Abstract
Vps28p and Vps32p act in both the endocytic and the pH signaling pathways in yeasts and are required for Candida albicans virulence. Here, we show that deletions of VPS28 and VPS32 increase the susceptibility of C. albicans to cell wall disruption agents, echinocandin and azole antifungal agents.
The incidence of invasive fungal infections is rising continuously, and Candida albicans is now a major cause of nosocomial infections (11, 15, 17). Although antifungal resistance among invasive C. albicans isolates is not common, there is concern about the development of azole resistance due to prophylactic use of fluconazole (13). Thus, in recent years, interest has focused on developing and licensing new agents, such as echinocandins or new triazoles (7, 10, 12, 23).
Vacuolar protein sorting (Vps) factors act in the endosomal sorting complexes required for transmembrane protein recycling and degradation during the last steps of endocytosis (1). Recently, some Vps factors have been documented as required for activation of the Rim pathway, which governs pH sensing in fungi (2, 3, 9, 16, 24). We previously established that Vps28p and Vps32p affect virulence in C. albicans through Rim-dependent and -independent pathways (3). Here, we show that, in addition to virulence, these Vps factors affect cell wall maintenance and the susceptibility of C. albicans to antifungal agents.
We analyzed strains with VPS deleted for sensitivity to two cell wall-perturbing agents: calcofluor white (CFW) and sodium dodecyl sulfate (SDS). Homozygous deletions of VPS28 (vps28−/−) and VPS32 (vps32−/−) were constructed in the BWP17 strain as previously described (3, 6). Complementation of each mutant was achieved by reintroduction of the wild-type allele at the HIS1 locus. As controls for any Rim-mediated effect, we analyzed the rim101−/− null mutant (4) and introduced RIM101SL, a truncated constitutively active allele of RIM101 (3, 5), into the vps28−/−, vps32−/−, and rim101−/− mutants and into the DAY286 control strain at the HIS1 locus (Table 1) .
TABLE 1.
Candida albicans susceptibilitiesa
| Strain | Geometric mean MIC (μg/ml)b
|
|||
|---|---|---|---|---|
| Fluconazole | Voriconazole | Caspofungin | Micafungin | |
| ATCC 90028 | 0.96 | 0.03 | 0.05 | 0.06 |
| DAY185 (control strain) | 0.62 | 0.02 | 0.06 | 0.03 |
| vps28−/− | 0.39 | 0.01 | 0.02 | 0.01 |
| vps32−/− | 0.32 | 0.01 | 0.02 | 0.01 |
| vps28−/−+VPS28 | 0.62 | 0.03 | 0.03 | 0.04 |
| vps32−/−+VPS32 | 0.68 | 0.02 | 0.03 | 0.04 |
| DAY286+RIM101SL | 0.75 | 0.03 | 0.04 | 0.02 |
| vps28−/−+RIM101SL | 0.75 | 0.02 | 0.007 | 0.001 |
| vps32−/−+RIM101SL | 0.62 | 0.01 | 0.005 | 0.001 |
| rim101−/− | 0.11 | 0.002 | 0.005 | 0.001 |
| rim101−/−+RIM101SL | 0.42 | 0.01 | 0.03 | 0.03 |
Calculated from at least 10 independent experiments. All strains derive directly from BWP17 (His− Ura− Arg−) (4) and are prototrophic. Double disruptions restored a Ura+ Arg+ phenotype. The His+ information was brought by the plamsids carrying VPS28, VPS32, or RIM101SL or by an empty vector, all integrated at HIS1. DAY286 is an Arg+ Ura+ derivative of DAY185.
Results are MIC minus 2 (prominent growth reduction or a 50% reduction in optical density) at 24 h for CAS and MCF and at 48 h for FLC and VRC.
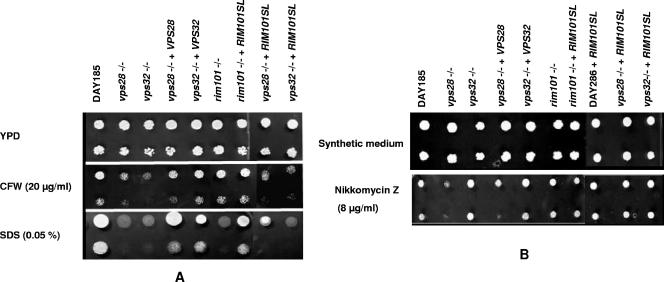
Both the vps28−/− and vps32−/− mutants were hypersensitive to CFW and SDS, with the wild-type phenotype partially restored by complementation (Fig. 1A). This suggests a cell wall disorder compensated for by chitin accumulation and corroborates previous reports of enhanced CFW binding to the cell walls of vps mutants (3). Indeed, SDS hypersensitivity reflects facilitated access of detergents to the mutant membrane, whereas hypersensitivity to CFW, a known inhibitor of chitin incorporation in the wall, results from disruption of the chitin-based compensatory mechanism (18, 19). Nikkomycin Z, a chitin synthase inhibitor, was used to investigate this cell wall defect: an increased sensitivity of the vps28−/− mutant was observed, whereas no major effect was observed in the case of the vps32−/− mutant, either on plates (Fig. 1B) or in liquid assays (not shown). This suggests that the compensatory mechanisms elicited by the two vps mutations (e.g., chitin overproduction versus chitin deposition) may differ. In Saccharomyces cerevisiae, deletions of VPS28 and VPS32 led to CFW hyperresistance, unlike other vps mutations affecting the same complexes, which also suggests subtle differences in the precise compensatory mechanisms triggered by vps mutations (22).
FIG. 1.
Sensitivities of C. albicans strains to CFW, SDS, and nikkomycin Z. The strains are indicated above the lanes. All strains are prototrophic derivatives of the Arg− Ura− His− strain BWP17 (4). DAY286 is a Ura+ Arg+ derivative of BWP17 that was transformed by a plasmid carrying RIM101SL and HIS1. (A) Droplets (3.5 μl) at two dilutions (105 and 104 cells/ml) were spotted on yeast extract-peptone-dextrose (YPD) medium and YPD medium supplemented with 20 μg/ml CFW (Sigma; fluorescent brightener 28) or 0.05% SDS. The plates were incubated at 30°C for 48 h, except for the plates with 0.05% SDS, which were incubated for 5 days. (B) Droplets (3.5 μl) at two dilutions (105 and 104 cells/ml) were spotted on synthetic complete medium (3) and synthetic medium supplemented with 8 μg/ml of nikkomycin Z (Sigma). The plates were incubated at 30°C for 48 h.
Mutations in the VPS genes affect Rim101p activation (3). Inactivation of RIM101 resulted in SDS, but not CFW or nikkomycin Z, susceptibility (Fig. 1, rim101−/−). SDS sensitivity elicited by vps mutations, however, is independent of Rim101p activation, since expression of RIM101SL, while suppressing the SDS sensitivity of the rim101−/− mutant, had no effect on vps28−/− and vps32−/− mutant susceptibilities (Fig. 1A). Similarly, the CFW sensitivities of vps mutants, which were not bypassed by RIM101SL and were not displayed by the rim101−/− mutant, appeared to be independent of an effect of vps genes on the Rim pathway. Expression of RIM101SL, surprisingly, increased the nikkomycin Z tolerance of the control strain (DAY286+RIM101SL) and of the vps28−/− mutant (Fig. 1B). This shows that, although inactivation of the Rim pathway has no effect on nikkomycin Z tolerance, constitutive activation of the pathway increases tolerance for unknown reasons.
The yeast cell wall is constructed around large, intricate glucan molecules (8). Since VPS genes affect cell wall integrity, we tested them for a possible effect on susceptibility to antifungal drugs, like echinocandins, which specifically target β-1,3-glucan synthesis.
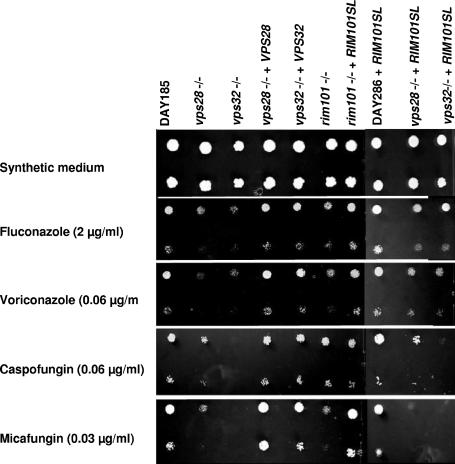
The MICs of the echinocandins caspofungin (CAS) and micafungin (MCF) and of the azoles fluconazole (FLC) and voriconazole (VRC) were determined using the CLSI (formerly NCCLS) broth microdilution method (12a). We discarded experiments showing reduced growth of the strains with deletions compared to the DAY185 control (4). Both vps28−/− and vps32−/− null mutants showed MICs different from that of DAY185 (Table 1), but the results were not consistently reproducible.
Indeed, although the CLSI protocol is recommended for clinical MIC testing, agar plate assays seem to be more reliable for mutant analysis (20, 21). Agar plate assays, carried out on synthetic complete medium containing semi-inhibitory drug concentrations, confirmed that both vps28−/− and vps32−/− null mutants were more susceptible to FLC, VRC, CAS, and MCF than the isogenic DAY185 control strain (Fig. 2). Growth inhibitions in these assays were clear, thus validating MIC observations (Fig. 2 shows one example). Growth inhibition of the strains with VPS deleted was observed over the following concentration ranges (in μg/ml): 1 to 4 for FLC, 0.03 to 0.12 for VRC and CAS, and 0.015 to 0.06 for MCF. The slight growth defect observed for the vps32−/− mutant on the no-drug control plates was confirmed by growth rate measurements (3) but was not significant enough (2.3 h versus 1.7 h) to account for the hypersensitivity of the strain. The susceptibilities of the strains with VPS deleted to amphotericin B and flucytosine were not affected (data not shown). Complementation restored the susceptibility of the control strain to all drugs tested (Fig. 2 and Table 1). The rim101−/− deletion strain was also hypersensitive to FLC, VRC, CAS, and MCF, and introduction of the constitutive allele RIM101SL restored wild-type susceptibility (the rim101−/− strain is hypersensitive to 0.12 μg/ml of CAS on agar plates [data not shown]). RIM101SL also restored normal resistance levels to azoles in VPS mutants (Fig. 2 and Table 1), confirming direct involvement of the Rim pathway in azole sensitivity, as in S. cerevisiae (14). The echinocandin hypersensitivity of the vps null mutants appeared to be independent of the Rim pathway, since the RIM101SL allele had no suppressive effect on the echinocandin hypersensitivity of vps28−/− and vps32−/− mutants.
FIG. 2.
Sensitivities of C. albicans strains to antifungal agents. The strains are indicated above the lanes. All strains are prototrophic derivatives of the Arg− Ura− His− strain BWP17 (4). Droplets (3.5 μl) at two dilutions (105 and 104 cells/ml) were spotted on synthetic complete medium (3) and synthetic complete medium buffered at pH 7.0 with 150 mM HEPES and supplemented with antifungal agents. The plates were incubated at 37°C for 48 h.
In conclusion, our data show that deletions of VPS28 and VPS32 increase C. albicans susceptibility to drugs that directly interfere with cell wall assembly at the level of chitin synthesis and/or deposition (nikkomycin Z and CFW) or at the level of β-1,3-glucan network construction (echinocandins) independently of the Rim pathway. These data suggest that vps mutations perturb localization and/or recycling of surface proteins involved in cell wall maintenance. They also lead to azole hypersensitivity, but the latter phenotype seems to result directly from their effect on the Rim pathway.
Acknowledgments
This work was supported by the Direction de la Recherche Clinique of the Assistance Publique-Hôpitaux de Paris, by the Centre National de la Recherche Scientifique, and by grants from Merck & Company and Fujisawa, Inc., through funding of M.C.
Strains with RIM101 deleted, the reference strain BWP17, and UAU1 carrying a plasmid were generous gifts from A. P. Mitchell.
REFERENCES
- 1.Babst, M. 2005. A protein's final ESCRT. Traffic 6:2-9. [DOI] [PubMed] [Google Scholar]
- 2.Blanchin-Roland, S., G. D. Costa, and C. Gaillardin. 2005. ESCRT-I components of the endocytic machinery are required for Rim101-dependent ambient pH regulation in the yeast Yarrowia lipolytica. Microbiology 151:3627-3637. [DOI] [PubMed] [Google Scholar]
- 3.Cornet, M., F. Bidard, P. Schwarz, G. Da Costa, S. Blanchin-Roland, F. Dromer, and C. Gaillardin. 2005. Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101-dependent and RIM101-independent pathways in Candida albicans. Infect. Immun. 73:7977-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, D. A., V. M. Bruno, L. Loza, S. G. Filler, and A. P. Mitchell. 2002. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enloe, B., A. Diamond, and A. P. Mitchell. 2000. A single-transformation gene function test in diploid Candida albicans. J. Bacteriol. 182:5730-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 8.Klis, F. M., P. de Groot, and K. Hellingwerf. 2001. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39(Suppl. 1):1-8. [PubMed] [Google Scholar]
- 9.Kullas, A. L., M. Li, and D. A. Davis. 2004. Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot. Cell. 3:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kullberg, B. J., J. D. Sobel, M. Ruhnke, P. G. Pappas, C. Viscoli, J. H. Rex, J. D. Cleary, E. Rubinstein, L. W. Church, J. M. Brown, H. T. Schlamm, I. T. Oborska, F. Hilton, and M. R. Hodges. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435-1442. [DOI] [PubMed] [Google Scholar]
- 11.Martin, G. S., D. M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546-1554. [DOI] [PubMed] [Google Scholar]
- 12.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 12a.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons, A. B., R. L. Brost, H. Ding, Z. Li, C. Zhang, B. Sheikh, G. W. Brown, P. M. Kane, T. R. Hughes, and C. Boone. 2004. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat. Biotechnol. 22:62-69. [DOI] [PubMed] [Google Scholar]
- 15.Patterson, T. F. 2005. Advances and challenges in management of invasive mycoses. Lancet 366:1013-1025. [DOI] [PubMed] [Google Scholar]
- 16.Penalva, M. A., and H. N. Arst, Jr. 2004. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 58:425-451. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 18.Popolo, L., and M. Vai. 1998. Defects in assembly of the extracellular matrix are responsible for altered morphogenesis of a Candida albicans phr1 mutant. J. Bacteriol. 180:163-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richard, M., P. De Groot, O. Courtin, D. Poulain, F. Klis, and C. Gaillardin. 2002. GPI7 affects cell-wall protein anchorage in Saccharomyces cerevisiae and Candida albicans. Microbiology 148:2125-2133. [DOI] [PubMed] [Google Scholar]
- 20.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 21.Schuetzer-Muehlbauer, M., B. Willinger, G. Krapf, S. Enzinger, E. Presterl, and K. Kuchler. 2003. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol. Microbiol. 48:225-235. [DOI] [PubMed] [Google Scholar]
- 22.Shiflett, S. L., D. M. Ward, D. Huynh, M. B. Vaughn, J. C. Simmons, and J. Kaplan. 2004. Characterization of Vta1p, a class E Vps protein in Saccharomyces cerevisiae. J. Biol. Chem. 279:10982-10990. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., H. Teppler, G. R. Donowitz, J. A. Maertens, L. R. Baden, A. Dmoszynska, O. A. Cornely, M. R. Bourque, R. J. Lupinacci, C. A. Sable, and B. E. dePauw. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391-1402. [DOI] [PubMed] [Google Scholar]
- 24.Xu, W., F. J. Smith, Jr., R. Subaran, and A. P. Mitchell. 2004. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell 15:5528-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]