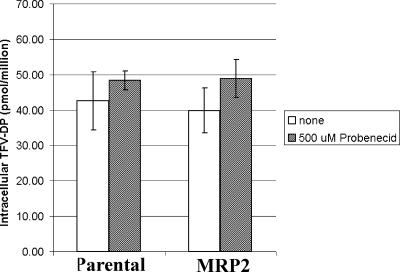
TFV and its metabolites did not accumulate to markedly different levels in parental versus MRP2-transfected 2008 cells. Incubation in the presence of the MRP2 inhibitor probenecid did not result in a selective increase in TFV or its phosphorylated metabolites in MRP2-transfected cells (Fig. 4). Treatment of MRP2-expressing 2008 cells with the MRP inhibitor MK571 at 100 μM also did not show a selective increase the intracellular levels of TFV (data not shown). Similar to results obtained with 2008 cells, studies with MRP2-overexpressing MDCKII cells showed no indication of the specific MRP2-mediated efflux of TFV (data not shown). Consistent with these observations, it was found that MRP2 expression did not alter the cytotoxicity of TFV in MDCKII cells. In contrast, the known MRP2 substrate vinblastine showed 2.9-fold lower cytotoxicity in MRP2-overexpressing cells relative to the parental cell line (Table 1).
FIG. 4.
Effect of MRP2 expression on TFV-diphosphate (DP) accumulation in 2008 cells. Parental or MRP2-overexpressing 2008 cells were incubated with 1 μM TDF in the presence or absence of the MRP2 inhibitor probenecid. No significant difference in TFV-DP levels was observed between parental and MRP2-overexpressing cells. Addition of probenecid caused a slight increase in TFV-DP levels in both parental and MRP2-overexpressing 2008 cells. Values represent the mean ± standard deviation of three independent experiments done in duplicate. Experiments with the positive control compound calcein AM showed fivefold lower accumulation in 2008 cells overexpressing MRP2 relative to nontransfected cells (data not shown).
TABLE 1.
Effect of MRP2 expression on cytotoxicity of vinblastine and TFV in MDCKII cells
| Compound | CC50 (μM)
|
Fold changea | |
|---|---|---|---|
| Wild type | MRP2 | ||
| Vinblastine | 0.0039 ± 0.0014 | 0.012 ± 0.004 | 2.9b |
| TFV | 17,600 ± 1,000 | 11,700 ± 500 | 0.7 |
Values represent the mean ± standard deviation of three independent experiments done in triplicate.
Significant (P < 0.05) decrease in toxicity due to MRP2 expression based on Student's unpaired two-tailed t test assuming equal variance.
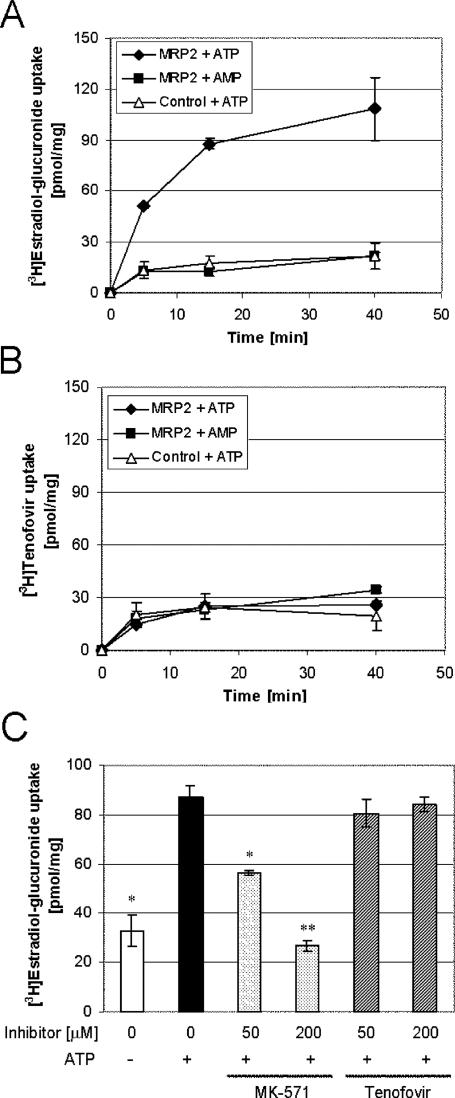
To further confirm the conclusion that MRP2 does not interact with TFV in a more controlled system not requiring initial cellular substrate permeation, experiments were performed with membrane vesicles isolated from insect cells overexpressing MRP2. Unlike a control MRP2 substrate, estradiol-17-β-d-glucuronide, addition of ATP did not stimulate the uptake of radiolabeled TFV into MRP2 vesicles (Fig. 5A and B). In addition, there was no difference between TFV accumulation in MRP2-containing vesicles and that in control vesicles in the presence of ATP. The proper function and specificity of transport in vesicles were further tested with the established MRP2 substrates vinblastine and methotrexate. Both drugs showed ATP-dependent accumulation in MRP2 vesicles but not in control vesicles (data not shown). It is expected that an MRP2 substrate should compete with the transport of other substrates. While MK571 was able to inhibit the transport of estradiol-17-β-d-glucuronide, TFV at concentrations of up to 200 μM showed no effect (Fig. 5C).
FIG. 5.
Uptake of estradiol-17-β-d-glucuronide and TFV into membrane vesicles derived from insect cells. Addition of ATP, but not AMP, stimulated the uptake of estradiol-17-β-d-glucuronide into MRP2 vesicles (A). No specific increase in the accumulation of TFV was noted in MRP2 vesicles upon addition of ATP (B). While addition of 200 μM MK571 was able to reduce the transport of estradiol-17-β-d-glucuronide to levels observed in the absence of ATP, TFV at a concentration of 50 or 200 μM was unable to inhibit estradiol-17-β-d-glucuronide accumulation (C). Values represent the mean ± standard deviation of two independent experiments performed in duplicate. Statistical significance was assessed by Student's unpaired two-tailed t test assuming equal variance (*, P < 0.05; **, P < 0.01).
Transport of TFV by MRP4.
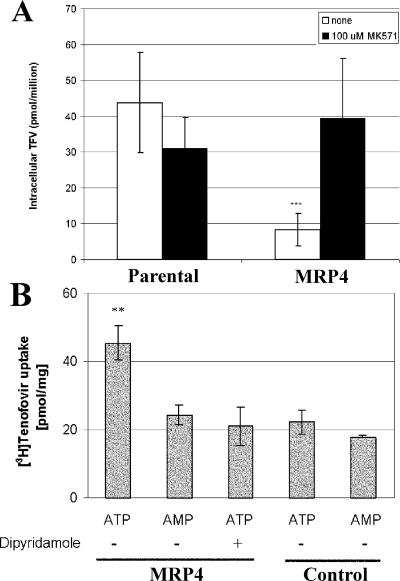
To determine the effect of MRP4 expression on the accumulation and cytotoxicity of TFV, experiments were performed with a CEM cell line previously selected for resistance to the structurally similar acyclic nucleotide analog adefovir (26). The selected cells have been shown to overexpress MRP4 through gene amplification (31). In contrast to results obtained with MRP2-overexpressing cells, TFV and its metabolites were found to accumulate to significantly lower levels in MRP4-overexpressing cells (CEM-R1) relative to parental cells (CEM-SS) following the treatment of both cell lines with TDF. Furthermore, the effect of MRP4 overexpression on the intracellular accumulation of TFV and its phosphorylated metabolites could be reversed by addition of the MRP inhibitor MK571 (Fig. 6A). Similar MK571-sensitive degrees of difference in TFV accumulation between MRP4-overexpressing and parental cells were observed when cells were incubated with 100 μM TFV (data not shown). Consistent with the effect of MRP4 on the intracellular accumulation of TFV and its metabolites, it was found that TFV and TDF were 2.0- to 2.5-fold less toxic in MRP4-overexpressing cells than in parental cells. Similar to previously published results (26), MRP4-overexpressing cells were also found to be resistant to cytotoxicity caused by adefovir (Table 2).
FIG. 6.
Effect of MRP4 expression on TFV accumulation in MRP4-overexpressing cells (A) or vesicles (B). Parental and MRP4-overexpressing cells (CEM-SS and -R1, respectively) were treated with TDF for 1 h in the presence or absence of the MRP inhibitor MK571 at 100 μM. MRP4-overexpressing cells were found to accumulate fivefold less TFV than parental cells (***, P < 0.001, based on Student's unpaired two-tailed t test assuming equal variance). Values are the mean ± standard deviation of six independent experiments done in duplicate. Addition of MK571 increased TFV concentrations in MRP4-overexpressing cells to levels similar to those observed in parental cells. Levels in the presence of MK571 represent the mean ± standard deviation of three independent experiments done in duplicate. Panel B shows MRP4-mediated uptake of TFV into membrane vesicles. MRP4 and control vesicles were incubated in parallel with 5 μM [3H]TFV in the presence of ATP or AMP for 10 min. Where indicated, 50 μM dipyridamole was added to the reaction mixture. TFV uptake into MRP4 vesicles in the presence of ATP was significantly higher than that observed in all of the other samples (**, P < 0.05, based on Student's unpaired two-tailed t test assuming equal variance). Values are the mean ± standard deviation of two independent experiments done in duplicate.
To further confirm conclusions from cell-based experiments in a more controlled system not requiring initial cellular substrate permeation, uptake studies were performed with membrane vesicles containing recombinant MRP4. Following incubation with 5 μM [3H]TFV, ATP-dependent accumulation of the substrate was detected only in MRP4 vesicles and not in control vesicles lacking the transporter (Fig. 6B). In addition, dipyridamole, a potent inhibitor of MRP4 (35), fully suppressed the ATP-stimulated vesicular uptake of TFV. Although the efficiency of TFV uptake into membrane vesicles was not sufficient to determine its affinity for MRP4, these results are consistent with the cell-based assays and directly support the conclusion that, unlike Pgp and MRP2, MRP4 is capable of mediating active cellular efflux of TFV.
DISCUSSION
In this study, we assessed the ability of highly expressed apical proximal-tubule efflux transporters to interact with TFV. It was found that MRP4, and not MRP2 or Pgp, was capable of transporting TFV. As renal elimination is the dominant mode of TFV clearance, these findings are important in expanding our knowledge of the molecular pharmacology of TFV.
Pgp is involved in the active tubular secretion of digoxin, and inhibition of Pgp-mediated renal clearance of digoxin has been implicated in drug interactions with Pgp inhibitors (7). Therefore, Pgp could play a role in the active tubular secretion of TFV and also mediate renal drug-drug interaction. However, our results obtained in Caco-2 permeability, calcein AM uptake, and ATPase studies show that TFV is not a substrate for Pgp. These data are consistent with TFV not fitting the normal structure-activity relationship for Pgp, which preferentially transports large, hydrophobic, and often basic molecules (32).
Results presented here illustrate that TFV is also not a substrate for MRP2. (i) MRP2 expression does not alter accumulation of TFV in cells or membrane vesicles, (ii) MRP2 expression does not decrease the toxicity of TFV, (iii) TFV does not interfere with the transport of known MRP2 substrates in whole cells or in membrane vesicles, and (iv) TFV was found not to stimulate MRP2 ATPase activity. These findings contradict previous reports stating that TFV and/or other acyclic nucleotide phosphonates are substrates for MRP2. One report concluded that the acyclic nucleoside phosphonates adefovir and cidofovir are substrates for MRP2 on the basis of slight inhibition of apical transport of a fluorescein-methotrexate conjugate in an intact killifish proximal-tubule model (19). However, while fluorescein-methotrexate has been shown to be an MRP2 and MRP3 substrate (18), methotrexate has also been shown to be an MRP4 substrate (4, 16). Thus, a plausible explanation for the weak inhibition of fluorescein-methotrexate secretion in killifish tubules, previously attributed to MRP2, is the competition between acyclic nucleoside phosphonates and fluorescein-methotrexate at the level of MRP4. In another report, other investigators concluded that inhibition of MRP2-mediated TFV transport increases the cytotoxicity of TFV in MDCKII cells (Louie et al., 6th Int. Workshop Clin. Pharmacol. HIV Ther.). However, this work did not directly establish TFV as a substrate for MRP2 or show any effect of MRP2 expression on TFV cytotoxicity. In fact, in the present study we showed, in the same MDCKII cell line system used in the previous report, that MRP2 overexpression does not decrease the toxicity of TFV. On the basis of our results in multiple independent in vitro assays and the caveats to other studies, we conclude that MRP2 does not mediate active tubular secretion of TFV.
In contrast to MRP2, we obtained experimental evidence in multiple independent assay systems that TFV is a substrate for MRP4. (i) MRP4 expression decreases the intracellular accumulation of TFV, (ii) TFV accumulation can be increased in MRP4-overexpressing cells in the presence of an MRP inhibitor, (iii) TFV is selectively taken up by MRP4-containing membrane vesicles in an ATP-dependent manner, and (iv) MRP4-overexpressing cells are less sensitive to both TFV- and TDF-induced cytotoxicity than are control cells. The cell line used in these studies was previously selected for resistance to adefovir and shows a large change in MRP4 expression through gene amplification (31) and a minor (twofold) decrease in phosphorylation that does not appear to play a major role in the drug resistance phenotype of these cells (26). Considering that the structures of adefovir and TFV only differ by a methyl-group substitution in the sugar-like aliphatic linker, it is not surprising that TFV is a substrate for MRP4. Interestingly, a difference was observed in the magnitude of resistance to cytotoxicity induced by TFV or adefovir caused by MRP4. The MRP4-overexpressing cells were found to be resistant to TFV cytotoxicity by only 2.0- to 2.5-fold and to adefovir cytotoxicity by >9.5-fold. This differential effect of MRP4 expression on cytotoxicity may be related to different mechanisms of cellular toxicity caused by TFV and adefovir. TFV has been noted to have low cytotoxicity in a number of cell types (5) and no evidence of mitochondrial toxicity (2). Alternatively, the difference in the degrees of change may be due to the high concentrations of TFV required to cause cellular toxicity in this system saturating the effect of efflux transport. Consistent with either of these hypotheses, both TFV and adefovir have been shown to have similar degrees of reduction in their anti-human immunodeficiency virus activity in response to MRP4 overexpression (14- and 27-fold, respectively) (28). Transport of TFV by MRP4 was also independently determined in membrane vesicles. The lack of transport by Pgp and MRP2 and the prior observation that MRP4 is expressed at approximately fivefold higher levels than MRP2 in proximal-tubule cells (33) suggest that MRP4 is a viable candidate for the major apical transporter involved in the active tubular secretion of TFV.
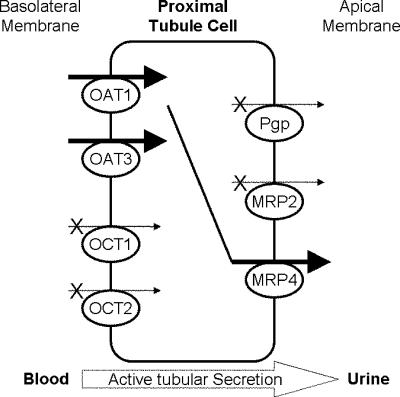
On the basis of the characterization of apical efflux pumps presented here and the previous work on basolateral influx transporters localized in proximal tubules (6; Cihlar et al., 44th ICAAC), we propose a molecular model for the active tubular secretion of TFV (Fig. 7). These studies suggest that transport of TFV through proximal-tubule cells via the coupled action of hOAT1-hOAT3 and MRP4 likely results in the pharmacokinetic observation of net active tubular secretion of TFV in patients treated with TDF. Recent in vivo findings on adefovir support this model, showing that MRP4 is responsible for most of the apical drug efflux on the basis of studies comparing renal clearance in wild-type and MRP4 knockout mice (T. Imaoka, H. Kusuhara, M. Adachi, J. Shuetz, and Y. Sugiyama, Abstr. 1st Asia Pacific Int. Soc. Study Xenobiotics, abstr. 11, 2006). While TFV is subject to net active tubular secretion, interaction with basolateral efflux pumps involved in reabsorption from the proximal tubule may also play a role in the renal handling of TFV. For instance, the basolateral efflux pump MRP5 has been shown to interact with adefovir (25). Besides providing a better understanding of the molecular pharmacology of TFV, delineation of the renal secretory pathway of TFV should allow assessment of the potential for renal drug-drug interactions between TFV and other coadministered agents.
FIG. 7.
Mechanism of active tubular secretion of TFV. TFV is transported from the blood into proximal-tubule cells by the parallel action of basolateral transporters hOAT1 and hOAT3. Subsequently, TFV is effluxed from proximal-tubule cells into urine by the MRP4 efflux pump expressed on the apical membrane. Neither hOCT1, hOCT2, MRP2, nor Pgp is involved in active tubular secretion of TFV.
Acknowledgments
We thank Ismael Hidalgo (Absorption Systems), Arnold Fridland (Gilead Sciences, Inc.), and Piet Borst (Netherlands Cancer Institute) for providing cells, advice on assays, and thoughtful discussion of the manuscript.
REFERENCES
- 1.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkus, G., M. J. M. Hitchcock, and T. Cihlar. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonate, P. L., K. Reith, and S. Weir. 1998. Drug interactions at the renal level. Implications for drug development. Clin. Pharmacokinet. 34:375-404. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Z. S., K. Lee, S. Walther, R. B. Raftogianis, M. Kuwano, H. Zeng, and G. D. Kruh. 2002. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 62:3144-3150. [PubMed] [Google Scholar]
- 5.Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. M. Hitchcock. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 54:37-45. [DOI] [PubMed] [Google Scholar]
- 6.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 7.Ding, R., Y. Tayrouz, K. D. Riedel, J. Burhenne, J. Weiss, G. Mikus, and W. E. Haefeli. 2004. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin. Pharmacol. Ther. 76:73-84. [DOI] [PubMed] [Google Scholar]
- 8.DiSaia, P. J., J. G. Sinkovics, F. N. Rutledge, and J. P. Smith. 1972. Cell-mediated immunity to human malignant cells. A brief review and further studies with two gynecologic tumors. Am. J. Obstet. Gynecol. 114:979-989. [DOI] [PubMed] [Google Scholar]
- 9.Drueckes, P., R. Schinzel, and D. Palm. 1995. Photometric microtiter assay of inorganic phosphate in the presence of acid-labile organic phosphates. Anal. Biochem. 230:173-177. [DOI] [PubMed] [Google Scholar]
- 10.Ernest, S., S. Rajaraman, J. Megyesi, and E. N. Bello-Reuss. 1997. Expression of MDR1 (multidrug resistance) gene and its protein in normal human kidney. Nephron 77:284-289. [DOI] [PubMed] [Google Scholar]
- 11.Evers, R., M. Kool, A. J. Smith, L. van Deemter, M. de Haas, and P. Borst. 2000. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br. J. Cancer 83:366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo, I. J., T. J. Raub, and R. T. Borchardt. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736-749. [PubMed] [Google Scholar]
- 13.Hooijberg, J. H., H. J. Broxterman, M. Kool, Y. G. Assaraf, G. J. Peters, P. Noordhuis, R. J. Scheper, P. Borst, H. M. Pinedo, and G. Jansen. 1999. Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res. 59:2532-2535. [PubMed] [Google Scholar]
- 14.Izzedine, H., V. Launay-Vacher, and G. Deray. 2005. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 45:804-817. [DOI] [PubMed] [Google Scholar]
- 15.Klokouzas, A., C. P. Wu, H. W. van Veen, M. A. Barrand, and S. B. Hladky. 2003. cGMP and glutathione-conjugate transport in human erythrocytes. Eur. J. Biochem. 270:3696-3708. [DOI] [PubMed] [Google Scholar]
- 16.Lee, K., A. J. Klein-Szanto, and G. D. Kruh. 2000. Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J. Natl. Cancer Inst. 92:1934-1940. [DOI] [PubMed] [Google Scholar]
- 17.Li, T., K. Ito, and T. Horie. 2003. Transport of fluorescein methotrexate by multidrug resistance-associated protein 3 in IEC-6 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G602-G610. [DOI] [PubMed] [Google Scholar]
- 18.Masereeuw, R., F. G. Russel, and D. S. Miller. 1996. Multiple pathways of organic anion secretion in renal proximal tubule revealed by confocal microscopy. Am. J. Physiol. 271:F1173-F1182. [DOI] [PubMed] [Google Scholar]
- 19.Miller, D. S. 2001. Nucleoside phosphonate interactions with multiple organic anion transporters in renal proximal tubule. J. Pharmacol. Exp. Ther. 299:567-574. [PubMed] [Google Scholar]
- 20.Motohashi, H., Y. Sakurai, H. Saito, S. Masuda, Y. Urakami, M. Goto, A. Fukatsu, O. Ogawa, and K. Inui. 2002. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 13:866-874. [DOI] [PubMed] [Google Scholar]
- 21.Ray, A. S. 2005. Intracellular interactions between nucleos(t)ide inhibitors of HIV reverse transcriptase. AIDS Rev. 7:113-125. [PubMed] [Google Scholar]
- 22.Ray, A. S., F. Myrick, J. E. Vela, L. Y. Olson, E. J. Eisenberg, K. Borroto-Esodo, M. D. Miller, and A. Fridland. 2005. Lack of a metabolic and antiviral drug interaction between tenofovir, abacavir and lamivudine. Antivir. Ther. 10:451-457. [PubMed] [Google Scholar]
- 23.Ray, A. S., L. Olson, and A. Fridland. 2004. Role of purine nucleoside phosphorylase in interactions between 2′,3′-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrob. Agents Chemother. 48:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray, A. S., J. E. Vela, L. Olson, and A. Fridland. 2004. Effective metabolism and long intracellular half life of the anti-hepatitis B agent adefovir in hepatic cells. Biochem. Pharmacol. 68:1825-1831. [DOI] [PubMed] [Google Scholar]
- 25.Reid, G., P. Wielinga, N. Zelcer, M. De Haas, L. Van Deemter, J. Wijnholds, J. Balzarini, and P. Borst. 2003. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 63:1094-1103. [DOI] [PubMed] [Google Scholar]
- 26.Robbins, B. L., M. C. Connelly, D. R. Marshall, R. V. Srinivas, and A. Fridland. 1995. A human T lymphoid cell variant resistant to the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine shows a unique combination of a phosphorylation defect and increased efflux of the agent. Mol. Pharmacol. 47:391-397. [PubMed] [Google Scholar]
- 27.Rollot, F., E. M. Nazal, L. Chauvelot-Moachon, C. Kelaidi, N. Daniel, M. Saba, S. Abad, and P. Blanche. 2003. Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: the role of lopinavir-ritonavir-didanosine. Clin. Infect. Dis. 37:e174-e176. [DOI] [PubMed] [Google Scholar]
- 28.Sampath, J., M. Adachi, S. Hatse, L. Naesens, J. Balzarini, R. M. Flatley, L. H. Matherly, and J. D. Schuetz. 2002. Role of MRP4 and MRP5 in biology and chemotherapy. AAPS Pharm. Sci. 4(E14):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkadi, B., E. M. Price, R. C. Boucher, U. A. Germann, and G. A. Scarborough. 1992. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J. Biol. Chem. 267:4854-4858. [PubMed] [Google Scholar]
- 30.Schaub, T. P., J. Kartenbeck, J. Konig, O. Vogel, R. Witzgall, W. Kriz, and D. Keppler. 1997. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J. Am. Soc. Nephrol. 8:1213-1221. [DOI] [PubMed] [Google Scholar]
- 31.Schuetz, J. D., M. C. Connelly, D. Sun, S. G. Paibir, P. M. Flynn, R. V. Srinivas, A. Kumar, and A. Fridland. 1999. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat. Med. 5:1048-1051. [DOI] [PubMed] [Google Scholar]
- 32.Seelig, A. 1998. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 251:252-261. [DOI] [PubMed] [Google Scholar]
- 33.Smeets, P. H., R. A. van Aubel, A. C. Wouterse, J. J. van den Heuvel, and F. G. Russel. 2004. Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J. Am. Soc. Nephrol. 15:2828-2835. [DOI] [PubMed] [Google Scholar]
- 34.St. Claire, R. L., III. 2000. Positive ion electrospray ionization tandem mass spectrometry coupled to ion-pairing high-performance liquid chromatography with a phosphate buffer for the quantitative analysis of intracellular nucleotides. Rapid Commun. Mass Spectrom. 14:1625-1634. [DOI] [PubMed] [Google Scholar]
- 35.van Aubel, R. A., P. H. Smeets, J. G. Peters, R. J. Bindels, and F. G. Russel. 2002. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J. Am. Soc. Nephrol. 13:595-603. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann, A. E., T. Pizzoferrato, J. Bedford, A. Morris, R. Hoffman, and G. Braden. 2006. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin. Infect. Dis. 42:283-290. [DOI] [PubMed] [Google Scholar]