Abstract
Antibacterial agents disrupt the ecological balance of the normal human microflora. Tigecycline, a member of a new class of antibiotics (glycylcyclines), has been shown to have a potent broad-spectrum activity against most gram-positive and gram-negative aerobic and anaerobic bacteria. The aim of the study was to investigate the ecological effects of tigecycline on the normal oropharyngeal and intestinal microflora of healthy subjects. Thirteen healthy white subjects (six females and seven males) between 20 and 31 years of age received 100 mg of tigecycline in the morning on day 1 as a 30-min intravenous infusion followed by a 50-mg dose of tigecycline every 12 h as a 30-minute infusion for 10 days. One subject was withdrawn on day 2 because of an adverse event (urticaria). Serum, saliva, and fecal samples were collected before, during, and after administration for microbiological cultivation and for assays of tigecycline. All new colonizing bacteria were tested for susceptibility (resistance of ≥8 mg/liter) during the investigation period. The fecal concentrations on day 8 were from 3.0 to 14.1 mg/kg, with a mean value of 6.0 mg/kg and a median value of 5.6 mg/kg. The saliva concentrations were generally low (0 to 0.12 mg/liter). A minor effect on the oropharyngeal microflora was observed. The numbers of enterococci and Escherichia coli cells in the intestinal microflora were reduced at day 8 (P < 0.05), while those of other enterobacteria and yeasts increased. There was a marked reduction of lactobacilli and bifidobacteria (P < 0.05) but no impact on bacteroides. No Clostridium difficile strains were isolated. Two Klebsiella pneumoniae strains and five Enterobacter cloacae strains resistant to tigecycline were found on day 8.
Tigecycline is a novel analog of minocycline that circumvents existing mechanisms of microbial resistance. It demonstrates a broad spectrum of antibacterial activity, inhibiting multiply resistant gram-positive and gram-negative aerobic and anaerobic bacteria (2, 6, 8, 9, 14). The safety and tolerability of tigecycline are consistent with those observed for other members of the tetracycline family of antibacterial agents. Preclinical and clinical data suggest that tigecycline is safe and effective. Thus, tigecycline has been shown to be effective for the treatment of complicated skin and skin structure infections (7) and for the treatment of complicated intra-abdominal infections (1) in large clinical trials. Tigecycline was approved for use in the United States in 2005 and in Europe in 2006.
The normal human microflora is relatively stable in each ecological habitat under normal circumstances and acts as a barrier against colonization by potentially pathogenic microorganisms. Disturbances in the normal microflora may occur due to changes in diet, radiation, or administration of antimicrobial agents. The administration of antimicrobial agents can cause several adverse effects on the microflora (16). Careful investigation of the impact of antibiotic treatment on the endogenous microflora is of importance, since alteration of the endogenous flora balance, qualitatively and/or quantitatively, may facilitate colonization by new potentially pathogenic strains or may enable microorganisms already present in the normal flora to develop resistance.
The primary objective of this study was to assess the impact of antimicrobial treatment on the oropharyngeal and intestinal microflora before, during, and after administration of tigecycline to healthy subjects, and the secondary objective was to explore the potential for development of resistance by measuring the susceptibility (MIC) of isolated microbial strains before, during, and after administration of tigecycline. The pharmacokinetics, safety, and tolerability of tigecycline were also assessed.
MATERIALS AND METHODS
Subjects.
Thirteen healthy white subjects (six women and seven men between 20 and 31 years of age) were enrolled, and 12 completed the study. One subject withdrew from the study due to an adverse event (urticaria) after receiving one 100-mg and three 50-mg doses of tigecycline. This subject was replaced. All subjects included in the study had normal findings from physical examinations, electrocardiograms, and laboratory tests (including tests of hematological and biochemical parameters, hepatitis and human immunodeficiency virus serological tests, tests for drug abuse, urinalysis, and pregnancy tests). Exclusion criteria were regular use of medications; smoking of more than 10 cigarettes per day; abuse of alcoholic beverages; symptoms of significant illness within 3 months before the study period; a history of gastrointestinal, liver, or kidney disease potentially interfering with absorption, metabolism, or excretion of drugs; a history of central nervous system disorders; allergy or hypersensitivity to the study drug; use of any investigational drug within 4 weeks of tigecycline administration; antibiotic treatment within 3 months before the study period; and pregnancy. Written informed consent was obtained from all subjects prior to the study. The study was approved by the Ethics Committee of Uppsala University, Uppsala, Sweden, and the Medical Products Agency, Uppsala, Sweden.
Drug administration.
Subjects received 100 mg of tigecycline in the morning on day 1 as a 30-min intravenous (i.v.) infusion followed by a 50-mg dose of tigecycline given every 12 h as a 30-min infusion for a total of 19 doses over 10 days (last dose on the morning of day 10). The subjects were fed standard medium-fat meals approximately 30 min before the administration. The standard medium-fat menu consisted of approximately 2,200 cal daily. The nutritional distribution was approximately 30% fat, 40% carbohydrates, and 30% protein. Fluids were taken ad lib. No other food was to be consumed except that provided by the study center, and food was not to be consumed at times other than the scheduled meal times.
Sampling of specimens.
Serum samples were collected on days 1, 2, and 5 before the morning i.v. infusion, on day 9 before the morning and evening i.v. infusions, on day 10 before the i.v. infusion, and then 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after the start of the infusion. The samples were kept on ice at all times and were centrifuged within 15 min at 2 to 8°C and 2,400 × g for 10 min. The resulting serum was transferred into two tubes and stored at −70°C until assayed.
Saliva samples were collected at the same time points as for the serum samples in addition to on days 2, 5, 10, 12, 15, 18, 24, and 31 for microbiological analyses and for assays of tigecycline. The samples of unstimulated mixed saliva were obtained by having the subjects spit into sterile tubes and were stored at −70°C until assayed.
Fecal samples were collected on day 1 before dose administration and then on days 2, 5, 8, 10, 12, 15, 18, 24, and 31 for microbiological cultivation and for assays of tigecycline (days 2, 5, 8, 10, 12, and 15). The first specimen passed on a given day was analyzed if several were produced on the same day. Fecal samples for microbiological analyses were collected in sterile plastic containers and were frozen at −70°C until processed.
Determination of tigecycline serum, saliva, and fecal concentrations.
The concentrations of tigecycline in sera, saliva samples, and feces were determined microbiologically using the agar plate diffusion method. The test agar medium was prepared with nutrient broth (BBL, Cockeysville, MD) and agarose (Sigma, St. Louis, MO), and the indicator strain was Bacillus cereus ATCC 11778 (American Type Culture Collection, Manassas, VA). Samples were run in duplicate, and a concomitant standard series was inoculated on each agar plate. The plates were incubated aerobically for 18 h at 30°C. Concentrations of tigecycline were determined relative to the diameters of the inhibition zones caused by the corresponding concentrations from the standard series. The detection limits were 0.01 mg/liter of serum or saliva and 0.01 mg/kg of feces. The concentrations of tigecycline were also determined by liquid chromatography-tandem mass spectrometry analysis (LC/MS-MS) (12).
Processing of saliva and fecal specimens for microbiological analysis.
The saliva and fecal specimens were suspended in prereduced peptone-yeast extract medium, diluted to 10−7, and inoculated on nonselective and selective media (5, 11). All processes were carried out in an anaerobic chamber. The following agar media were used: blood agar (5% horse blood; Kemila, Lab M, Bury, United Kingdom) for total aerobes and anaerobes, cystine lactose electrolyte-deficient agar (Merck, Darmstadt, Germany) for the detection of Enterobacteriaceae, Enterococcosel agar (BBL) for the detection of enterococci, Sabouraud agar (Difco) for the detection of yeasts, Rogosa agar (Difco Laboratories, Detroit, MI) for the cultivation of lactobacilli, BL agar (BBL and Difco) for the cultivation of bifidobacteria, kanamycin-vancomycin-blood agar for the cultivation of Bacteroides and Prevotella species, neomycin-vancomycin-blood agar for the cultivation of fusobacteria, veillonella agar (Difco) for the cultivation of Veillonella cocci, egg yolk agar (Oxoid, Basingstoke, United Kingdom) for the cultivation of clostridia, and taurocholate-cycloserine-cefoxitin-fructose agar (peptone from casein/proteose peptone no. 3 at 40 mg/ml, sodium hydrogen phosphate at 5 mg/ml, potassium-dihydrogen phosphate at 1 mg/ml, sodium chloride at 2 mg/ml, magnesium sulfate at 0.2 mg/ml, Bacto agar at 20 mg/ml, taurocholic acid at 1 mg/ml, neutral red at 0.03 mg/ml, 15% fructose, Clostridium difficile supplement d-cycloserine, cefoxitin) for the detection of C. difficile. The aerobic agar plates and the anaerobic freshly prepared plates were incubated for 48 h at 37°C in GasPak anaerobic jars (BBL). After incubation, different colony types were counted, isolated in pure culture, and identified to the genus level. All isolates were analyzed according to Gram reaction and cell and colony morphology, and different biochemical tests were done subsequently. An API-20E test kit (BioMérieux, Marcy l'Etoile, France) was used for the identification of Enterobacteriaceae. The anaerobic microorganisms were identified by gas-liquid chromatography of metabolites from glucose. The lower limit of detection was 102 microorganisms per ml of saliva or per gram of feces.
Tigecycline susceptibility tests.
All new colonizing bacteria were isolated from each subject on days −1 to 31 to study the susceptibility to tigecycline during the investigation period. The MICs for tigecycline were determined by the agar dilution method according to guidelines established by the Clinical Laboratory and Standards Institute (3, 4). The following reference strains of the American Type Culture Collection were used: Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Bacteroides fragilis ATCC 25285, Bacteroides thetaiotaomicron ATCC 29741, and Eubacterium lentum ATCC 43055. The agar plates were incubated aerobically or anaerobically at 37°C for 24 or 48 h, respectively. The breakpoint for resistance used was ≥8 mg/liter (13).
Pharmacokinetic methods.
The single-dose tigecycline serum concentration data for each individual patient were analyzed using conventional, noncompartmental methods (10). Specifically, the peak serum concentrations and times to peak concentration were taken directly from the observed data. The area under the concentration-time curve (AUC) from time zero to the last reported concentration (CT) (AUCT) was calculated using the logarithmic trapezoidal rule for decreasing concentrations and the linear trapezoidal rule for increasing concentrations. The area under the concentration-time curve from time zero to infinity (AUC0-∞) was determined by the formula AUCT + CT/λz, where λz is the monoexponential elimination rate constant determined from the terminal portion of the concentration-time curve. The half-life was calculated as 0.693/λz. Systemic clearance was determined as the ratio of dose/AUC. The volume of distribution at steady state was determined as the ratio of (dose × AUMC)/AUC2, where AUMC (the area under the first moment of the concentration-time curve) was determined as Σ(Ci/λz2).
Statistical methods.
Statistics were calculated for the values estimated for the saliva and fecal specimens as log numbers of microorganisms per g feces or per liter saliva and as saliva and fecal concentrations of tigecycline, as well as for the pharmacokinetic paralysis, by use of Wilcoxon's signed-rank test and the Mann-Whitney U test.
RESULTS
Tigecycline concentrations in serum.
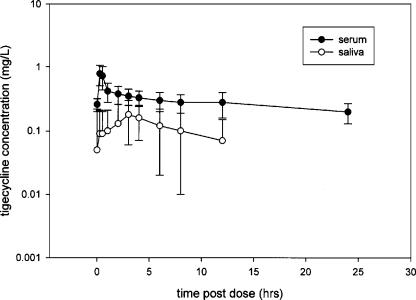
The serum concentrations of tigecycline on day 9 are shown for the subjects in Fig. 1. Table 1 summarizes tigecycline steady-state pharmacokinetic parameters as determined by both LC/MS-MS and microbiological analysis for the 12 subjects included in the analysis.
FIG. 1.
Tigecycline serum and saliva concentrations (mean ± standard deviation) in 12 subjects (healthy volunteers) after 50 mg i.v. every 12 h for 10 days.
TABLE 1.
Tigecycline steady-state serum pharmacokinetic parameters
| Type of analysis | Mean ± SD (geometric mean) for indicated pharmacokinetic parametera
|
||||
|---|---|---|---|---|---|
| Cmax (mg/liter) | Tmax (h) | AUC (mg · h/liter) | CL (liter/h) | Vss (liter) | |
| LC/MS-MS | 1.0 ± 0.3 (1.0) | 0.48 ± 0.07 (0.47) | 3.4 ± 1.3 (3.2) | 16.5 ± 4.7 (15.7) | 605 ± 259 (550) |
| Microbiological | 0.8 ± 0.3 (0.8) | 0.4 ± 0.1 (0.3) | 4.0 ± 1.2 (3.9) | 13.3 ± 3.0 (12.9) | 546 ± 164 (523) |
Abbreviations: CL, systemic clearance; Cmax, peak serum concentration; Tmax, time to maximum concentration of drug in serum; Vss, volume of distribution at steady state.
Tigecycline concentrations in feces.
Table 2 summarizes the tigecycline concentrations in feces. No measurable concentrations were found at baseline or on days 18 to 31, while the concentrations on day 8 were in the range 3.0 to 14.1 mg/kg, with a mean value of 6.0 mg/kg and a median value of 5.6 mg/kg.
TABLE 2.
Fecal concentrations of tigecycline determined by microbiological analysis for 12 healthy subjects receiving 50-mg doses of tigecycline i.v. every 12 h for 10 days
| Day | No. of subjects | Fecal concentration (mg/kg)
|
|||
|---|---|---|---|---|---|
| Mean | SD | Median | Range | ||
| 2 | 12 | 4.7 | 3.4 | 4.5 | 0-9.9 |
| 5 | 12 | 5.0 | 3.7 | 3.5 | 0-11.3 |
| 8 | 12 | 6.0 | 2.9 | 5.6 | 3.0-14.1 |
| 10 | 12 | 4.1 | 1.9 | 3.7 | 1.1-7.2 |
| 12 | 12 | 2.4 | 1.1 | 2.8 | 0.5-4.2 |
| 15 | 3 | 0.1 | 0.2 | 0 | 0-0.4 |
Tigecycline concentrations in saliva.
The saliva concentrations of tigecycline are summarized in Fig. 1. No measurable concentrations were found at baseline or on days 12 to 31. The saliva concentrations were generally low (0 to 0.12 mg/liter).
Effect of tigecycline on aerobic intestinal microflora.
The left panel of Fig. 2 presents the effect of tigecycline on the aerobic intestinal microflora. The numbers of enterococci and E. coli organisms were significantly reduced between days 2 and 12 (102 microorganisms/g feces), while there were increases in the numbers of other enterobacteria, such as Klebsiella and Enterobacter spp. (107 microorganisms/g feces), and of yeasts (P < 0.05). Six of 12 subjects were colonized by tigecycline-resistant (≥8 mg/liter) Klebsiella pneumoniae and Enterobacter cloacae strains on day 8, and eight volunteers were colonized by Candida albicans strains (≥8 mg/liter) on day 8. The aerobic microflora was normalized within 2 weeks.
FIG. 2.
Effects of tigecycline administration on the aerobic (left panel) and anaerobic (right panel) intestinal microfloras of 12 subjects. —, median value of logarithmic number of microorganisms/g feces.
Effect of tigecycline on anaerobic intestinal microflora.
The effect of tigecycline on the anaerobic intestinal microflora is shown in Fig. 2, right panel. There were marked reductions in the numbers of lactobacilli and bifidobacteria between days 2 and 12 (P < 0.05), while the numbers of bacteroides and clostridia were not affected by the administration of tigecycline. None of the subjects were colonized by C. difficile during the investigation period. On day 31, the anaerobic microflora, except for the bifidobacteria, was normalized.
Effect of tigecycline on aerobic oropharyngeal microflora.
The left panel of Fig. 3 shows the effect of tigecycline on the aerobic oropharyngeal microflora. There were minor disturbances in the microflora. The number of Streptococcus salivarius organisms decreased, while the number of micrococci increased. No significant alterations in the number of Streptococcus mitis, Neisseria species, or Haemophilus species organisms or staphylococci (not shown) were observed. No new colonizing microorganisms were isolated. The aerobic microflora was normalized on day 31.
FIG. 3.
Effects of tigecycline administration on the aerobic (left panel) and anaerobic (right panel) oropharyngeal microfloras of 12 subjects. —, median value of logarithmic number of microorganisms/ml saliva.
Effect of tigecycline on anaerobic oropharyngeal microflora.
The effect of tigecycline on the anaerobic oropharyngeal microflora is presented in Fig. 3, right panel. There were minor changes in the numbers of lactobacilli, bifidobacteria, and fusobacteria, while there were no alterations in the numbers of anaerobic cocci and Prevotella spp. (not shown). The number of bacteroides strains increased at day 31. No new colonizing anaerobic bacteria were observed. The anaerobic oropharyngeal microflora was normalized 2 weeks after the discontinuation of the administration of tigecycline.
Tigecycline susceptibility tests.
Two Klebsiella pneumoniae strains and five Enterobacter cloacae strains resistant to tigecycline (≥8 mg/liter) were found on day 8.
Safety and tolerability.
One or more treatment-emergent adverse events were reported by all subjects enrolled in the study. All adverse events were considered mild to moderate in severity. The most frequently reported adverse events were nausea (92.3%), vomiting (69.2%), diarrhea (38.5%), headache (38.5%), abdominal pain (30.8%), dizziness (30.8%), asthenia (23.1%), pharyngitis (23.1%), anorexia (15.4%), and pruritus (15.4%).
DISCUSSION
Tigecycline has good in vitro activity against aerobic and anaerobic gram-positive and gram-negative bacteria, including antibiotic-resistant strains. It was developed for the treatment of complicated skin and skin structure infections as well as complicated intra-abdominal infections, and clinical trials with tigecycline have shown favorable results (1, 7).
The administration of antibiotics causes disturbances in the ecological balance between the host and the microorganisms. These disturbances are dependent on the spectrum of activity, the dose, the route of administration, the pharmacokinetic and pharmacodynamic properties, and the in vivo inactivation of the agent. Secretion of an antibiotic by intestinal mucosa, bile, or salivary glands may interfere with the normal microflora at different sites. Antibiotic-resistant microorganisms may increase in number as a consequence. The effect of tigecycline on the normal microflora in humans has not been studied before. The mean concentration of tigecycline in saliva was lower than that in serum and was below the MIC50 (<0.01 mg/liter) for most oropharyngeal bacteria in the normal flora. The minor impact on the oropharyngeal microflora is thus explained by the low saliva concentration, which is ecologically favorable in the treatment of lower respiratory tract infections because it results in less selective pressure for the emergence of colonization by resistant microorganisms. The high fecal concentrations of tigecycline during the administration period, which are a direct consequence of the biliary elimination of the drug, are in accordance with the changes seen in the intestinal flora that affect mainly enterococci, E. coli, lactobacilli, and bifidobacteria. Other noncultivable microorganisms may also be involved in the ecological disturbances of the microflora. No C. difficile strains were isolated, due to the fact that fecal concentrations of tigecycline were far above the MIC for C. difficile (6). Another explanation is that the subjects were not colonized or exposed to C. difficile. Patients may also be at risk for the acquisition of C. difficile during the recovery of the normal microflora.
No major metabolites of tigecycline have been identified, indicating that metabolic elimination is minor (12). The major component of tigecycline clearance is probably biliary secretion resulting in high intestinal concentrations of tigecycline, as found in the present investigation.
The most common adverse events are nausea and vomiting, which are typical for administration of the tetracyclines. In clinical trials of tigecycline done with patients with skin and skin structure infections or with intra-abdominal infections, the incidence of nausea and vomiting was lower than that registered for healthy subjects (15). Tigecycline is excreted in the bile in humans and may have an irritant effect on the intestinal mucosa, resulting in nausea and vomiting. In the present investigation, some volunteers experienced nausea and vomiting during the administration of tigecycline, as expected. However, no serious adverse events were noticed during the study.
In conclusion, the effect of tigecycline on the oropharyngeal microflora was minor due to the low saliva concentrations of tigecycline. Tigecycline caused minor ecological changes in the oropharynx. Tigecycline is ecologically favorable for the treatment of lower respiratory tract infections caused by bacteria. The effect of tigecycline on the intestinal microflora was as expected, due to the spectrum of antibacterial activity and the intestinal concentrations of tigecycline. No C. difficile strains were found, which is ecologically favorable. Most intestinal microflora returned to a normalized state by the end of the study period.
REFERENCES
- 1.Babinchak, T., E. Ellis-Grosse, N. Dartois, G. M. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin. Infect. Dis. 41:S354-S367. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, H. W., C. B. Wennersten, and G. M. Eliopoulos. 2000. In vitro activities of the glycylcycline GAR-936 against gram-positive bacteria. Antimicrob. Agents Chemother. 44:2225-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute/NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, vol. 23, 6th ed. Approved standard, CLSI document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 4.Clinical and Laboratory Standards Institute/NCCLS. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria, vol. 24, 6th ed. Approved standard, CLSI document M11-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 5.Edlund, C., G. Beyer, M. Hiemer-Bau, S. Ziege, H. Lode, and C. E. Nord. 2000. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand. J. Infect. Dis. 32:81-85. [DOI] [PubMed] [Google Scholar]
- 6.Edlund, C., and C. E. Nord. 2000. In vitro susceptibility of anaerobic bacteria to GAR-936, a new glycylcycline. Clin. Microbiol. Infect. 6:158-163. [DOI] [PubMed] [Google Scholar]
- 7.Ellis-Grosse, E. J., T. Babinchak, N. Dartois, G. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 41:S341-S353. [DOI] [PubMed] [Google Scholar]
- 8.Gales, A. C., and R. N. Jones. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19-36. [DOI] [PubMed] [Google Scholar]
- 9.Jacobus, N. V., L. A. McDermott, R. Ruthazer, and D. R. Snydman. 2004. In vitro activities of tigecycline against the Bacteroides fragilis group. Antimicrob. Agents Chemother. 48:1034-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jusko, W. J. 1992. Guidelines for collection and analysis of pharmacokinetic data, p. 2-1-2-43. In W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics: principles of therapeutic drug monitoring, 3rd ed. Applied Therapeutics, Vancouver, Canada.
- 11.Lund, B., C. Edlund, B. Rynnel-Dagöö, Y. Lundgren, J. Sterner, and C. E. Nord. 2001. Ecological effects on the oro- and nasopharyngeal microflora in children after treatment of acute otitis media with cefuroxime axetil or amoxycillin-clavulanate as suspensions. Clin. Microbiol. Infect. 7:230-237. [DOI] [PubMed] [Google Scholar]
- 12.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pankey, G. A. 2005. Tigecycline. J. Antimicrob. Chemother. 56:470-480. [DOI] [PubMed] [Google Scholar]
- 14.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinstein, E., and D. Vaughan. 2005. Tigecycline. A novel glycylcycline. Drugs 65:1317-1336. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan, Å., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]