Abstract
Daptomycin, a novel lipopeptide, is bactericidal against a broad range of gram-positive strains, including methicillin- (MRSA) and vancomycin-resistant Staphylococcus aureus. Daptomycin is approved at 4 mg/kg of body weight given intravenously once daily for the treatment of complicated skin and skin structure infections and at 6 mg/kg for the treatment of S. aureus bloodstream infections (bacteremia), including right-sided endocarditis caused by methicillin-susceptible S. aureus and MRSA. The present study was designed to evaluate the multiple-dose pharmacokinetics and safety of daptomycin at doses of 6 to 12 mg/kg in healthy volunteers. Three cohorts of 12 subjects each were given daptomycin (10 mg/kg) or placebo once daily for 14 days, daptomycin (12 mg/kg) or placebo once daily for 14 days, or daptomycin (6 or 8 mg/kg) once daily for 4 days. Daptomycin produced dose-proportional increases in the area under the plasma concentration-time curve and in trough daptomycin levels and nearly dose-proportional increases in peak daptomycin concentrations. Other pharmacokinetic parameters measured on day 1 and at steady state were independent of the dose, including the half-life (approximately 8 h), weight-normalized plasma clearance (9 to 10 ml/h/kg), and volume of distribution (approximately 100 ml/kg). Plasma protein binding was 90% to 93% and was independent of the daptomycin concentration. Daptomycin did not produce electrocardiographic abnormalities or electrophysiological evidence of muscle or nerve toxicity. Daptomycin was well tolerated in subjects dosed with up to 12 mg/kg intravenously for 14 days. Doses of daptomycin higher than 6 mg/kg once daily may be considered in further studies to evaluate the safety and efficacy of daptomycin in difficult-to-treat infections.
Daptomycin is the first member of the novel cyclical- lipopeptide class, which provides rapid, concentration-dependent bactericidal activity against a broad range of gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) (2, 6, 9, 10). Daptomycin causes bacterial cell death by a mechanism unique among available antimicrobial agents (8, 11). It binds to the cell membranes of gram-positive bacteria, thereby disrupting their membrane potential, initiating potassium efflux with rapid cell death.
The safety and efficacy of daptomycin in treating complicated skin and skin structure infections were demonstrated in two randomized, controlled clinical trials (1). When administered at a dose of 4 mg/kg of body weight intravenously once daily for 7 to 14 days, daptomycin provided clinical success rates comparable to those of vancomycin and penicillinase-resistant penicillins and showed safety and tolerability similar to those of the comparators. On the basis of these studies, daptomycin was approved by the FDA and the European Medicines Agency for the treatment of complicated skin and skin structure infections caused by susceptible gram-positive strains, including MRSA (3). Because of its antibacterial profile, daptomycin may also be useful in treating other serious infections by gram-positive bacteria. A clinical trial designed to evaluate the safety and efficacy of daptomycin at 6 mg/kg once daily in patients with bacteremia and infective endocarditis due to S. aureus met its primary endpoint of noninferiority versus conventional therapy (7). This study served as a basis for the approval of daptomycin as a once-a-day therapy at 6 mg/kg for the treatment of S. aureus bloodstream infections (bacteremia), including right-sided endocarditis caused by methicillin-susceptible S. aureus and MRSA (4).
It was previously determined that daptomycin displays linear and time-dependent pharmacokinetics at doses up to 6 mg/kg once daily, with slight nonlinearity noted at 8 mg/kg (5), but little information has been available about the pharmacokinetics or safety of doses higher than 8 mg/kg once daily (5, 10, 12). Accordingly, the present study was designed primarily to assess the multiple-dose pharmacokinetic profile and linearity of daptomycin in healthy volunteers over the dosage range of 6 to 12 mg/kg once daily. The secondary objective was to assess the safety and tolerability of daptomycin given intravenously at doses of 10 and 12 mg/kg once daily for 14 days.
MATERIALS AND METHODS
Study design.
This study was a single-center, randomized, double-blind, placebo-controlled, ascending-dose phase 1 study conducted with healthy volunteers. The study protocol and informed-consent form were approved by an independent institutional review board. The study involved three cohorts, each consisting of 12 healthy subjects. Cohort 1 was randomly assigned in a 3:1 ratio to receive daptomycin (10 mg/kg) once daily or placebo (0.9% sodium chloride, USP) for 14 days. After a blinded review of safety data from cohort 1, cohort 2 was randomized in a 3:1 ratio to receive daptomycin (12 mg/kg) or placebo once daily for 14 days. Cohort 3 was randomized in a 1:1 ratio to receive daptomycin (6 or 8 mg/kg) once daily for 4 days in order to establish a pharmacokinetic baseline for comparisons with the higher daptomycin doses. Daptomycin or placebo was infused intravenously over 30 min in a 50-ml volume once daily for the specified duration of treatment.
Subjects were screened up to 2 weeks before the first dose of the study treatment and then entered a self-contained research unit on the day before the first dose (the baseline was day −1). The subjects remained confined to the research unit until completion of all posttreatment assessments (3 days postdose for subjects in cohorts 1 and 2 and 1 day postdose for subjects in cohort 3). The subjects in cohorts 1 and 2 returned 12 to 16 days posttreatment for follow-up electrophysiological testing and adverse-event monitoring.
Subjects.
Healthy subjects, aged 18 to 45 years, with body mass indexes (BMI) of 18.5 to 30 kg/m2 and body weights of 65 to 120 kg (men) or 45 to 90 kg (women) were eligible if they had no active medical findings and normal renal function (creatinine clearance of ≥80 ml/min as determined by the Cockcroft-Gault formula with actual body weight).
Pharmacokinetic assessments.
Plasma daptomycin concentrations were determined using blood samples collected at predose, the end of infusion, and nine other time points on days 1, 4, 7, and 14 in cohorts 1 and 2 and days 1 and 4 in cohort 3. Additional samples were collected at 36 and 48 h after the last infusion on day 14 for cohorts 1 and 2. Trough daptomycin levels were collected within 30 min of dosing on days 3 and 11 in cohorts 1 and 2 (day 3 only in cohort 3). Urine daptomycin concentrations were determined in pooled samples of urine collected over the 0- to 12- and 12- to 24-hour intervals from the start of the infusion on days 1, 7, and 14 (only day 1 in cohort 3). Serum protein binding of daptomycin was determined using blood samples collected on day 1 at 0.5, 2.5, and 8.5 h after the start of infusion.
Daptomycin concentrations in plasma and urine samples were analyzed at PPD analytical laboratory (PPD, Inc., Richmond, VA) using reverse-phase high-performance liquid chromatography with UV detection at 214 nm over the concentration range of 3 to 500 μg/ml. Interassay precision and accuracy for the 3.0-μg/ml pool were 4.8% and a −3.8% difference, respectively, from the theoretical value. The remaining quality control pools had interassay coefficients of variation ranging from 1.86 to 2.07% and percentages of difference within 1.16% of theoretical values.
The protein binding of daptomycin was analyzed by Avantix Laboratories, Inc. (Newcastle, DE). Serum protein binding of daptomycin was determined by equilibrium dialysis. Samples were extracted by solid-phase extraction and were subsequently analyzed by liquid chromatography-mass spectrophotometry. The range of the daptomycin standard curve was 0.2 to 200 μg/ml, and the coefficient of variation of the quality control samples ranged from 1.0 to 15.1%. Descriptions of the analytical methods were previously reported (5).
Pharmacokinetic parameters.
Pharmacokinetic parameters were calculated, using SAS version 8.02 software (SAS Institute, Cary, NC), from the measured plasma and urine daptomycin concentrations. The maximum plasma concentration (Cmax) and the time at which it occurred (Tmax) were determined directly from the measured plasma concentration-time data without interpolation. The trough plasma concentration (Cmin) was determined from the measured daptomycin concentration immediately prior to dosing. The area under the concentration-time curve from dosing to infinity (AUC0-∞) was determined on day 1 as the sum of AUC0-24, and the residual area after 24 h was calculated as Ct/kel. Ct is the measured plasma concentration at 24 h, and kel is the apparent elimination rate constant determined by regression analysis of the log-linear segment of the plasma concentration-time curve. AUC0-tau is the area under the concentration-time curve calculated by linear trapezoidal rule from time zero to the end of the dosing interval (i.e., 24 h) at steady state.
Plasma clearance (CLp) was determined by dividing the daptomycin dose by AUC0-∞ on day 1 or by AUC0-tau on days 4, 7, and 14 and then normalizing it for body weight (CLwp). The elimination half-life (t1/2) was calculated as the ratio of ln 2/kel.
Safety assessments.
Safety assessments were performed at baseline, during treatment, and during posttreatment. Unless otherwise indicated, the assessments in cohort 3 were made at the same time points, provided they were contained within the treatment (days 1 to 4) and posttreatment (day 1 posttreatment) periods. Standard 12-lead electrocardiograms (ECGs) were obtained within 1 h before study drug dosing on days 1, 4, 7, 10, and 14 and within 20 min following infusion on days 7 and 10. The ECGs were reviewed by an independent cardiologist. A physical examination and laboratory testing (including creatine phosphokinase [CPK]) were done at baseline and on days 4, 7, and 14 and day 3 (posttreatment) for cohorts 1 and 2 and at baseline and day 4 for cohort 3. Serum creatinine was also measured on day 1, and CPK was additionally measured on days 4, 9, and 11. Finally, urine creatinine levels were determined in urine samples collected for the 0- to 12- and 12- to 24-hour intervals on days 1, 7, and 14.
Electrophysiological testing was performed only in cohorts 1 and 2 at baseline, on the last day of dosing, and at the follow-up assessment (12 to 16 days posttreatment). Motor nerve function was assessed using the NC-Stat Nerve Conduction Monitoring System (NeutroMetrix, Waltham, MA) combined with a median nerve biosensor. The test was performed on the median motor nerve on the nondominant side, with stimulation applied approximately 2 cm proximal to the wrist crease. The electrophysiological parameters determined by this test included the compound muscle action potential (CMAP) in the abductor pollicis brevis muscle of the hand following supramaximal stimulation of the median nerve, peak amplitude of the evoked CMAP, and minimal latency of the associated F-wave. The amplitude of the CMAP reflects the number and synchrony of active motor units driven by maximal nerve stimulation and is sensitive to myopathy, alterations in neuromuscular transmission, or denervation of the muscle due to distal axonopathy. The distal latency and F-wave latency reflect the maximal conduction velocity and are sensitive to changes in the distal axon and myelin.
All adverse events that occurred after the start of the study treatment and up to 30 days after the last dose were recorded, and their severity and relationship to the study treatment were rated by the investigator.
Statistical analysis.
Demographic, safety, and pharmacokinetic data were analyzed using descriptive statistics. The achievement of steady state was tested by regressing the trough concentrations of daptomycin measured on days 3, 4, 7, 11, and 14. Because at least three trough values are required for this analysis, the Cmin values in cohort 3 included those determined on days 3 and 4, and the plasma concentration at 24 h on day 4 was considered the Cmin value for day 5. The model Cmin = a + b · day + error was used to estimate the slope and corresponding 90% interval. Steady state was not reached if the slope b was positive and significantly different from zero at the 5% level. The dose proportionalities of AUC0-∞, Cmax, and Cmin were determined on days 1 and 4 after log transformation of the power regression model into the following equation: ln (pharmacokinetic parameter) = β · ln (dose) + α. Dose proportionality was concluded to exist if the 90% confidence interval constructed for the slope estimate (β) included the value of 1.0.
All randomized subjects who received at least one dose of the study drug were included in the safety analysis.
RESULTS
Subject disposition and demographics.
Thirty-six subjects were enrolled in the study, with 12 subjects per cohort. All subjects received daptomycin or placebo and were therefore included in the safety analysis. In addition, all subjects completed the study and follow-up assessment, except for one subject who received placebo in cohort 2, who withdrew consent. Accordingly, all 30 subjects who received daptomycin were available for inclusion in the pharmacokinetic analyses.
The subjects ranged in age from 18 to 45 years and included equal numbers of men and women. The majority (89%) were Hispanic/Latino. In general, the demographic characteristics of the study subjects were comparable among the three cohorts. For the groups receiving daptomycin, the mean age ranged from 31 to 35 years, the mean BMI ranged from 24 to 27 kg/m2, and the mean weight ranged from 63 to 72 kg. All subjects had a BMI and body weight within the range defined by the study inclusion criteria.
Pharmacokinetics.
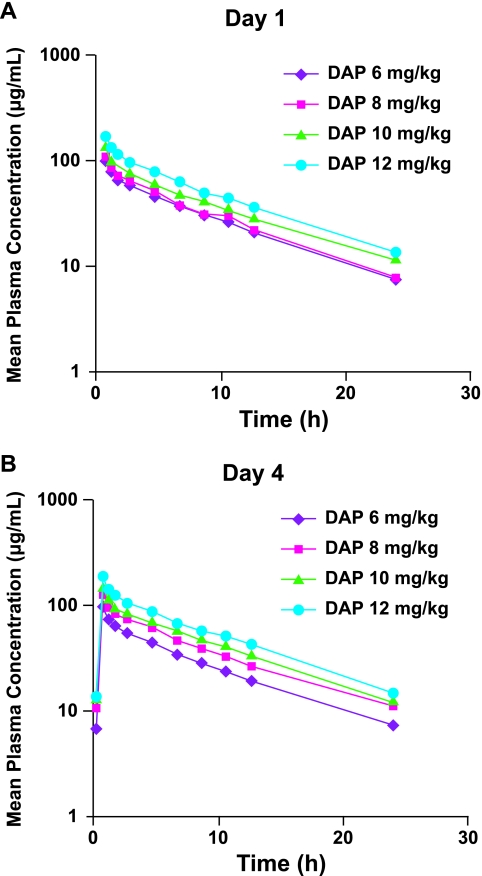
The single-dose pharmacokinetics of daptomycin were determined on day 1. Plasma concentrations of daptomycin and exposure to daptomycin (AUC0-∞) increased with the dose over the range of 6 to 12 mg/kg (Fig. 1). Cmax values were attained at the end of the 30-minute infusion period (Tmax) at all dose levels (Table 1). Daptomycin was highly bound to plasma proteins, with the fraction bound being independent of the drug concentration. Protein binding was consistent across all dose levels at 90% to 93% (i.e., mean unbound fraction values of 0.07 to 0.10). Daptomycin was eliminated mainly by the kidneys, with 37% to 68% of the administered dose being excreted as unchanged drug within 24 h. The high protein binding and predominant renal excretion led to a relatively low CLwp, ranging from 9.9 to 10.1 ml/h/kg, and a long t1/2, ranging from 7.3 to 8.4 h. Daptomycin had a small volume of distribution (V) ranging from 103 to 117 ml/kg.
FIG. 1.
Plasma daptomycin (DAP) concentration-time curves on day 1 (A) and at steady state (day 4) (B).
TABLE 1.
Pharmacokinetics of daptomycin on day 1, at steady state (day 4), and on day 14a
| Daptomycin dose (mg/kg) | Cmax (μg/ml) | AUC0-∞b (μg · h/ml) | t1/2 (h) | CLwp (ml/h/kg) | V (ml/kg) |
|---|---|---|---|---|---|
| Day 1 | |||||
| 6 | 95.7 (31.8) | 729.8 (32.2) | 7.5 (10.9) | 9.9 (12.5) | 105.9 (13.3) |
| 8 | 106.2 (20.0) | 773.3 (20.3) | 7.3 (18.4) | 10.1 (24.0) | 102.9 (11.8) |
| 10 | 129.7 (11.3) | 1,013.5 (16.2) | 8.4 (12.0) | 9.9 (20.7) | 117.2 (11.5) |
| 12 | 164.8 (7.4) | 1,269.2 (22.2) | 7.8 (12.1) | 10.0 (23.7) | 111.1 (13.7) |
| Day 4 | |||||
| 6 | 93.9 (6.4) | 631.8 (12.3) | 7.9 (12.8) | 9.1 (16.9) | 101.4 (7.1) |
| 8 | 123.3 (13.0) | 858.2 (24.9) | 8.3 (26.1) | 9.0 (33.0) | 101.3 (12.8) |
| 10 | 141.1 (17.0) | 1,038.8 (17.2) | 7.9 (8.0) | 8.8 (25.3) | 98.3 (17.2) |
| 12 | 183.7 (13.6) | 1,277.4 (19.8) | 7.7 (13.0) | 9.0 (30.5) | 97.6 (18.3) |
| Day 14 | |||||
| 10 | 139.3 (13.9) | 1,082.1 (15.3) | 7.9 (6.1) | 7.5 (18.6) | 85.8 (16.7) |
| 12 | 181.7 (13.2) | 1,290.5 (22.0) | 7.9 (13.8) | 9.0 (32.3) | 99.2 (18.7) |
All values are means, with coefficients of variation (percent) in parentheses.
AUC0-tau (day 4 and day 14).
Upon repeated daily dosing, steady state was reached by day 3 at all dose levels. Cmax and AUC0-tau values increased with the dose. Steady-state AUC0-tau values were generally similar to the values for AUC0-∞ measured after the first dose (Fig. 1 and Table 1). The mean Cmax observed at steady state on day 4 was slightly higher, by about 10% to 15%, than that observed after single doses of 8 to 12 mg/kg. A comparable increase was not observed in the 6-mg/kg group, presumably because a very high Cmax value was measured on day 1 in one subject. At doses of 10 mg/kg and 12 mg/kg, the AUC0-tau values at steady state on day 4 and on day 14 were comparable to the AUC0-∞ values found on day 1. At the other two dose levels, the AUC0-tau on day 4 was 13% lower (6 mg/kg) and 11% higher (8 mg/kg) than the day 1 AUC0-∞. The other pharmacokinetic parameters measured at steady state, including t1/2, CLwp, and V, were comparable to those measured on day 1. Again, each was independent of the dose.
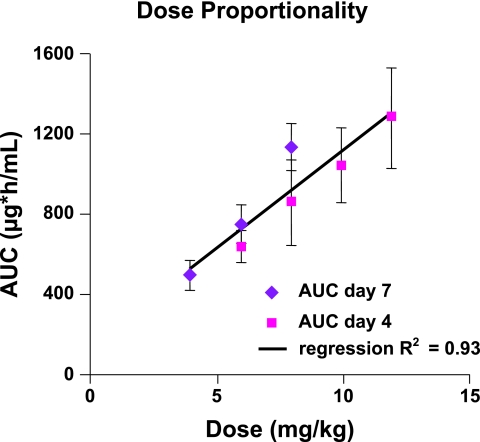
Dose proportionality was demonstrated for AUC0-∞ on day 1, AUC0-tau on day 4, and Cmin on day 4. For each of these parameters, the 90% confidence interval (CI) of the β-estimate in the power regression model included 1.0. The β values ± standard errors were 0.885 ± 0.102 (90% CI, 0.712 to 1.058; P = 0.27) for AUC0-∞ on day 1, 0.895 ± 0.116 (90% CI, 0.698 to 1.091; P = 0.37) for AUC0-tau on day 4, and 0.966 ± 0.257 (90% CI, 0.529 to 1.403; P = 0.90) for Cmin on day 4. In comparison, the increase in Cmax with dose level was slightly less than dose proportional, as the upper bound of the 90% confidence interval of the β-estimate was just below 1.0. The β values ± standard errors were 0.849 ± 0.073 (90% CI, 0.725 to 0.973; P = 0.047) for Cmax on day 1 and 0.821 ± 0.084 (90% CI, 0.677 to 0.964; P = 0.042) for Cmax on day 4.
Safety.
All subjects receiving daptomycin at 10 or 12 mg/kg were exposed to 14 daily doses, whereas all subjects receiving daptomycin at 6 or 8 mg/kg were exposed to 4 daily doses. Five of the six placebo subjects of cohorts 1 and 2 combined were exposed to 14 daily placebo infusions, whereas the sixth subject was exposed to 8 infusions before withdrawing consent.
There were no serious adverse events or discontinuations due to adverse events. Myalgia was not reported by any subject during the study. Most subjects in cohorts 1 and 2 reported adverse events, including nine (100%) in the 10-mg/kg group, seven (78%) in the 12-mg/kg group, and five (83%) in the placebo group. The most common adverse events (two or more subjects at the preferred term level) reported with daptomycin at 10 and 12 mg/kg combined were headache (5/18; 27.8%), adenoviral upper respiratory infection (4/18; 22%), aphthous stomatitis (2/18; 11.1%), constipation (2/18; 11.1%), arthralgia (2/18; 11.1%), and urinary tract infection not otherwise specified (NOS) (2/18; 11.1%). All these adverse events, except for arthralgia and urinary tract infection NOS, were reported by one or more of the placebo subjects. Other adverse events that were reported by one subject (1/18; 5.6%) from either cohort 1 or 2 but which went unreported in the placebo group were abnormal bowel sounds, chapped lips, nausea, vomiting NOS, injection site phlebitis, fungal infection NOS, upper respiratory tract infection NOS, muscle twitching, hypoesthesia, paresthesia, vaginal discharge, erythema, rash NOS, and rash papular. Similarly, subjects in the placebo group reported abdominal pain NOS (2/6; 33.3%) and herpes simplex (1/6; 16.7%), which were not reported by cohort 1 or 2 subjects. All events were rated as mild in severity by the investigator. One subject in the daptomycin 10-mg/kg group experienced mild numbness in the hands on day 3, which did not recur during continued treatment, and one subject in the daptomycin 12-mg/kg group experienced mild paresthesia on day 4, which resolved after 7 days without discontinuation of daily daptomycin administration.
Hematology, clinical chemistry, and vital-signs parameters were within the normal range at all assessments for all treatment groups, and the changes from baseline in these parameters were not clinically meaningful. CPK values were within the normal range for men and women at each assessment for all treatment groups (Table 2), and there was no apparent relationship between the daptomycin dose and the CPK values measured during the study. No clinically significant ECG abnormalities were reported, and changes in the QTc interval were not considered clinically meaningful. None of the subjects receiving daptomycin at 10 or 12 mg/kg had a prolonged QTc interval (>450 ms for males and >470 ms for females) at any study assessment.
TABLE 2.
Summary of serum creatine phosphokinase results
| Day | Mean (SD) serum creatine phosphokinase (U/liter)
|
||||
|---|---|---|---|---|---|
| Cohort 3 (daptomycin)
|
Cohorts 1 and 2
|
||||
| Daptomycin
|
Placebo (n = 6)a | ||||
| 6 mg/kg (n = 6) | 8 mg/kg (n = 6) | 10 mg/kg (n = 9) | 12 mg/kg (n = 9) | ||
| −1 (baseline) | 106 (37) | 129 (38) | 96 (42) | 100 (55) | 97 (47) |
| 4 | 67 (22) | 93 (40) | 58 (22) | 66 (30) | 61 (26) |
| 7 | 59 (20) | 72 (29) | 61 (25) | ||
| 14 | 64 (27) | 89 (42) | 64 (26) | ||
One subject receiving placebo in cohort 2 withdrew consent after dosing on day 8.
On electrophysiological testing, the changes from baseline in distal motor latency, CMAP, and F-wave latency were small and not clinically meaningful, and they did not differ significantly among treatment groups with daptomycin at 10 mg/kg or 12 mg/kg or placebo. Moreover, no apparent trends in these parameters over time or treatment were evident. At each assessment, the mean values for these electrophysiological parameters and the associated standard deviations were within normal limits.
DISCUSSION
The results of this study demonstrate that daptomycin has linear, dose-proportional pharmacokinetics over the 6- to 12-mg/kg dose range. Dose proportionality was demonstrated for the AUC after a single dose and at steady state and for steady-state trough daptomycin concentrations (Cmin). Moreover, the t1/2 and CLwp were unaffected by the daptomycin dose, a finding consistent with linear pharmacokinetics. The Cmax values after a single dose and at steady state were slightly less than dose proportional. It is important to note, however, that Cmax is a point estimate of the patients' rate of exposure to the study drug, which has larger intersubject variation than the AUC, which measures the extent of exposure.
The pharmacokinetic parameters determined in this study are generally consistent with those in a previous multiple-dose pharmacokinetic study of healthy volunteers given daptomycin at doses of 4, 6, and 8 mg/kg (Fig. 2) (5) and with a single-dose pharmacokinetic study at doses of 0.5 to 6 mg/kg (12). The Cmax values at steady state were about 10% to 15% higher than on day 1 in this study at 8 to 12 mg/kg and in the previous multiple-dose study at 6 to 8 mg/kg (5). In some groups (8 mg/kg in this study and 4 to 6 mg/kg in the previous multiple-dose study), the AUC0-tau at steady state was also slightly higher than the AUC0-∞ determined on day 1. However, in the other groups, notably with daptomycin doses of 10 mg/kg and 12 mg/kg, the AUC0-tau at steady state was comparable to the AUC0-∞ on day 1. Minimal drug accumulation occurs after the first dose, with average AUCtau about 18% greater than AUC0-24 for the 8-, 10-, and 12-mg/kg doses. Once steady state was achieved, no further drug accumulation was seen in the present study at 10 to 12 mg/kg or in the previous study at 8 mg/kg when evaluated after 14 days of dosing.
FIG. 2.
Relationship between daptomycin dose and steady-state AUC in this study (6 to 12 mg/kg) and a previous phase 1 study by Dvorchik et al. (4 to 8 mg/kg) in healthy volunteers (5). The line shows a projection of dose proportionality over the dose range covered by these studies.
The binding of daptomycin to plasma proteins was independent of the daptomycin dose and plasma concentration. Daptomycin was 90% to 93% bound to plasma proteins at the doses tested in this study and 92% bound to plasma proteins at the doses (4 to 8 mg/kg) tested in the previous study (5). Similarly, the clearance of daptomycin was independent of the dose and treatment duration. The low clearance is consistent with the high plasma protein binding and the predominant elimination of daptomycin in urine as unchanged drug. As a result, the elimination t1/2 was also independent of the dose and treatment duration. The t1/2 was approximately 8 h in this study at 6 to 12 mg/kg and approximately 9 h in the previous study at 4 to 8 mg/kg (5). Given the concentration-dependent bactericidal activity of daptomycin, these t1/2 values are sufficient to allow administration once every 24 h. Finally, the V of 0.1 liter/kg determined in the present and previous pharmacokinetic studies suggests that daptomycin remains primarily in the plasma and interstitial fluid. Considered together, these studies indicate that daptomycin offers consistent and linear pharmacokinetics over the range of 4 to 12 mg/kg in healthy volunteers.
Daptomycin is primarily eliminated by the kidneys as unchanged drug. In the present study, 37% to 68% of the administered dose was excreted unchanged in urine within 24 h, whereas in the previous study at 4 to 8 mg/kg, an average of 54% of the administered dose was found unchanged in the urine by 24 h (5).
Daptomycin was well tolerated at doses up to 12 mg/kg administered once daily for 14 days. No serious adverse events or treatment discontinuations due to adverse events were reported in this study. In the present study, no patients experienced elevations in CPK above the normal range (i.e., >204 U/liter), and no evidence of adverse skeletal muscle effects were seen after 14 days of dosing at up to 12 mg/kg once daily. Moreover, electrophysiological muscle testing and ECG testing did not identify any abnormalities in the study subjects. The median changes in the QTc interval were small, not clinically significant, and comparable to those seen with placebo. Thus, the present study found no evidence of adverse skeletal- muscle, nerve, or cardiac effects when daptomycin was administered at doses up to 12 mg/kg once daily for 14 days to healthy volunteers.
In vitro, daptomycin exhibits concentration-dependent bactericidal activity. Its efficacy may correlate best with the Cmax or AUC (which are increased by higher daptomycin doses) relative to the MIC than with time above MIC (9). Accordingly, higher doses of daptomycin may provide optimal treatment of difficult infections.
In conclusion, the present study demonstrates that daptomycin was well tolerated in subjects dosed at up to 12 mg/kg once daily for 14 days. Doses of daptomycin higher than 6 mg/kg once daily may be considered in further studies to evaluate the safety and efficacy of daptomycin in difficult-to-treat infections.
Acknowledgments
We thank Maria Gutierrez and the staff at Comprehensive Phase 1, where the clinical portion of the study was conducted; PPD Development and Avantix Laboratories, which performed the high-performance liquid chromatography and the liquid chromatography-mass spectrometry-mass spectrometry analysis; Xiaochui (Michelle) Li and J. Michael Davenport of PPD for the pharmacokinetic and statistical analyses; Joseph Arezzo of the Albert Einstein College of Medicine for independently reviewing the electrophysiological test results and confirming the accuracy of the automated scoring; and Westat, Inc., for performing the data management and statistical analyses.
This study was supported by Cubist Pharmaceuticals, Lexington, MA. M.B., D.P.B., S.Y., and G.V. are employees of Cubist.
REFERENCES
- 1.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter, C. F., and H. F. Chambers. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant Gram-positive pathogens. Clin. Infect. Dis. 38:994-1000. [DOI] [PubMed] [Google Scholar]
- 3.Cubist Pharmaceuticals. 2003. Cubicin (daptomycin for injection) prescribing information. Cubist Pharmaceuticals, Lexington, Mass.
- 4.Cubist Pharmaceuticals. 2006. Cubicin (daptomycin for injection) prescribing information. Cubist Pharmaceuticals, Lexington, Mass.
- 5.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton, C., G. M. Keating, and M. P. Curran. 2004. Daptomycin. Drugs 64:445-455. [DOI] [PubMed] [Google Scholar]
- 7.Fowler, V., S. Cosgrove, E. Abrutyn, H. Boucher, H. Chambers, G. Corey, I. Demeyer, S. Filler, D. Levine, A. Link, M. Rupp, A. Karchmer, and the S. aureus Endocarditis and Bacteremia Study Group. 2005. Daptomycin vs. standard therapy for Staphylococcus aureus bacteremia (SAB) and infective endocarditis (SAIE), abstr. K-426a. Program Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 8.Hancock, R. E. 2005. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 5:209-218. [DOI] [PubMed] [Google Scholar]
- 9.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schriever, C. A., C. Fernandez, K. A. Rodvold, and L. H. Danziger. 2005. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am. J. Health Syst. Pharm. 62:1145-1158. [DOI] [PubMed] [Google Scholar]
- 11.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodworth, J. R., E. H. Nyhart, Jr., G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]