Abstract
Therapeutic strategies aimed at inhibiting human immunodeficiency virus type 1 (HIV-1) replication employ a combination of drugs targeted to two viral enzymes (reverse transcriptase and protease) and to the viral entry/fusion step. However, the high propensity of HIV-1 to develop resistance makes the development of novel compounds targeting different steps of the HIV-1 life cycle essential. Among these, integrase (IN) inhibitors have successfully passed the early phases of clinical development. By preventing integration, IN inhibitors preclude viral replication while allowing production of extrachromosomal forms of viral DNA (E-DNA). Here, we describe an improved and standardized assay aimed at evaluating IN inhibitors by taking advantage of the transcriptional activity of E-DNA produced by HIV-derived vectors in the absence of replication-competent virus. In this context, the use of the firefly luciferase gene as a reporter gene provides a rapid and quantitative measure of viral-vector infectivity, thus making it a safe and cost-effective assay for evaluating novel IN inhibitors.
The development of chemotherapeutic agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection has focused primarily on two viral enzymes, reverse transcriptase (RT) and protease, and on the viral entry/fusion step (15, 31). Highly active antiretroviral therapy, consisting of multiple drug regimens attacking different targets and steps in the viral life cycle, has profoundly suppressed the levels of plasma viremia in patients infected with HIV-1, preventing opportunistic infections and reducing patient mortality. However, the emergence of multidrug-resistant variants remains problematic, suggesting the requirement for new antiviral agents (18, 20). Integrase (IN) inhibitors, targeting a key enzyme of HIV-1 essential for its replication and persistence in the host genome, are one of the most promising classes of anti-HIV compounds. Importantly, two of them recently completed phase II clinical trials (B. Grinsztejn, B. Y. Nguyen, C. Katlama, J. Gatell, A. Lazzarin, D. Vittecoq, C. Gonzalez, J. Chen, R. Isaacs, and the Protocol 005 Study Team, Abstr. 13th Conf. Retroviruses Opportunistic Infect., abstr. 159LB, 2006, and E. DeJesus, D. Berger, M. Markowitz, C. Cohen, T. Hawkins, P. Ruane, R. Elion, C. Farthing, A. Cheng, B. Kearney, and the 183-0101 Study Team, Abstr. 13th Conf. Retroviruses Opportunistic Infect., abstr. 160LB, 2006).
Soon after infection and reverse transcription, viral linear DNA is transported into the nucleus of the infected cell, where it may either integrate into the host cell's genome or remain extrachromosomal and circularize to form episomes containing either one or two long-terminal repeats (one-LTR or two-LTR circles) (8). The integration step is a process dependent on the viral IN enzyme, which is essential for the subsequent steps of viral replication (8). Thus, integration of the viral genome into the cell's chromosome is a crucial step for completion of the HIV-1 life cycle, and its inhibition is an attractive target for anti-HIV drug development (32). By preventing integration, the anti-IN compounds preclude the subsequent steps of viral replication and spread while allowing the production of the extrachromosomal forms of viral DNA (E-DNA) (17, 26, 28, 40, 41). This recapitulates the effects obtained in cell cultures after infection with IN-defective viruses, which produce only E-DNA in the absence of integrated provirus (1, 5, 11). Importantly, E-DNA has been shown to be transcriptionally active, albeit at lower levels than its integrated counterpart, producing only spliced RNA for the viral proteins Env, Tat, Rev, and Nef (14, 46).
Until recently, high-throughput screening for potential IN inhibitors has been performed primarily in cell-free systems, using purified IN protein either alone or within the context of a partially purified preintegration complex (12, 16, 17, 19). However, inhibitors identified in this manner are frequently cytotoxic or do not exhibit antiviral activity in cell culture (29, 33). Conversely, the cell culture-based HIV-1 drug susceptibility assay in use measures the extent to which a drug inhibits HIV-1 p24 antigen production in peripheral blood mononuclear cells (PBMC) or HIV-permissive T-cell lines acutely infected with viral isolates or laboratory-adapted viruses, such as HIV-1IIIB. The limitations of this assay concern the use of PBMC, with the consequent variability among different donors, the requirement for infectious virus and different virus inocula, and the costs and time involved. In addition, when potential IN inhibitors exhibit antiviral activity in this system, the molecular target of virus inhibition may not be solely the integration reaction, and further analyses need to be performed.
During the past few years, several authors have described different assays that can be used to evaluate potential IN inhibitors in cell culture systems. These methods are based on the detection and quantification of integrated HIV-1 DNA, eventually in combination with the quantitative analysis of the two-LTR circular forms of E-DNA (2, 4, 41, 42). These assays, usually based on Alu nested or real-time DNA PCR, have the advantage of specifically evaluating HIV-1 integration in the presence or absence of a given compound but require expensive instruments and advanced technology. More recently, a luciferase-based assay relying on a single-cycle infectious virus for testing antiviral activities of compounds has been reported (40).
Here, we report on the development and standardization of a 96-well microtiter assay to evaluate the potential anti-IN activities of new compounds in a cell-based system by taking advantage of the transcriptional activities of IN-competent and IN-defective HIV-derived vectors expressing the luciferase reporter gene, mimicking the parental virus life cycle in the absence of replication-competent virus. By using this method, we evaluated several potential novel compounds with in vitro anti-IN activity and found that three of them possessed strong activity in cell culture in the absence of cellular toxicity.
MATERIALS AND METHODS
Cell cultures.
Human kidney epithelial HEK293 cells, referred to as 293 cells here, were maintained in Dulbecco's modified Eagle's medium (Euroclone, Life Sciences Division, Pero, Italy) with 10% fetal calf serum (GIBCO Invitrogen, Paisley, United Kingdom), penicillin (50 U/ml), and streptomycin (50 μg/ml). PBMC were isolated from healthy blood donors by using a Ficoll Hypaque density gradient and cultured in RPMI medium (Euroclone, Life Sciences Division, Pero, Italy) with 20% fetal calf serum (GIBCO Invitrogen, Paisley, United Kingdom), l-glutamine (2 mM), penicillin (50 U/ml), and streptomycin (50 μg/ml). Before infections and drug treatment, PBMC were activated with 5 μg phytohemagglutinin (PHA) (Sigma-Aldrich) ml−1 for 3 days (see below).
HIV-1 drug susceptibility assay.
HIV-1IIIB virus (34-36), obtained through the NIH Repository Reagents (Bethesda, MD), was expanded and titrated on PHA-stimulated donor PBMC and used in a HIV-1 drug susceptibility assay following standard methodologies (10a). Briefly, stock HIV-1IIIB virus (1,000 50% tissue culture infective doses per 106 PBMC, equivalent to 3.5 × 102 cpm of RT activity) was used to infect PBMC from healthy blood donors stimulated with PHA for 3 days. Briefly, following 2 h of incubation, the cells were washed, plated at 2 × 103/well, and cultured in the absence (control) or presence of scalar concentrations of each of the tested drugs. The known anti-IN aryldiketo acid (DKA) L-731,988 (17) was always used as an internal positive control. Cell culture supernatants were harvested after 7 days of culture, and HIV-1 p24 antigen was quantified using an antigen capture enzyme-linked immunosorbent assay with a limit of detection of 20 pg/ml (Innotest; Innogenetics N.V., Ghent, Belgium). The 50% and 90% inhibitory concentrations (IC50 and IC90) of each drug were determined by the median effect equation (7). Each condition was tested in five replicates, and each drug was evaluated for toxicity by a trypan blue cell viability count. The percentage of recovered live cells present in each treated sample was calculated with the following formula: (number of live cells in treated sample/number of live cells in untreated sample) × 100.
Construction of the modified lentiviral vectors.
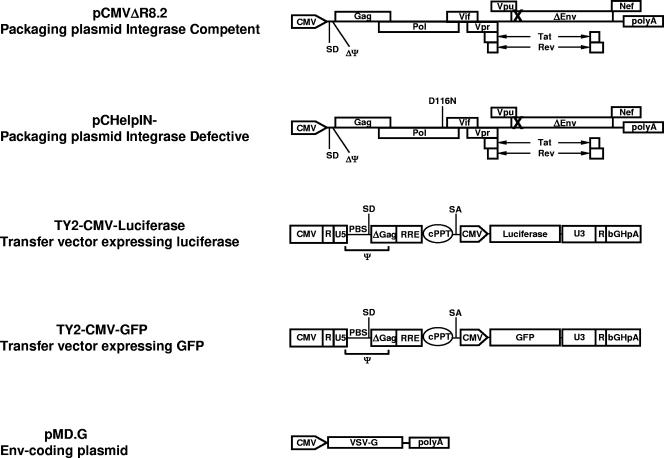
A schematic depiction of the vectors used in this study is provided in Fig. 1. Briefly, the self-inactivating TY-EF1α-GFP lentiviral vector was obtained through the NIH Repository Reagents (Bethesda, MD) (6, 9, 21, 48). A ClaI/KpnI fragment of DNA from pHR′CMV-GFP (44) containing the cytomegalovirus (CMV) promoter was cloned in place of the EF1α promoter to produce the TY-CMV-GFP self-inactivating lentiviral vector. A BamHI/SalI fragment of DNA containing the coding sequence of luciferase (44) was cloned in place of green fluorescent protein (GFP) to produce the TY-CMV-Luciferase plasmid. A BssHII/ClaI fragment from the pHR′cPPT plasmid, containing the central polypurine tract (cPPT) and a shorter packaging signal (Ψ), was inserted in the corresponding sites of the TY-CMV-GFP and TY-CMV-Luciferase plasmids to obtain the self-inactivating TY2-CMV-GFP and TY2-CMV-Luciferase lentiviral vectors. Plasmid pCHelpIN−, kindly provided by J. Reiser, produces all HIV-1 viral proteins, with the exception of Env. In addition, the IN produced by the pCHelpIN− plasmid contains a point mutation in the middle of a reading frame of the IN protein (D116N), preventing the functions characteristic of the IN protein, as previously described (11, 44). Plasmid pCMVΔR8.2 (27), obtained from I. Verma (Salk Institute, La Jolla, CA), produces all HIV-1 viral proteins, including wild-type IN, with the exception of Env. Plasmid pMD.G (27), obtained from D. Trono, produces the vesicular stomatitis virus envelope glycoprotein G (VSV.G) to increase recombinant virus stability and tropism for infection of 293 target cells.
FIG. 1.
Schematic representation of the vector used in this study. IN-competent (pCMVΔR8.2) and IN-defective (pCHelpIN−) packaging plasmids and a VSV.G envelope-coding plasmid (pMD.G) were used to produce recombinant viruses expressing luciferase (TY2-CMV-Luciferase) or GFP (TY2-CMV-GFP). The packaging signal (ψ), the primer binding site (PBS), the deleted packaging signal (ΔΨ), the major splice donor (SD) and acceptor (SA) sites, the bovine growth hormone polyadenylation signal (bGHpA), and the cPPT are indicated. X indicates nonfunctional envelope and/or integrase proteins in the packaging vectors.
IN inhibitors.
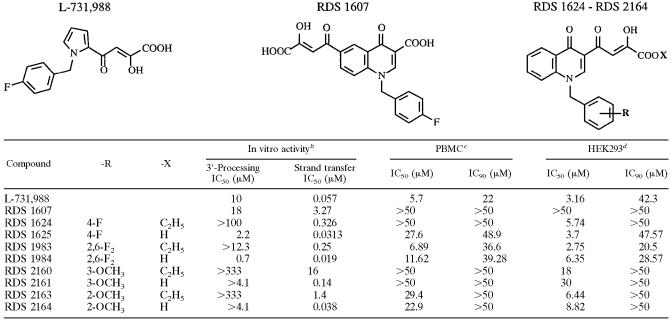
The IN inhibitors described in this paper are L-731,988 (17), 4-[1-(4-fluorophenylmethyl)-3-carboxy-4(1H)-quinolinon-6-yl]-2,4-dioxobutanoic acid (RDS 1607) and 4-[1-arylmethyl-4(1H)-quinolinon-3-yl]-2,4-dioxobutanoic acid derivatives (RDS 1624, RDS 1625, RDS 1983, RDS 1984, RDS 2160, RDS 2161, RDS 2163, and RDS 2164), and they belong to the DKA family (R. Di Santo, Y. Pommier, C. Marchand, M. Artico, and R. Costi, 22 September 2005, international patent application WO2005087759). The chemical structures of these compounds are characterized by the presence of a diketobutanoic acid chain linked to a 4(1H)-quinolinone ring. We chose the quinolinone skeleton because (i) the activity and safety of 4(1H)-quinolinone derivatives as antibacterial agents are well known and (ii) this structure is useful to obtain a high number of congeners to be tested in biological assays (10). The synthesis, structure-activity relationships, and molecular-modeling studies of these novel anti-IN agents will be reported elsewhere.
In vitro HIV-1 recombinant integrase assay.
Compounds were evaluated for their IN inhibition in a gel-based assay as described previously (25). In the assay, a 5′-end-labeled 21-mer double-stranded DNA oligonucleotide corresponding to the last 21 bases of the U5 viral LTR was used to follow both the 3′-processing and strand transfer steps of the integration reaction (32). Briefly, a DNA-enzyme complex was preformed by mixing 500 nM of recombinant HIV-1 IN and 20 nM of 5′-labeled double-stranded DNA template in a buffer containing 50 mM MOPS (morpholinepropanesulfonic acid), pH 7.2, 7.5 mM MnCl2, and 14.3 mM β-mercaptoethanol for 15 min on ice. The integration reaction was then initiated by addition of each compound and continued in a total volume of 10 μl for 60 min at 37°C. The reaction was stopped by adding the same volume of electrophoresis denaturing dye, containing 99% formamide (Sigma-Aldrich, Milwaukee, WI), 1% sodium dodecyl sulfate, 0.2 mg/ml bromophenol blue (Sigma-Aldrich, Milwaukee, WI), and 0.2 mg/ml xylene cyanol blue (Sigma-Aldrich, Milwaukee, WI). Samples were loaded on a 20% 19:1 acrylamide-bis-acrylamide denaturing gel (Accugel; National Diagnostics, Atlanta, GA) containing 7 M urea (Gibco BRL Life Technologies, Rockville, MD) in 1× Tris-borate-EDTA (Gibco BRL Life Technologies, Rockville, MD). The gels were exposed overnight and analyzed using a Molecular Dynamics (Sunnyvale, CA) PhosphorImager.
Lentiviral-vector production and 293 cell transduction.
Lentiviral vectors were produced in 293 cells by a calcium phosphate transient-transfection method as previously described (27). Briefly, the day before transfection, cells were plated at a density of 3 × 106 in a 100-mm-diameter petri dish at 80% confluence. A total of 28 μg of plasmid DNA was used for each plate in a 3:2:0.7 (transfer vector-packaging vector-envelope vector) ratio using the calcium phosphate-based Profection Mammalian Transfection System (Promega Corporation, Madison, WI). After 48 h, the viral supernatants were collected, spun at 1,500 rpm, and passed through a 0.45-μm-pore-size filter. Supernatants containing recombinant viruses were normalized for RT activity using standard radioactive methodologies (45). For transduction experiments, 293 cells were plated at a concentration of 1 × 104 cells/well in a 96-well plate (luciferase experiments) or at 5 × 104 cells/well in a 24-well plate (GFP experiments). The next day, the cells were infected with 1 × 104 or 5 × 104 cpm/well (the 96-well plate and 24-well plate, respectively) of either the IN-competent or the IN-defective TY2-CMV-Luciferase (96-well plate) or TY2-CMV-GFP (24-well plate) virus in the presence of increasing amounts of each drug. Four hours after infection, the cells were washed and the drugs were replaced. Cells infected with TY2-CMV-GFP viruses were observed with the fluorescence microscope every day and photographed. Seventy-two hours posttransduction, cells infected with TY2-CMV-GFP viruses were collected for PCR analysis, while luciferase activity was evaluated in cells infected with TY2-CMV-Luciferase viruses. Cell viability was evaluated by the trypan blue exclusion method. The percentage of recovered live cells present in each treated samples was calculated with the following formula: (number of live cells in treated sample/number of live cells in untreated sample) × 100.
Evaluation of intracellular luciferase activity in 293 cells was performed directly on a 96-well white plate with a clear bottom (Costar Corning Incorporated), using the Britelite Ultra-High Sensitivity Luminescence Reporter Gene Assay System (Perkin-Elmer, Groningen, The Netherlands) on Packard Top Count NXT (Packard, Berkshire, United Kingdom), following the manufacturer's protocol. Each condition was tested in three replicates.
GFP fluorescence analysis.
Cells transduced with recombinant GFP vectors were fixed with 4% formaldehyde at 3 days posttransduction, and GFP fluorescence was measured with a FACSCalibur analytical flow cytometer with CellQuest software (BD Biosciences Immunocytometry Systems, San Jose, CA).
RESULTS
HIV-1 drug susceptibility assay on PBMC.
The HIV-1 drug susceptibility assay measures the extent to which a given compound inhibits HIV-1 p24 antigen production on PHA-stimulated PBMC from normal donors acutely infected with a HIV-1 molecular clone, a laboratory-adapted or primary isolate.
For HIV-1 drug susceptibility assay validation in our model system, we tested the abilities of known antiviral compounds, the DKA IN inhibitor L-731,988 (17) and two RT inhibitors, zidovudine (AZT) and dideoxyinosine (ddI), to inhibit p24 release in the supernatants of PHA-stimulated HIV-1IIIB-infected PBMC. Following infection, p24 antigen release in the cell culture supernatants was evaluated on day 7, and the IC50s and IC90s of the drugs were determined using the median effect equation (7). As shown in Fig. 2, p24 values decreased with increasing amounts of each drug, and IC50 and IC90 values obtained for L-731,988 (IC50, 3.15 μM, and IC90, 24 μM), AZT (IC50, 0.022 μM, and IC90, 0.4 μM), and ddI (IC50, 0.4 μM, and IC90, 2.45 μM) were comparable to those described in the literature (17, 22, 24, 37, 42). Drug toxicity was tested by cell viability, using the trypan blue exclusion method, and was found to be negligible at the indicated IC50s and IC90s (Fig. 2).
FIG. 2.
Inhibition of p24 release in the supernatants of PHA-stimulated HIV-1IIIB-infected PBMC using increasing amounts of known antiviral compounds. (a) Anti-IN L-731,988. (b and c) RT inhibitors AZT and ddI, respectively. At day 7 after infection, p24 antigen in the cell culture supernatants (bars) was evaluated with an enzyme-linked immunosorbent assay kit, and the percent inhibition (lines) was calculated, assuming 0% inhibition in the non-drug-treated PBMC. Each condition was tested in five replicates, and mean values with standard deviations are indicated.
The IN inhibitor L-731,988 does not hinder expression from unintegrated templates.
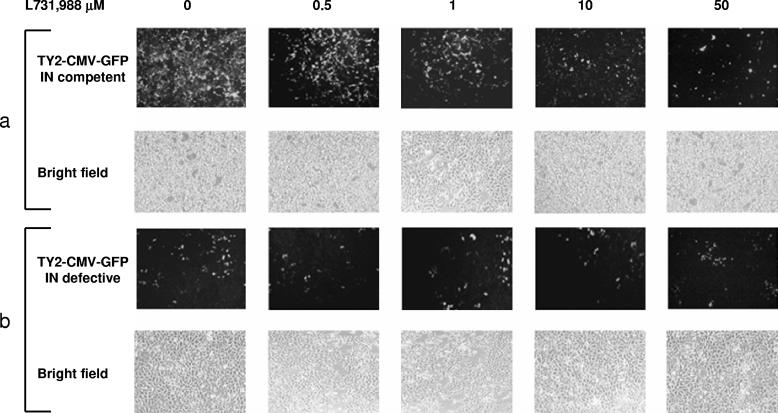
Recently, a few reports showed that viral proteins are expressed in the presence of anti-IN DKA compounds due to the transcriptional activity of E-DNA (14, 46). To validate this assumption in our model system, we performed infections of 293 cells using IN-competent and IN-defective TY2-CMV-GFP lentiviral vectors in the presence of increasing amounts of the anti-IN compound L-731,988. GFP expression was monitored daily with a fluorescence microscope to evaluate the infection efficiency and compound activity, and fluorescence-activated cell sorter analysis of GFP-expressing cells was performed at 3 days posttransduction. As expected, differences in GFP expression between IN-competent and IN-defective viruses were clearly visible in the absence of the anti-IN compound (Fig. 3a and b, left, and Table 1), consistent with a lower transcription activity of E-DNA templates, as previously reported (44). Concerning GFP expression in the IN inhibitor-treated cells, infection performed in the presence of the IN-competent virus showed a decrease in GFP expression proportional to the increase in the L-731,988 concentration (Fig. 3a and Table 1). Conversely, GFP expression remained stable in the IN-defective lentiviral-vector-infected cells regardless of the anti-IN inhibitor concentration, confirming a steady transcriptional activity, albeit lower than that of the integrated counterpart of E-DNA, as previously reported (Fig. 3b and Table 1) (3, 14, 46). Similar to what has been found by other groups (4, 17, 40, 42), the presence of E-DNA was confirmed by two-LTR amplification of DNA extracted from the infected cells, using a primer pair spanning the junction between the two LTRs, as described previously (44), indicating that production of E-DNA was not affected by the IN inhibitor, allowing GFP expression from unintegrated viral templates (data not shown).
FIG. 3.
Transduction of 293 cells with IN-competent (a) and IN-defective (b) GFP-expressing self-inactivating lentiviral vectors. The cells were infected in a 24-well plate format with 1 cpm/cell equivalent/well and treated with increasing amounts (range, 0.5 to 50 μM) of anti-IN drug (L-731,988). Three days postinfection, GFP expression was evaluated by fluorescence microscopy (a and b, top rows). Phase-contrast micrographs of the same fields are shown (bottom rows).
TABLE 1.
Quantitative GFP analysis of 293 cells transduced with IN-competent and IN-defective TY2-CMV-GFP vectors
| L-731,988 amt (μM) | TY2-CMV-GFP (integrase competent)
|
TY2-CMV-GFP (integrase defective)
|
||
|---|---|---|---|---|
| % Positivea | MFIb | % Positivea | MFIb | |
| 0 | 99.5 ± 0.1 | 110.8 ± 3.3 | 88.8 ± 7.6 | 16.1 ± 2.3 |
| 0.5 | 97.2 ± 3.1 | 35.7 ± 9.4 | 76.5 ± 13.1 | 23.0 ± 1.9 |
| 1 | 55.1 ± 4.4 | 49.2 ± 23.1 | 73.8 ± 10.2 | 27.3 ± 4.9 |
| 10 | 33.2 ± 15.4 | 30.3 ± 14.5 | 79.8 ± 8.5 | 22.8 ± 0.4 |
| 50 | 36.1 ± 0.1 | 10.9 ± 0.2 | 85.1 ± 4.1 | 19.4 ± 6.9 |
The percentage of GFP-positive 293 cells was analyzed by fluorescence-activated cell sorter 3 days posttransduction with the indicated vectors. Each condition was tested in three replicates, and mean values with standard deviations are shown.
Mean fluorescence intensity (MFI) with standard deviations.
Use of lentiviral vectors expressing luciferase in 293 cells for evaluation of IN inhibitors.
To extend this system, we set up a high-throughput system for ex vivo analysis of compounds with known in vitro anti-HIV activities, using a microtiter plate format based on luciferase activity, allowing a rapid and sensitive evaluation of viral inhibitors on 293 cells. To this end, 293 cells were seeded in a 96-well white plate with a clear bottom and infected with equal amounts of IN-competent and IN-defective TY2-CMV-Luciferase lentiviral vectors. At the time of infection, increasing amounts of the IN inhibitor L-731,988 (range, 0.5 to 50 μM) and the RT inhibitors AZT (range, 10 to 1,000 μM) and ddI (range, 0.0005 to 10 μM) were added to the medium. Four hours postinfection, the medium was removed and replaced with fresh medium supplemented with each drug. After 72 h, the luciferase activity was evaluated directly on the 96-well white plate. Following data analysis, the IC50s and IC90s in both the IN-competent and IN-defective TY2-CMV-Luciferase-infected cells were calculated. As shown in Fig. 4a, luciferase values decreased in the IN-competent TY2-CMV-Luciferase-infected cells with increasing amounts of L-731,988, confirming the results obtained with the IN-competent TY2-CMV-GFP HIV-derived vector. Importantly, the calculated IC50 and IC90 values were 6.6 μM and 40.7 μM, respectively, both in the same log range of activity attained in the PBMC experiment (Fig. 4). In particular, luciferase expression decreased proportionally to the increase in drug concentrations, declining over 22-fold and approaching the values obtained in cells transduced with the IN-defective vector. Conversely, in cells infected with the IN-defective vector, luciferase expression was not modified, with calculated IC50 and IC90 values of >50 μM, suggesting that luciferase production from E-DNA was not affected by the addition of the anti-IN drug, as shown for the IN-defective TY2-CMV-GFP HIV-derived vector. Compound toxicity was tested using the trypan blue exclusion method and was found to be negligible (below 10%) at the indicated IC50 and IC90 (data not shown).
FIG. 4.
Luciferase activity in 293 cells infected with IN-competent (squares; left y axes) or IN-defective (diamonds; right y axes) luciferase-expressing self-inactivating lentiviral vectors. The cells were infected in a 96-well plate format with 1 cpm/cell equivalent/well and treated with increasing amounts of the anti-IN drug L-731,988 (range, 0.5 to 50 μM) (a) and the RT inhibitors AZT (range, 10 to 1,000 μM) (b) and ddI (range, 0,0005 to 10 μM) (c). Three days posttransduction, cell-associated luciferase activity was evaluated on a 96-well white plate with a clear bottom, using the Britelite Ultra-High Sensitivity Luminescence Reporter Gene Assay System. RLU, relative light units. Each condition was tested in three replicates, and mean values with standard deviations are shown.
As a further control to validate IN as the molecular target accountable for the antiviral activity in our system, the two nucleoside analogues, AZT and ddI, that interfered with viral reverse transcription, a step preceding nuclear import and circle formation, were tested in the same system. As shown in Fig. 4b and c, luciferase activity decreased with increasing amounts of either AZT or ddI in both the IN-competent and IN-defective lentiviral-vector-infected cells in the absence of evident cellular toxicity, indicating that drugs that inhibit the reverse-transcription step preceding E-DNA synthesis do not allow transcription from unintegrated templates. However, in the 293 cell system, a larger amount of each drug was necessary to reach measurable IC50 and IC90 values than in the HIV-1 drug susceptibility assay performed on PBMC, suggesting that 293 cells are somewhat refractory to nucleoside analogue internalization (Fig. 1).
A luciferase-based assay for the evaluation of new DKA-derived compounds.
The anti-IN activities of DKA-derived compounds were measured by standard biochemical assays (Table 2). The results indicated that all tested compounds were potent IN inhibitors in enzyme assays and were selective against strand transfer versus 3′-processing reactions, as has been described with most DKA-derived compounds (47). In general, preliminary structure-activity relationships referred to strand transfer reaction, leading us to conclude that (i) the acids (RDS 1625, RDS 1984, RDS 2161, and RDS 2164) were 10 to 100 times more potent inhibitors than the corresponding esters (RDS 1624, RDS 1983, RDS 2160, and RDS 2163), (ii) the 2,6-F2 derivatives were more potent inhibitors than the 4-F derivatives, (iii) the 2-OCH3 derivatives were 4- to 10-fold more active than their 3-OCH3 counterparts, and (iv) the shift of the DKA chain of RDS 1625 in the 6 position of the quinolinone ring led to compound RDS 1607, which showed a decreased inhibitory activity compared to that of the parental compound. Interestingly, three out of nine derivatives were more potent than the reference compound, L-731,988.
TABLE 2.
Comparison of IC50 and IC90 values of novel IN inhibitors by using biochemical and cell-based assaysa
Structures of compounds L-731,688 and RDS 1607 and the parental structure of derivatives of RDS 1624 to RDS 2164 are shown above the table.
In vitro IC50 values in 3′-processing and strand transfer reactions for the indicated DKA derivatives.
For PBMC infections, HIV-1 p24 antigen release in the supernatant was quantified using an enzyme-linked immunosorbent assay kit. The IC50 and IC90 of each drug were determined by the median effect equation (7). Each condition was tested in five replicates.
For 293 cell infections, cell-associated luciferase activity was evaluated on a 96-well white plate with a clear bottom. The IC50 and IC90 of each drug were determined by the median effect equation. Each condition was tested in three replicates.
Subsequent to the in vitro analysis, all compounds were evaluated for antiviral activity by comparing the HIV-1 drug susceptibility assay performed on PBMC and the luciferase-based assay described above. First, all compounds were tested on PBMC (range, 1.0 μM to 50 μM), using the in vitro HIV-1 drug susceptibility assay. As shown in Table 2, compounds RDS 1625, RDS 1983, and RDS 1984 attained measurable IC50s and IC90s, while compounds RDS 2163 and RDS 2164 produced only measurable IC50s but not IC90s. Of note, the IC50 and IC90 were never reached at any given drug concentration with compounds RDS 1607, RDS 1624, RDS 2160, and RDS 2161.
We further evaluated all compounds with our luciferase-based system by infecting 293 cells with equal amounts of IN-competent and IN-defective lentiviral vectors expressing luciferase. The reference drug, L-731,988, was added under the same conditions previously tested, while the range of concentrations used for all the other compounds was 1.0 μM to 50 μM (Fig. 5). Seventy-two hours later, luciferase activity was evaluated directly on a 96-well white plate, and the IC50 and IC90 were calculated (Table 2). A good correlation was observed between the luciferase-based system and the HIV-1 drug susceptibility assay performed on PBMC; in fact, L-731,988, RDS 1625, RDS 1983, and RDS 1984 inhibitors exhibited IC50s and IC90s in the same log range of magnitude obtained in the PBMC assay, while IC50, but not IC90, values were measurable for compounds RDS 1624, RDS 2160, RDS 2161, RDS 2163, and RDS 2164. Furthermore, the IC50 and IC90 were never reached at any given drug concentration with compound RDS 1607, similar to the PBMC assay. Moreover, L-731,988 values were comparable to those obtained in previous experiments, confirming test reproducibility. In particular, luciferase expression was reduced with increasing amounts of each tested drug, nearing the luciferase values obtained in cells transduced with the IN-defective vector. Conversely, luciferase expression in cells infected with the IN-defective vector was not modified, indicating that luciferase production from E-DNA was not affected by the addition of the anti-IN drugs. Compound toxicity was tested using the trypan blue exclusion method by evaluating the percentage of live cells recovered with respect to the untreated samples; in particular, we did not notice cytotoxic effects above 10% with the anti-IN compounds in 293 cells at any of the tested concentrations, but rather a dose-dependent generalized cytostatic effect in the drug-treated cultures that was evident with some of the compounds used (RDS 1624, RDS 2160, RDS 2161, RDS 2163, and RDS 2164) (Fig. 5).
FIG. 5.
Luciferase activities in 293 cells infected with IN-competent (squares; left y axes) or IN-defective (diamonds; right y axes) luciferase-expressing self-inactivating lentiviral vectors. The cells were infected in a 96-well plate format with 1 cpm/cell equivalent/well and treated with increasing amounts of the anti-IN drug L-731,988 (range, 0.5 to 50 μM) or the anti-IN DKA derivatives indicated (range, 1 to 50 μM). Three days posttransduction, cell-associated luciferase activity was evaluated on a 96-well white plate with a clear bottom, using the Britelite Ultra-High Sensitivity Luminescence Reporter Gene Assay System. RLU, relative light units. Each condition was tested in three replicates, and mean values with standard deviations are shown.
DISCUSSION
HIV-1 IN is an attractive target for antiviral therapy; indeed, several compounds with anti-IN activities have been identifiedover the past 10 years. However, continuous development and testing of novel compounds with anti-IN activities in the absence of toxicity remains important. To evaluate anti-IN activity, a number of assays have been established, generally relying on the ability of the compound under scrutiny to inhibit IN activity in in vitro biochemical assays (32). Nevertheless, at present, specific and appropriate cell-based assays required for determining the antiviral activity of IN inhibitors are not available or are costly and time-consuming.
Here, we have reported on the development and standardization of a 96-well microtiter assay for the fast screening of novel agents with potential anti-IN activities in a cell-based system. For the assay, adherent 293 cells were infected with an IN-competent luciferase-expressing HIV-derived vector and cultured in the presence of different concentrations of the anti-IN compounds analyzed. Values were compared to the luciferase activity recovered in 293 cells infected with an IN-defective luciferase-expressing HIV-derived vector, which was always used as a reference for background luciferase activity from E-DNA and for the toxicity of the compounds. Since the anti-IN compounds decreased the amount of integrated provirus, and consequently the transcription, we could evaluate the anti-IN activities of several compounds by determining whether luciferase transcription from the IN-competent virus approached the values obtained from the IN-defective virus, whose expression was driven only by E-DNA.
In this setting, the use of an IN-defective lentiviral vector expressing a reporter gene allowed us to evaluate whether the anti-IN compounds under analysis were acting at steps prior to circle formation. In fact, following infection of 293 cells with the IN-defective lentiviral vector, E-DNA production and GFP and luciferase expression did not change in the presence of IN inhibitors in the absence of cellular toxicity. This was as expected in the presence of a true IN inhibitor. In contrast, compounds such as AZT or ddI, acting at steps preceding circle formation, had the same effect on E-DNA production from IN-competent or IN-defective viruses, since E-DNA synthesis was inhibited (13, 23, 30, 38, 39) and luciferase expression fell to negligible levels. This assay was validated by comparative evaluation with the HIV-1 drug susceptibility assay performed on PBMC. IC50 and IC90 values obtained in the HIV-1IIIB-infected PBMC and in the 293 cells infected with the IN-competent lentiviral vector were comparable and similar to those reported in the literature.
Importantly, this method allowed us to evaluate the ex vivo anti-IN activities of novel compounds for which the anti-IN activity had been first demonstrated using biochemical assays. Using the well-known anti-IN drug L-731,988 as the reference control, all the new compounds were tested in the HIV-1 drug susceptibility assay of PBMC in comparison with 293 cells infected with the luciferase-expressing lentiviral vectors. The results showed that only compounds RDS 1625, RDS 1983, and RDS 1984 confirmed their activities as IN inhibitors in both assays, while all other compounds were either unable to block HIV-1IIIB replication in the drug susceptibility assay of PBMC or ineffective in reducing luciferase expression in the 293 cell-based system. These data confirm the need to evaluate in a cell-based assay compounds previously tested in vitro and further confirm the validity of the method we described.
In conclusion, the microtiter assay for the detection of IN inhibitors described here allows high reproducibility and standardization, avoiding the limitation of donor variability and thus providing a uniform and cost-effective method for the evaluation of novel anti-IN compounds. Moreover, the use of HIV-1-derived vectors (IN competent and IN defective) avoids the requirement for handling infectious HIV-1, since lentiviral vectors are competent to infect target cells but do not support further rounds of viral replication. In addition, since these vectors mimic the HIV-1 life cycle, including the formation of E-DNA (43, 44), the use of IN-defective virus expressing luciferase allows investigators to check whether the anti-IN compounds under evaluation are acting at steps prior to circle formation. Finally, the presence of the firefly luciferase gene as a reporter gene provides a rapid, sensitive, and quantitative measure of virus infectivity and expression.
Acknowledgments
We thank P. Cocco, D. Diamanti, and F. Costa for technical support. We also thank Donatella Negri for critical reading and comments. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pTY-EFeGFP from Lung-Ji Chang and HTLV-IIIB/H9 virus from Robert Gallo.
This work was supported by grants from the AIDS National Program, Istituto Superiore di Sanità, Rome, Italy.
REFERENCES
- 1.Ansari-Lari, M. A., L. Donehower, and R. A. Gibbs. 1995. Analysis of human immunodeficiency virus type 1 integrase mutants. Virology 211:332-335. [DOI] [PubMed] [Google Scholar]
- 2.Brussel, A., and P. Sonigo. 2003. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J. Virol. 77:10119-10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brussel, A., and P. Sonigo. 2004. Evidence for gene expression by unintegrated human immunodeficiency virus type 1 DNA species. J. Virol. 78:11263-11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. [DOI] [PubMed] [Google Scholar]
- 5.Cara, A., F. Guarnaccia, M. S. Reitz, Jr., R. C. Gallo, and F. Lori. 1995. Self-limiting, cell type-dependent replication of an integrase-defective human immunodeficiency virus type 1 in human primary macrophages but not T lymphocytes. Virology 208:242-248. [DOI] [PubMed] [Google Scholar]
- 6.Chang, L.-J., V. Urlacher, T. Iwakuma, Y. Cui, and J. Zucali. 1999. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 6:715-728. [DOI] [PubMed] [Google Scholar]
- 7.Chou, T. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-98. In T. Chou and D. Ridout (ed.), Synergism and antagonism in chemotherapy. Academic Press, San Diego, Calif.
- 8.Coffin, J. M., S. H. Hughes, and H. E. Varmus (ed.). 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 9.Cui, Y., T. Iwakuma, and L.-J. Chang. 1999. Contributions of viral splice sites and cis-regulatory elements to lentivirus vector function. J. Virol. 73:6171-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Santo, R., R. Costi, A. Roux, M. Artico, A. Lavecchia, L. Marinelli, E. Novellino, L. Palmisano, M. Andreotti, R. Amici, C. M., Galluzzo, L. Nencioni, A. T., Palamara, C., Marchand, and Y. Pommier. 2006. Novel bifunctional quinolonyl diketo acid derivatives as HIV-1 integrase inhibitors: design, synthesis, biological activities, and mechanism of action. J. Med. Chem. 49:1939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Division of AIDS, National Institute of Allergy and Infection Disease. 1997. DAIDS virology manual for HIV laboratories. Publication NIH-97-3828. U.S. Department of Health and Human Services, Washington, D.C.
- 11.Engelman, A., G. Englund, J. M., Orenstein, M. A., Martin, and R. Craigie. 1995. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 69:2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farnet, C. M., B. Wang, J. R. Lipford, and F. D. Bushman. 1996. Differential inhibition of HIV-1 preintegration complexes and purified integrase protein by small molecules. Proc. Natl. Acad. Sci. USA 93:9742-9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geleziunas, R., E. J. Arts, F. Boulerice, H. Godman, and M. A. Wainberg. 1993. Effect of 3′-azido-3′-deoxythymidine on human immunodeficiency virus type 1 replication in human fetal brain macrophages. Antimicrob. Agents Chemother. 37:1305-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillim-Ross, L., A. Cara, and M. E. Klotman. 2005. Nef expressed from human immunodeficiency virus type 1 extrachromosomal DNA downregulates CD4 on primary CD4+ T lymphocytes: implications for integrase inhibitors. J. Gen. Virol. 86:765-771. [DOI] [PubMed] [Google Scholar]
- 15.Gulick, R. M. 2003. New antiretroviral drugs. Clin. Microbiol. Infect. 9:186-193. [DOI] [PubMed] [Google Scholar]
- 16.Hansen, M. S., G. J. Smith III, T. Kafri, V. Molteni, J. S., Siegel, and F. D. Bushman. 1999. Integration complexes derived from HIV vectors for rapid assays in vitro. Nat. Biotechnol. 17:578-582. [DOI] [PubMed] [Google Scholar]
- 17.Hazuda, D. J., P. Felock, M. Witmer, A. Wolfe, K. Stillmock, J. A. Grobler, A. Espeseth, L. Gabryelski, W. Schleif, C. Blau, and M. D. Miller. 2000. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287:646-650. [DOI] [PubMed] [Google Scholar]
- 18.Hermans, P. 2001. Current review and clinical management of patients with primary HIV-1 infection: limits and perspectives. Biomed. Pharmacother. 55:301-307. [DOI] [PubMed] [Google Scholar]
- 19.Hwang, Y., D. Rhodes, and F. Bushman. 2000. Rapid microtiter assays for poxvirus topoisomerase, mammalian type IB topoisomerase and HIV-1 integrase: application to inhibitor isolation. Nucleic Acids Res. 28:4884-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamichi, T. 2004. Action of anti-HIV drugs and resistance: reverse transcriptase inhibitors and protease inhibitors. Curr. Pharm. Des. 10:4039-4053. [DOI] [PubMed] [Google Scholar]
- 21.Iwakuma, T., Y. Cui, and L.-J. Chang. 1999. Self-inactivating lentiviral vectors with U3 and U5 mutations. Virology 261:120-132. [DOI] [PubMed] [Google Scholar]
- 22.Japour, A. J., D. L. Mayers, V. A., Johnson, D. R., Kuritzkes, L. A. Beckett, J. M. Arduino, J. Lane, R. J. Black, P. S. Reichelderfer, R. T. D'Aquila, C. S. Crumpacker, the RV-43 Study Group, and the AIDS Clinical Trials Group Virology Committee Resistance Working Group. 1993. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother. 37:1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kok, T. W., P. Li, and C. J. Burrel. 1998. Further characterization of HIV RNA synthesis early after cell-to-cell transmission infection. Arch. Virol. 143:1911-1926. [DOI] [PubMed] [Google Scholar]
- 24.Maag, H., R. M. Rydzewski, M. J. McRoberts, D. Crawford-Ruth, J. P. Verheyden, and E. J. Prisbe. 1992. Synthesis and anti-HIV activity of 4′-azido- and 4′-methoxynucleosides. J. Med. Chem. 35:1440-1451. [DOI] [PubMed] [Google Scholar]
- 25.Marchand, C., X. Zhang, G. C. Pais, K. Cowansage, N. Neamati, T. R. Burke, Jr., and Y. Pommier. 2002. Structural determinants for HIV-1 integrase inhibition by beta-diketo acids. J. Biol. Chem. 277:12596-12603. [DOI] [PubMed] [Google Scholar]
- 26.Nair, V. 2002. HIV integrase as a target for antiviral chemotherapy. Rev. Med. Virol. 12:179-193. [DOI] [PubMed] [Google Scholar]
- 27.Naldini, L., U. Blömer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 28.Ojwang, J. O., R. W. Buckheit, Y. Pommier, A. Mazumder, K. De Vreese, J. A. Este, D. Reymen, L. A. Pallansch, C. Lackman-Smith, T. L. Wallace, E. De Clercq, M. S. McGrath, and R. F. Rando. 1995. T30177, an oligonucleotide stabilized by an intramolecular guanosine octet, is a potent inhibitor of laboratory strains and clinical isolates of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 39:2426-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pais, G. C. G., X. C. Zhang, C. Marchand, N. Neamati, K. Cowansage, E. S. Svarovskaia, V. K. Pathak, Y. Tang, M. Nicklaus, Y. Pommier, and T. R. Burke. 2002. Structure activity of 3-aryl-1,3-diketo-containing compounds as HIV-1 integrase inhibitors. J. Med. Chem. 45:3184-3194. [DOI] [PubMed] [Google Scholar]
- 30.Pauza, C. D., P. Trivedi, T. S. McKechnie, D. D. Richman, and F. M. Graziano. 1994. 2-LTR circular viral DNA as a marker for human immunodeficiency virus type 1 infection in vivo. Virology 205:470-478. [DOI] [PubMed] [Google Scholar]
- 31.Pereira, C. F., and J. T. Paridaen. 2004. Anti-HIV drug development—an overview. Curr. Pharm. Des. 10:4005-4037. [DOI] [PubMed] [Google Scholar]
- 32.Pommier, Y., A. A. Johnson, and C. Marchand. 2005. Integrase inhibitors to treat HIV/AIDS. Nat. Rev. Drug Discov. 4:236-248. [DOI] [PubMed] [Google Scholar]
- 33.Pommier, Y., and N. Neamati. 1999. Inhibitors of human immunodeficiency virus integrase. Adv. Virus Res. 52:427-458. [DOI] [PubMed] [Google Scholar]
- 34.Popovic, M., E. Read-Connole, and R. C. Gallo. 1984. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet ii:1472-1473. [DOI] [PubMed] [Google Scholar]
- 35.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 36.Ratner, L., W. Haseltine, R. Patarca, K. J. Livak, B. Starcich, S. F. Josephs, E. R. Doran, J. A. Rafalski, E. A. Whitehorn, K. Baumeister, L. Ivanoff, S. R. Petteway, Jr., M. L. Pearson, J. A. Lautenberger, T. S. Papas, J. Ghrayab, N. T. Chang, R. C., Gallo, and F. Wong-Staal. 1985. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313:277-283. [DOI] [PubMed] [Google Scholar]
- 37.Schinazi, R. F., J. P. Sommadossi, V. Saalmann, D. L. Cannon, M. Y. Xie, G. C. Hart, G. A. Smith, and E. F. Hahn. 1990. Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 34:1061-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharkey, M. E., I. Teo, T. Greenough, N. Sharova, K. Luzuriaga, J. L. Sullivan, R. P. Bucy, L. G., Kostrikis, A. Haase, C. Veryard, R. E. Davaro, S. H. Cheeseman, J. S. Daly, C. Bova, R. T. Ellison III, B. Mady, K. K. Lai, G. Moyle, M. Nelson, B. Gazzard, S. Shaunak, and M. Stevenson. 2000. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 6:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharmeen, L., T. McQuade, A. Heldsinger, R. Gogliotti, J. Domagala, and S. Gracheck. 2001. Inhibition of the early phase of HIV replication by an isothiazolone. Antiviral Res. 49:101-114. [DOI] [PubMed] [Google Scholar]
- 40.Svarovskaia, E. S., R. Barr, X. Zhang, G. C. G. Pais, C. Marchand, Y. Pommier, T. R. Burke, Jr., and V. K. Pathak. 2004. Azido-containing diketo acid derivatives inhibit human immunodeficiency virus type 1 integrase in vivo and influence the frequency of deletions at two-long-terminal-repeat-circle junctions. J. Virol. 78:3210-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandegraaff, N., R. Kumar, C. J. Burrell, and P. Li. 2001. Kinetics of human immunodeficiency virus type 1 (HIV) DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. J. Virol. 7:11253-11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandegraaff, N., R. Kumar, H. Hocking, T. R. Burke, Jr., J. Mills, D. Rhodes, C. J. Burrell, and P. Li. 2001. Specific inhibition of human immunodeficiency virus type 1 (HIV-1) integration in cell culture: putative inhibitors of HIV-1 integrase. Antimicrob. Agents Chemother. 45:2510-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Maele, B., J. De Rijck, E. De Clercq, and Z. Debyser. 2003. Impact of the central polypurine tract on the kinetics of human immunodeficiency virus type 1 vector transduction. J. Virol. 77:4685-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas, J., Jr., G. L. Gusella, V. Najfeld, M. E. Klotman, and A. Cara. 2004. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum. Gene Ther. 15:361-372. [DOI] [PubMed] [Google Scholar]
- 45.Weiss, R., N. Teich, H. Varmus, and J. Coffin (ed.). 1982. Molecular biology of tumor viruses, p. 1205-1218. In R. Weiss, N. Teich, H. Varmus, and J. Coffin (ed.), RNA tumor viruses, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 46.Wu, Y., and J. W. Marsh. 2003. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J. Virol. 77:10376-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, X., G. C. G. Pais, E. S. Svarovskaia, C. Marchand, A. A. Johnson, R. G. Karki, M. C. Nicklaus, V. K. Pathak, Y. Pommier, and T. R. Burke, Jr. 2003. Azido-containing aryl beta-diketo acid HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 13:1215-1219. [DOI] [PubMed] [Google Scholar]
- 48.Zolotukhin, S., M. Potter, W. W. Hauswirth, J. Guy, and N. A. Muzyczka. 1996. “Humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 70:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]