Abstract
This study reports the discovery of erm genes in seven species of rapidly growing mycobacteria (RGM): Mycobacterium boenickei, M. goodii, M. houstonense, M. mageritense, M. neworleansense, M. porcinum, and M. wolinskyi. This study further substantiates the role of erm genes in intrinsic macrolide resistance in RGM.
Several rapidly growing mycobacteria (RGM) are significant human pathogens, with approximately 90% of RGM infections being caused by Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum (3, 4). Other members of the M. fortuitum and Mycobacterium smegmatis groups cause most of the remaining RGM infections. Although the incidence of infections caused by some species of slowly growing nontuberculous mycobacteria appears to be declining, there is evidence that RGM infections may be increasing or increasingly recognized (5). Many pathogenic RGM species are considered susceptible to the newer macrolides (e.g., clarithromycin), and these agents are important components in the treatment of RGM infections (13). However, several RGM species express intrinsic macrolide resistance, including Mycobacterium goodii, Mycobacterium houstonense, Mycobacterium mageritense, and Mycobacterium wolinskyi (2). Previous studies have shown that the novel rRNA methylase genes erm(38) and erm(39) confer inducible macrolide resistance on M. fortuitum and M. smegmatis, respectively (8, 9). Thus, the objective of this study was to determine if other clinically important RGM have similar erm genes.
Southern blot analysis with an erm(38)-derived probe (8, 9) indicated that M. mageritense strain 961635 had DNA similar to that of erm(38) of M. smegmatis. Genetic complementation of the erm(38) knockout variant M. smegmatis ermKO4, using methods described elsewhere (9), isolated a 2.8-kbp M. mageritense DNA fragment that conferred resistance (MIC ≥ 128 μg/ml) to clarithromycin, clindamycin, erythromycin, spiramycin, and the ketolide HMR3004, but not to quinupristin (streptogramin B). Sequencing of the M. mageritense DNA fragment identified a gene encoding a 251-amino-acid polypeptide that was 77% identical to Erm(39) of M. fortuitum (GenBank accession no. AAR92235) and 71% identical to the first 264 amino acids of Erm(38) of M. smegmatis (GenBank accession no. AAN86837). The M. mageritense erm gene sequence was submitted to the Nomenclature Center for MLS Genes maintained by Marilyn C. Roberts (11, 12) and was designated erm(40). Using erm(40)-specific primers (MM1 and MM2) (Table 1) and methods described elsewhere (9), this gene was detected by PCR in 11/11 clinical isolates of M. mageritense.
TABLE 1.
PCR primers used in this studya
| Primer | Sequence | Target |
|---|---|---|
| MM1 | CGCCGGGTTGCTGACCATCA | erm of M. mageritense |
| MM2 | GGACCCACAATCTGCGCCACAC | erm of M. mageritense |
| CME-1Y | ACGTGGTGGTGGGCAAYCTG | erm consensus |
| CME-2 | AATTCGAACCACGGCCACCACT | erm consensus |
| GERM-4 | GTGACGCTGCCCGCACCGTTCA | 3342 consensus |
| MAB-7 | GGTGGCCTCGCGCAGCTACTG | 3342 consensus |
| MM6 | GCGTCGTACTTGCCGTTGAA | 3342 consensus |
| GERM-1 | CCTCGCGGGTCACGTTCTCCAG | TR consensus |
| GERMTR-1 | TCGATGCTGCGCCGCATGCGTTC | TR consensus |
| MP-3 | GATCGCGGCGAACTCGTTCTTG | TR consensus |
| GERM-2 | CCGGTGACGTCGCCCCCGAC | folD consensus |
| MAB-8 | ACGTGGCCGAGGATGTCTGGGA | folD consensus |
| MFERM-2 | CCCGTCAGGCCGACATCATCA | folD consensus |
| MSX-5L | GGTCACCGCGGACATGGTCAAG | folD consensus |
Organized by target gene.
The sequences of the erm(38), erm(39), and erm(40) regions (GenBank accession no. AY154657, AY487229, and AY570506) provided the basis for designing consensus primers (Table 1) targeting the RGM erm genes and flanking DNA. Using these primers, erm genes were detected by PCR in Mycobacterium boenickei (10 strains or isolates tested), M. goodii (1 strain or isolate tested), M. houstonense (3 strains or isolates tested), Mycobacterium neworleansense (1 strain or isolate tested), Mycobacterium porcinum (2 strains or isolates tested), and M. wolinskyi (4 strains or isolates tested). No evidence of a related erm gene was found for M. abscessus (7 strains or isolates tested), M. chelonae (2 strains or isolates tested), Mycobacterium flavescens (1 strain or isolate tested), Mycobacterium immunogenum (1 strain or isolate tested), Mycobacterium peregrinum (5 strains or isolates tested), Mycobacterium senegalense (4 strains or isolates tested), and Mycobacterium thermoresistibile (1 strain or isolate tested).
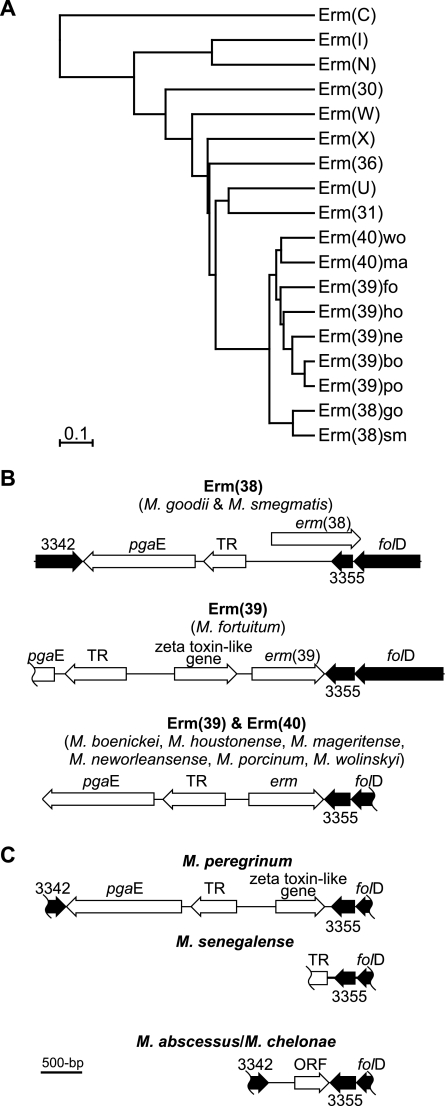
Sequencing of the erm genes and surrounding DNA of the erm-positive species was enabled by using different combinations of the consensus primers (Table 1). The encoded amino acid sequences of the erm genes of M. boenickei, M. goodii, M. houstonense, M. neworleansense, M. porcinum, and M. wolinskyi, along with the previously described erm genes of M. fortuitum (9) and M. smegmatis (8), were found to be highly conserved, with a mean identity of 77% (range, 68 to 99%) (see Table S1 in the supplemental material). Following the guidelines of Roberts (11), the erm alleles in different RGM fall into three existing Erm classes: Erm(38) for M. goodii and M. smegmatis; Erm(39) for M. boenickei, M. fortuitum, M. houstonense, M. neworleansense, and M. porcinum; and Erm(40) for M. mageritense and M. wolinskyi. Furthermore, the predicted phylogenic relationship between the amino acid sequences of the RGM erm alleles and other closely related Erm classes (Fig. 1A) showed that the RGM erm genes formed a distinct clade of the erm phylogenic tree. This clade was consistent with the phylogeny of RGM predicted by molecular analysis of other genes (6). Furthermore, the RGM Erm node appears to have “emerged” more recently than the other Erm classes, which are largely derived from antibiotic-producing Actinomycetales (e.g., Streptomyces). This suggests that the RGM erm genes were acquired after the divergence of the RGM from other members of this order.
FIG. 1.
(A) Phylogram showing the predicted relationships of the RGM Erm proteins, with the representative alleles of the eight most closely related Erm classes, and rooted on Erm(C) of Staphylococcus aureus (GenBank accession no. AAA20192). The Erm class representatives (with GenBank accession numbers) are Erm(30) of Streptomyces venezuelae (AAC69328), Erm(31) of S. venezuelae (AAC69327), Erm(36) of Micrococcus luteus (AAL68827), Erm(I) of Streptomyces mycarofaciens (A60725), Erm(N) of Streptomyces fradiae (CAA66307), Erm(U) of Streptomyces lincolnensis (CAA55770), Erm(W) of Micromonospora griseorubida (P43433), and Erm(X) of Corynebacterium jeikeium (AAK28910). For the RGM Erm proteins, Erm(38), Erm(39), and Erm(40), the allele (or RGM species) is indicated by the letters after the right parenthesis: bo, M. boenickei; fo, M. fortuitum; go, M. goodii; ho, M. houstonense; ma, M. mageritense; ne, M. neworleansense; po, M. porcinum; sm, M. smegmatis; and wo, M. wolinskyi. The scale bar shows the evolutionary distance (i.e., the amount of divergence, or time since splitting, from a theoretical common ancestor). (B and C) The genetic organization of the folD regions of erm gene-positive and erm gene-negative RGM, respectively. The shaded genes are similar (>64% amino acid identity) to M. tuberculosis strain H37Rv genes Rv3342 (GenBank accession no. CAA17114), Rv3355c (GenBank accession no. NP_217872), and folD (GenBank accession no. NP_217873). Other genes are those encoding TR, a putative tetR family transcriptional regulator, and pgaE, a putative polyketide synthase, and zeta toxin-like, containing a zeta toxin conserved domain (Pfam06414) and ∼50% amino acid identity to conserved hypothetical genes of Polaromonas spp. (GenBank accession no. EAM36411) and Xanthomonas oryzae (GenBank accession no. AAW75612). ORF, open reading frame with a likely ribosome binding site (AAGGGA).
Conservation of the RGM erm genes was not only reflected in the coding regions, but also in the flanking genes (Fig. 1B), i.e., the erm gene regions of the RGM show synteny. Specifically, the erm genes were bound by the 3355 and folD genes downstream and the TR (putative transcriptional regulator) and pgaE genes upstream. Furthermore, PCR mapping indicated that the 3342 gene was upstream of the pgaE gene in all erm-positive RGM species. Using the consensus primers to amplify and sequence the 3342-folD regions of the erm-negative RGM species M. peregrinum (GenBank accession no. DQ435604) and M. senegalense (GenBank accession no. DQ435605), a gene organization similar to that of erm-positive RGM species, minus the erm gene, was revealed (Fig. 1C). Intriguingly, M. peregrinum, like M. fortuitum, has a zeta toxin-like gene absent in other RGM. Zeta toxin/antitoxin genes provide a mechanism for plasmid maintenance (7). Thus, the zeta toxin-like gene may be the “smoking gun” for the acquisition event of the erm gene (and possibly the pgaE and TR genes) from a plasmid.
Sequencing of the folD regions of M. abscessus (isolates MAB30 and MC719) and M. chelonae (strain ATCC 35752T) revealed that the 3342 and folD genes were separated by 0.8 kbp of DNA (GenBank accession no. DQ144643 and DQ144644) (Fig. 1C). Although a possible open reading frame was identified in this region, BLAST analysis (1) revealed no convincing alignments with other sequences (all had <30% identity). In fact, BLAST searching of the current completely sequenced mycobacteria (through the NCBI website [http://www.ncbi.nlm.nih.gov/]) suggested that the pgaE and TR genes were found only in erm-positive RGM species and M. peregrinum and M. senegalense. This in turn suggested that M. peregrinum and M. senegalense originally had erm genes that were subsequently deleted.
As previously found for M. fortuitum and M. smegmatis (8, 9), M. boenickei, M. houstonense isolate 14133, M. neworleansense, and M. porcinum appeared susceptible to clarithromycin (MIC < 4 μg/ml) on standard susceptibility testing (10). However, as demonstrated with M. houstonense isolate 14133, overnight incubation in clarithromycin (0.1 μg/ml) increased the clarithromycin MIC to > 128 μg/ml. The clarithromycin MIC remained at ≤2 μg/ml for organisms incubated in the absence of macrolide. Thus, these results provide further evidence that macrolide resistance in RGM species can be inducible. Intriguingly, M. houstonense isolate 2014 and M. mageritense isolate 961635 were constitutively resistant to macrolide-lincosamide-ketolide agents (MIC > 128 μg/ml), yet reverse transcription-PCR analysis (using methods described elsewhere [8, 9]) indicated that the erm mRNA levels were ∼10-fold higher in organisms preincubated for 2 h in 0.1 μg/ml clarithromycin or HMR3004 (data not shown). Thus, the results were consistent with RGM erm genes being inducible at the molecular level, even in organisms deemed constitutively resistant by standard susceptibility testing.
In conclusion, erm genes are present in a range of pathogenic RGM species, including species that are considered macrolide susceptible as currently defined by the Clinical and Laboratory Standards Institute (formerly NCCLS) (10). This, with the finding that all RGM erm genes are inducible at the molecular level, suggests that the results of routine susceptibility testing with macrolides should be interpreted with caution, especially for species of the M. fortuitum and M. smegmatis groups. Furthermore, induction of macrolide-lincosamide-ketolide resistance needs to be considered when assessing new macrolide-ketolide agents for potential antimycobacterial activity.
Nucleotide sequence accession numbers.
The RGM erm regions and the erm gene-encoded polypeptides have been submitted to GenBank under the following accession numbers: M. boenickei, DQ144638 and AAZ76615; M. goodii, DQ144639 and AAZ76618; M. houstonense, DQ144640 and AAZ76622; M. mageritense, AY570506 and AAS76623; M. neworleansense, DQ144641 and AAZ76625; M. porcinum, DQ447745 and ABE01848; and M. wolinskyi, DQ144642 and AAZ76628. The folD regions of M. abscessus, M. peregrinum, and M. senegalense have been submitted to GenBank under accession numbers DQ144643, DQ435604, and DQ435605, respectively.
Supplementary Material
Acknowledgments
Funding for this study was provided by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant RO1-AI052291; the Department of Pathology and Laboratory Medicine, Childrens Hospital Los Angeles; and the Department of Microbiology, The University of Texas Health Center, Tyler, TX.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Brown, B. A., B. Springer, V. A. Steingrube, R. W. Wilson, G. E. Pfyffer, M. J. Garcia, M. C. Menendez, B. Rodriguez-Salgado, K. C. Jost, Jr., S. H. Chiu, G. O. Onyi, E. C. Bottger, and R. J. Wallace, Jr. 1999. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Bacteriol. 49:1493-1511. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Elliott, B. A., D. E. Griffith, and R. J. Wallace, Jr. 2002. Newly described or emerging human species of nontuberculous mycobacteria. Infect. Dis. Clin. N. Am. 16:187-220. [DOI] [PubMed] [Google Scholar]
- 4.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. 1999. Nontuberculous mycobacteria reported to the public health laboratory information system by state public health laboratories United States, 1993-1996. Centers for Disease Control and Prevention, Atlanta, Ga.
- 6.Devulder, G., M. Perouse de Montclosand, and J. P. Flandrois. 2005. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int. J. Syst. Evol. Microbiol. 55:293-302. [DOI] [PubMed] [Google Scholar]
- 7.Meinhart, A., J. C. Alonso, N. Strater, and W. Saenger. 2003. Crystal structure of the plasmid maintenance system epsilon/zeta: functional mechanism of toxin zeta and inactivation by epsilon 2 zeta 2 complex formation. Proc. Natl. Acad. Sci. USA 100:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash, K. A. 2003. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38). Antimicrob. Agents Chemother. 47:3053-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash, K. A., Y. Zhang, B. A. Brown-Elliott, and R. J. Wallace, Jr. 2005. Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J. Antimicrob. Chemother. 55:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCLS. 2003. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic actinomycetes, 1st ed. Approved standard M24-A, vol. 23. NCCLS, Villanova, Pa. [PubMed]
- 11.Roberts, M. C. 21 February 2005, accession date. Nomenclature Center for MLS Genes. [Online.] http://faculty.washington.edu/marilynr/.
- 12.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace, R. J., Jr. 1997. J. Glassroth, D. E. Griffith, K. N. Olivier, J. L. Cook, and F. Gordin. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:1S-25S. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.