Lincosamide antibiotics include lincomycin, a compound produced by several actinomycetes, and its semisynthetic chlorinated derivative clindamycin. These antibiotics block the peptidyltransferase activity of the 50S subunit of the bacterial ribosome, inhibiting protein synthesis, and are active against most gram-positive cocci and anaerobes. However, they are not generally effective against gram-negative bacilli due to intrinsic resistance (3, 5).
Resistance to lincosamides is most commonly due to N6 dimethylation of an adenine residue in the 23S rRNA, which usually confers broad-spectrum cross-resistance to macrolides, lincosamides, and streptogramin B antibiotics or to efflux (5). However, antibiotic inactivation by nucleotidylation (1, 2) has also been described as a mechanism of resistance. Despite the fact that lincosamides are not used to treat enterobacterial infections, a gene, linF, that confers low levels of resistance to both lincomycin (fourfold) and clindamycin (twofold) was recently found in a gene cassette in a class 1 integron recovered from an Escherichia coli blood isolate (4). The linF gene encodes a 273-amino-acid lincosamide nucleotidyltransferase. The LinF protein shares approximately 35% identity with the nucleotidyltransferases encoded by the linB gene from Enterococcus faecium (1) (GenBank accession no. AF110130) and linB′ from Enterococcus faecalis (GenBank accession no. AF408195).
We have identified a second lin gene in a gene cassette. This cassette was recovered from a multiply antibiotic-resistant Salmonella enterica serovar Stanley strain (SRC54) isolated in 2001 from a traveler who had recently returned from Thailand. This strain was resistant to chloramphenicol, gentamicin, kanamycin, spectinomycin, streptomycin, sulfathiazole, and tetracycline but susceptible to ampicillin and nalidixic acid at levels described previously (6). It displayed intermediate resistance to ciprofloxacin. The gene cassette array was amplified by using standard primers (L2 and R1) in the 5′ conserved sequence and 3′ conserved sequence of class 1 integrons, and the 2.25-kb amplicon was cloned into pPCRscript and sequenced as previously described (see reference 6 for primer details). E. coli strain DH5α containing pPCR-Script with the cassette array was at least 10-fold more resistant to lincomycin (MIC, ≥2,000 μg/ml) than DH5α containing only pPCR-Script (MIC, 180 μg/ml).
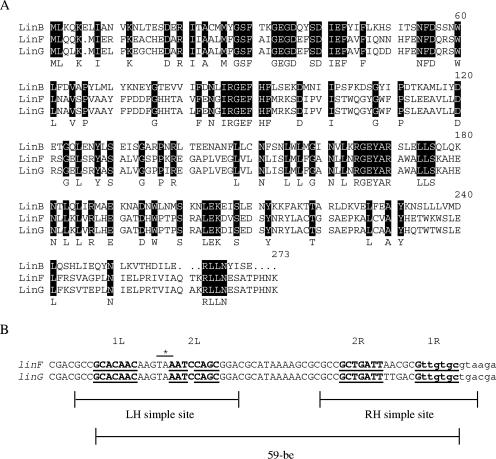
The first cassette in the array was identical to the aadA2 cassette in GenBank accession no. L06822. The second cassette was 937 bp long and 93.4% identical to the linF gene cassette. It encoded a 273-amino-acid protein that is 93.1% identical to LinF (17 amino acid differences). An alignment of these proteins with LinB is shown in Fig. 1A. The sequence of the aadA2-linG cassette array was identical to a region found in GenBank accession no. AY522431. The 59-base elements (59-be; attC sites) of the linG and linF cassettes are 58 bp long (Fig. 1B) and not closely related to any other known 59-be. They retain the critical features of 59-be, namely, complementary sites 1L-1R and 2L-2R (7).
FIG. 1.
Analysis of LinG sequences. (A) Alignment of Lin proteins. Amino acids that are completely conserved across all sequences are shown as white letters on a black background. The protein sequences of LinB and LinF were from GenBank accession numbers AF110130 and AJ561197, respectively. The LinG protein sequence is from this study. (B) Alignment of linF and linG 59-be. The core sites are in bold and are labeled 1L, 1R, 2L, and 2R (7). The boundaries of the LH (left-hand) and RH (right-hand) simple sites and the 59-be are indicated by bars. The bases in lowercase are those derived from the beginning of the cassette. The stop codons of the LinG and LinF proteins are indicated by the asterisk. The sequence of each 59-be came from the sources mentioned for panel A.
Nucleotide sequence accession numbers.
The sequence of the aadA2-linG cassette array has been deposited in GenBank under accession no. DQ836009.
REFERENCES
- 1.Bozdogan, B., L. Berrezouga, M. Kuo, D. A. Yurek, K. A. Farley, B. J. Stockman, and R. Leclercq. 1999. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 43:925-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brisson-Noel, A., P. Delrieu, D. Samain, and P. Courvalin. 1988. Inactivation of lincosamide antibiotics in Staphylococcus. Identification of lincosaminide O-nucleotidyltransferases and comparison of the corresponding resistance genes. J. Biol. Chem. 263:15880-15887. [PubMed] [Google Scholar]
- 3.Dhawan, V. K., and H. Thadepalli. 1982. Clindamycin: a review of fifteen years of experience. Rev. Infect. Dis. 4:1133-1153. [DOI] [PubMed] [Google Scholar]
- 4.Hier, E., B.-A. Lindstedt, T. M. Leegaard, E. Gjernes, and G. Kapperud. 8 July 2004. Prevalence and characterization of integrons in blood culture Enterobacteriaceae and gastrointestinal Escherichia coli in Norway and reporting of a novel class 1 integron-located lincosamide resistance gene. Ann. Clin. Microbiol. Antimicrob. 3:12. doi: 10.1186/1476-0711-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclercq, R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34:482-492. [DOI] [PubMed] [Google Scholar]
- 6.Levings, R. S., S. R. Partridge, D. Lightfoot, R. M. Hall, and S. P. Djordjevic. 2005. New integron-associated gene cassette encoding a 3-N-aminoglycoside acetyltransferase. Antimicrob. Agents Chemother. 49:1238-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stokes, H. W., D. B. O'Gorman, G. D. Recchia, M. Parsekhian, and R. M. Hall. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26:731-745. [DOI] [PubMed] [Google Scholar]