Abstract
Evaluating new therapeutic agents for invasive aspergillosis requires animal models that are reproducible among different laboratories. We therefore evaluated a murine model of aerosol infection in two independent laboratories and found a high level of both intra- and interlaboratory reproducibility of survival, fungal burden over time, and the efficacy of liposomal amphotericin B.
Murine models of invasive pulmonary aspergillosis have been invaluable for the assessment of novel therapeutics and diagnostics, as well as for the study of disease pathogenesis. A wide variety of these models have been described, including intravenous (3, 6), intranasal (5), intratracheal (13), and inhalation (10-12) infections. Although the availability of such a diverse group of model systems is useful for many specific questions, the methodological differences among models have made direct comparisons of individual studies difficult. Furthermore, there have been very few studies of the interlaboratory variability of results obtained with the same animal model. Demonstration of interlaboratory reproducibility is a critical benchmark in the development of a standardized model of invasive aspergillosis. The availability of such a standardized model would provide a useful benchmark for the evaluation of new diagnostic and therapeutic strategies across geographically separated laboratories.
We recently described a simple and reproducible animal model of invasive pulmonary aspergillosis in which mice are infected by inhalation using an aerosol chamber (10). To evaluate the reproducibility of this model, we used it to compare the time course of mortality and fungal burden and the efficacy of liposomal amphotericin B in two different laboratories.
Strains and culture conditions.
Aspergillus fumigatus Af293 was used for all studies. To obtain conidia for the infections, organisms were grown for 10 days on Sabouraud dextrose agar at 37°C. Conidia were harvested by flooding the plate with phosphate-buffered saline supplemented with 0.1% Tween 80 and concentrated by centrifugation to the desired concentration (10).
Animal model.
Male BALB/c mice, 20 to 22 g (National Cancer Institute) were used for all experiments. Mice were infected in both laboratories by inhalation in an acrylic aerosol chamber using an identical protocol as described previously (10). Briefly, mice were immunosuppressed with cyclosphosphamide (250 mg/kg on day −2, relative to infection, and 200 mg/kg on day +3) and cortisone acetate (200 mg/kg on day −2 and day +3). On the day of infection, mice were exposed in an acrylic chamber for 1 h to an aerosol generated from 12 ml of phosphate-buffered saline supplemented with 0.1% Tween 80 containing 109 A. fumigatus conidia per ml. Mice were monitored daily and sacrificed when moribund. To prevent bacterial infection, all immunosuppressed mice received ceftazidime (5 mg/day subcutaneously) from days 1 to 6 after infection. For treatment studies, liposomal amphotericin B (Ambisome; Fujisawa) was administered daily at 10 mg/kg/d by intraperitoneal injection for 8 days, beginning the day after infection (1, 4). All procedures involving mice were approved by both institutional animal care and use committees according to the National Institutes of Health guidelines for animal housing and care.
For the determination of fungal burden, groups of 10 mice were sacrificed, and their lungs were removed and homogenized either using blunt crushing of tissue in 5 ml of sterile 0.85% saline within Whirl-Pak plastic sample bags (Fisher Scientific) (10) or, alternately, in the same volume using a Wheaton tissue homogenizer (VWR) set at 1,200 rpm. A Safe-Grind plastic-coated tissue grinder with a Teflon pestle (10 ml; catalog no. 358007 [Wheaton]) was used for the homogenization.
Aliquots were removed and serially diluted for quantitative culture in Sabouraud dextrose agar. Plates were incubated at 37°C, and the CFU were counted after 24 h of incubation. All homogenizations and cultures were performed on site at each individual laboratory.
Intra- and interlaboratory reproducibility of survival.
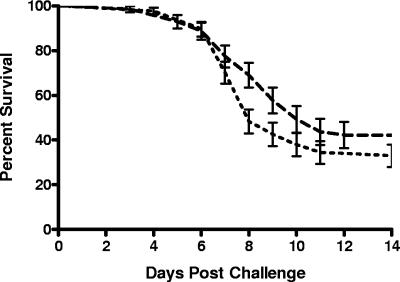
We first compared the survival of untreated mice that were infected according to the standard protocol at both laboratory sites. Seven independent experiments were performed at laboratory 1, and nine were performed at laboratory 2 over a 1-year period. The overall mortality of untreated, mice infected with strain Af293 by aerosol was very similar over multiple experiments performed in both laboratories (Fig. 1, overall survival of 42.7% at laboratory 1 versus 33.4% at laboratory 2).
FIG. 1.
Intralaboratory reproducibility of the survival of mice infected in the aerosol model at two different laboratories. The composite survival of mice infected with strain Af293 at two independent laboratories was charted. The results include seven independent experiments at laboratory 1 and nine experiments at laboratory 2. The data are presented as the mean ± the standard deviation for each laboratory. Upper dashed curve, laboratory 1 (n = 71); lower dashed curve, laboratory 2 (n = 87). P = 0.1550.
Interlaboratory reproducibility of the response to liposomal amphotericin B.
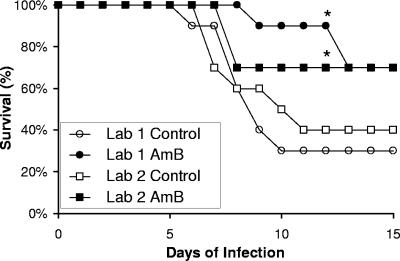
Next, we compared the ability of systemic antifungal therapy to improve survival in this model of invasive aspergillosis at both laboratories. The administration of liposomal amphotericin B at 10 mg/kg/d resulted in a significant increase in survival at both institutions (Fig. 2). Indeed, the overall mortality in these treatment groups was identical at both sites (30%). Collectively, the results suggest a high degree of laboratory-to-laboratory reproducibility for this aerosol model of infection, both from the perspective of virulence and from the perspective of the evaluation of pharmaceutical agents.
FIG. 2.
Interlaboratory reproducibility of the efficacy of liposomal amphotericin B. Survival of mice infected with A. fumigatus Af293 by aerosol and treated with liposomal amphotericin B (AmB) or vehicle alone (Control). Ten mice were infected in each group at each laboratory. An asterisk indicates a significant difference compared to the untreated control group at the same laboratory as determined by log-rank testing with P < 0.05.
Interlaboratory reproducibility of tissue fungal burden.
In addition to the survival studies, we also performed independent experiments to compare the pulmonary fungal burden of liposomal amphotericin B-treated and untreated mice by the quantitative culture of lungs harvested after 6 days of infection. The results from the two laboratories were somewhat different, with an ∼0.5-log-higher pulmonary fungal burden in mice infected in laboratory 2 (Table 1) . Despite this overall difference, treatment with liposomal amphotericin B resulted in a modest but significant reduction in pulmonary CFU in both laboratories.
TABLE 1.
Interlaboratory reproducibility of the effects of liposomal amphotericin B on pulmonary fungal burden as determined by quantitative culture 6 days after infection
| Laboratory | No. of mice | Method | Mean log10 CFU/g of mouse lung tissue ± SEa
|
MECb | |
|---|---|---|---|---|---|
| Control | AmBisome 10 | ||||
| 1 | 10 | Whirl-Pak | 2.92 ± 0.13 | 2.13 ± 0.11* | −0.79 |
| 2 | 20 | Grinder | 3.81 ± 0.09 | 3.42 ± 0.09* | −0.47 |
*, P = 0.001 versus controls.
MEC, mean effected change.
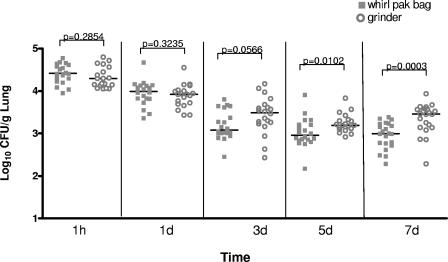
The only difference in experimental protocols between the two laboratories was that the lungs were homogenized using the blunt-crush Whirl-Pak method in laboratory 1, whereas a tissue grinder was used in laboratory 2. Therefore, we hypothesized that the differences in fungal burden were a consequence of differences in the method of tissue homogenization. To test this hypothesis, we compared the time course of pulmonary fungal burden using the blunt-crush versus the tissue grinder methods of tissue disruption at both laboratories in an independent set of experiments. As we have previously shown, there was an apparent decrease in fungal burden as determined by quantitative culture over the first 24 h (9). This probably represents the decreased efficiency of homogenization of large complex hyphal lesions compared to single conidia and short hyphae that are present in the lungs immediately after infection (9). For both the Whirl-Pak and the grinder methods, there was no significant difference between the average fungal burden between the two institutions (P > 0.05 for both methods as determined by the Wilcoxon-rank sum test). In contrast to the interlaboratory reproducibility of quantitative culture, we observed a systematic difference between the results of quantitative culture of homogenates obtained by the blunt-crush method compared to the tissue grinder method. The tissue grinder method yielded significantly higher quantitative culture results from day 3 of infection onward (Fig. 3). Importantly, these differences in quantitative fungal burden are similar to the difference in the baseline pulmonary fungal burden observed in the initial liposomal amphotericin study at 6 days after infection (Table 1).
FIG. 3.
Comparison of two methodologies of tissue homogenization for the determination of pulmonary fungal burden. The fungal burden at each time point by each method is shown, combining the results from both laboratories (20 mice per homogenization method per time point). Bars indicate the mean fungal burden. For each laboratory, all mice were infected at the same time and then randomly divided into groups for evaluation using each method.
Collectively, these results suggest that the same method of tissue homogenization should be used to ensure reproducible results between laboratories. We found that tissue disruption using a tissue grinder results in consistently higher fungal CFU than does the blunt crush method at the later time points of infection. This observation may reflect more efficient tissue disruption by the mechanical grinder, which leads to increased dispersion of fungal aggregates into individual CFU. These results also highlight the inherent limitations of quantitative culture methods. The use of alternate techniques, such as quantitative PCR (2, 7, 9), might prove useful as alternative measures of disease progression.
In contrast to the aerosol model described here, previous comparisons of animal models of invasive aspergillosis have found wide variability across laboratories. Graybill et al. compared an inhalation model of invasive aspergillosis at two different laboratory sites and observed a systematic difference in quantitative fungal burden between the two laboratories of over 1 log using this model despite the use of an almost identical protocol (8). Further, when the efficacy of amphotericin B was evaluated at both sites, a significant survival advantage was demonstrated at only one of the two laboratories (8).
The aerosol model of invasive aspergillosis described here was reproducible between two laboratories with respect to overall survival, the time course of pulmonary fungal burden, and the efficacy of liposomal amphotericin B. Collectively, these results suggest that this model provides a useful tool in the evaluation of therapeutic agents for invasive aspergillosis.
Acknowledgments
This study was supported with federal funds from the National Institute of Allergy and Infectious Diseases under contract no. N01-AI-30041 1. D.C.S. is supported by a Burroughs-Welcome Fund Career Award in the Biomedical Sciences and a Clinician Scientist award from the Canadian Institutes of Health. We also thank Marcos Olivo and Destiny Molina for technical assistance.
REFERENCES
- 1.Bellocchio, S., R. Gaziano, S. Bozza, G. Rossi, C. Montagnoli, K. Perruccio, M. Calvitti, L. Pitzurra, and L. Romani. 2005. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signaling from TLR-2 to TLR-4. J. Antimicrob. Chemother. 55:214-222. [DOI] [PubMed] [Google Scholar]
- 2.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford, S., and L. Friedman. 1967. Experimental study of the pathogenicity of aspergilli for mice. J. Bacteriol. 94:928-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaziano, R., S. Bozza, S. Bellocchio, K. Perruccio, C. Montagnoli, L. Pitzurra, G. Salvatori, R. De Santis, P. Carminati, A. Mantovani, and L. Romani. 2004. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 48:4414-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graybill, J. R., S. R. Kaster, and D. J. Drutz. 1983. Treatment of experimental murine aspergillosis with BAY n7133. J. Infect. Dis. 148:898-906. [DOI] [PubMed] [Google Scholar]
- 6.Hanson, L. H., K. V. Clemons, D. W. Denning, and D. A. Stevens. 1995. Efficacy of oral saperconazole in systemic murine aspergillosis. J. Med. Vet. Mycol. 33:311-317. [DOI] [PubMed] [Google Scholar]
- 7.MacCallum, D. M., J. A. Whyte, and F. C. Odds. 2005. Efficacy of caspofungin and voriconazole combinations in experimental aspergillosis. Antimicrob. Agents Chemother. 49:3697-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najvar, L. K., A. Cacciapuoti, S. Hernandez, J. Halpern, R. Bocanegra, M. Gurnani, F. Menzel, D. Loebenberg, and J. R. Graybill. 2004. Activity of posaconazole combined with amphotericin B against Aspergillus flavus infection in mice: comparative studies in two laboratories. Antimicrob. Agents Chemother. 48:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard, D. C., K. A. Marr, D. N. Fredricks, L. Y. Chiang, T. Doedt, and S. G. Filler. 2006. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin. Microbiol. Infect. 12:376-380. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinbach, W. J., D. K. Benjamin, Jr., S. A. Trasi, J. L. Miller, W. A. Schell, A. K. Zaas, W. M. Foster, and J. R. Perfect. 2004. Value of an inhalational model of invasive aspergillosis. Med. Mycol. 42:417-425. [DOI] [PubMed] [Google Scholar]
- 12.Stephens-Romero, S. D., A. J. Mednick, and M. Feldmesser. 2005. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 73:114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams, D. M., M. H. Weiner, and D. J. Drutz. 1981. Immunologic studies of disseminated infection with Aspergillus fumigatus in the nude mouse. J. Infect. Dis. 143:726-733. [DOI] [PubMed] [Google Scholar]