Abstract
Leprosy responds very slowly to the current multidrug therapy, and hence there is a need for novel drugs with potent bactericidal activity. PA-824 is a 4-nitroimidazo-oxazine that is currently undergoing phase I clinical trials for the treatment of tuberculosis. The activity of PA-824 against Mycobacterium leprae was tested and compared with that of rifampin in axenic cultures, macrophages, and two different animal models. Our results conclusively demonstrate that PA-824 has no effect on the viability of M. leprae in all three models, consistent with the lack of the nitroimidazo-oxazine-specific nitroreductase, encoded by Rv3547 in the M. leprae genome, which is essential for activation of this molecule.
Mycobacterium leprae is one of the important pathogens of the genus Mycobacterium, since it is responsible for causing leprosy in humans. Leprosy is caused by a chronic granulomatous infection of the skin and peripheral nerves by bacteria (26). The damage to peripheral nerves results in sensory and motor impairment, leading to deformities and disability. Currently, leprosy is treated with multidrug therapy (MDT) (27). Paucibacillary leprosy patients are treated with dapsone and rifampin for 6 months, and those with multibacillary disease are treated with a combination of dapsone, clofazimine, and rifampin for 12 to 24 months. Despite the success of MDT regimens, newer regimens are required to shorten the duration of chemotherapy and to reduce the relapse rates in multibacillary leprosy cases (4).
PA-824 is a 4-nitroimidazo-oxazine compound active against Mycobacterium tuberculosis that is effective against both actively replicating and aerobically growing M. tuberculosis and oxygen-limited, nonreplicating, persistent M. tuberculosis (17, 24). Thus, PA-824 is an important lead compound with the potential for reducing the duration of antitubercular chemotherapy. Recently, the Global Alliance for TB Drug Development has initiated phase I clinical trials with PA-824 (www.tballiance.org).
PA-824 has a unique spectrum of antibacterial activity. PA-824 is highly active against the M. tuberculosis complex (consisting of M. tuberculosis, Mycobacterium bovis, M. bovis BCG, Mycobacterium africanum, and Mycobacterium microti) but has poor or no activity against mycobacteria outside the M. tuberculosis complex (Mycobacterium avium, Mycobacterium smegmatis, Mycobacterium chelonae, and Mycobacterium fortuitum [24] and Mycobacterium ulcerans [15]). Interestingly, PA-824 is effective against Helicobacter pylori, Clostridium difficile, and a few other microaerophilic and anaerobic bacteria that are distantly related to mycobacteria (1); however, it has no activity against closely related actinomycetes like Nocardia spp. and Streptomyces spp. (U. H. Manjunatha and C. E. Barry, unpublished results). As part of our program to understand the mechanism of action of PA-824 and in view of the loss of a significant amount of genetic information from the leprosy bacillus (7), we sought to determine the susceptibility of M. leprae to PA-824.
MATERIALS AND METHODS
Bacterial strains and chemicals.
Nude mouse-derived M. leprae (isolate Thai-53) was maintained in serial passage in the footpads of athymic nu/nu mice. The mice were inoculated in the plantar surfaces of both hind feet with 5 × 107 fresh, viable, nu/nu mouse-derived M. leprae bacilli. Both hind footpads were harvested at 4 to 6 months to obtain the intracellular M. leprae bacilli as described previously (25), and the bacilli were enumerated by direct microscopic count of acid-fast bacilli (AFB) according to the method of Shepard and McRae (23). The bacterial suspension was passed a few times through a 27-gauge needle to remove clumps immediately prior to use. Freshly harvested bacilli were always employed in experiments within 24 h of harvest.
PA-824 was synthesized as described in U.S. patent 5,668,127 (1). Cyclodextran, lecithin, ampicillin, HEPES, glutamine, rifampin, and sodium hydroxide were obtained from Sigma. BACTEC 7H12B and BACTEC PZA media were obtained from Becton Dickinson. RPMI 1640 and fetal calf serum (FCS) were obtained from Invitrogen. The BALB/c and athymic nude (nu/nu) mice used in this study were obtained from Harlan (Harlan, Indianapolis, Indiana).
Radiorespirometry and axenic culture experiment.
The metabolic activity of a suspension of M. leprae was measured by evaluating the oxidation of [14C]palmitic acid to 14CO2 by radiorespirometry as described previously (9). Briefly, for experiments in axenic medium, 1 × 107 M. leprae bacilli were suspended in 1.0 ml of BACTEC 7H12B medium to which different concentrations of PA-824 (0 to 10 μg/ml) were added. Rifampin (2 μg/ml) was used as a positive control. The radiorespirometry vials were incubated for 7 days, and the day 7 cumulative counts per minute were determined as described previously (16). For radiorespirometry experiments with M. tuberculosis (strain Erdman [ATCC 35801]), the BACTEC method was employed as described previously (8).
Macrophage experiments.
Resident peritoneal macrophages from Swiss mice were harvested and allowed to adhere for 6 h in RPMI 1640 supplemented with 25 mM HEPES, 50 μg/ml ampicillin, 2 mM glutamine, and 10% (vol/vol) FCS. Adherent cells were infected overnight at 33°C with fresh footpad-derived M. leprae from nude mice at a multiplicity of infection (MOI) of 20:1. After extracellular bacilli were washed off, the infected macrophages were incubated with PA-824 or rifampin in RPMI 1640 for 7 days. The macrophages were lysed with 0.1 N NaOH, and the intracellular M. leprae metabolic activity was measured by radiorespirometry in 4 ml of acidified BACTEC PZA medium (21). For M. tuberculosis, macrophages were infected at a MOI of 2:1 for 30 min at 37°C. Extracellular bacilli were washed off, and the M. tuberculosis-infected macrophages were incubated at 37°C with PA-824 or rifampin for 7 days. On day 7, the infected macrophages were lysed with 0.1% sodium dodecyl sulfate and the bacilli were counted by serial dilution plating on 7H11 agar medium.
Mouse footpad experiments. (i) BALB/c mouse model.
The kinetic method of Shepard was employed to test the efficacy of PA-824 treatment on the growth of M. leprae in the mouse footpad model (22). Sixty immunocompetent BALB/c mice were each inoculated in both hind footpads with 1 × 104 (30 μl/footpad) viable M. leprae bacilli freshly prepared from infected nu/nu mice and randomly allocated into five groups: an untreated control group with 20 mice and four treated groups with 10 mice each. Eight weeks postinfection, the animals were treated orally with drugs (Table 1) in a CM-2 formulation for 4 weeks (5 days/week) and then discontinued. The CM-2 formulation consists of 10% hydroxypropyl-β-cyclodextrin and 10% lecithin in water (24). The concentrations of PA-824 (25 mg/kg and 100 mg/kg) were based on mouse efficacy studies with M. tuberculosis (24). The animals showed no adverse effects from drug treatment. The footpads from control animals were harvested at 8, 12, 20, and 24 weeks postinfection (five mice per time point). Based on the growth in untreated animals, the footpads from all treated animals were harvested when the AFB growth was near plateau and 4 weeks later (five mice per time point). After harvest, the bacilli were enumerated by direct microscopic count (23).
TABLE 1.
Experimental design for PA-824 efficacy against M. leprae in BALB/c and nude mouse footpad models
| Model and treatment (mg/kg) | No. of mice | Harvest time(s) postinfection (wk) |
|---|---|---|
| BALB/c mice | ||
| Untreated control | 20 | 8, 12, 20, 24 |
| CM-2 control | 10 | 20, 24 |
| Rifampin (8) | 10 | 20, 24 |
| PA-824 (100) | 10 | 20, 24 |
| PA-824 (25) | 10 | 20, 24 |
| Nude mice | ||
| Untreated control | 5 | 24 |
| Rifampin (8) | 5 | 24 |
| PA-824 (100) | 5 | 24 |
(ii) Nude mouse model.
Fifteen athymic BALB/c nu/nu nude mice were infected in both hind footpads with 5 × 107 bacilli and divided into three groups (Table 1). At 2 months postinfection, the animals were treated orally with drugs in a CM-2 formulation for 4 weeks. After 6 months, the footpads from treated and control animals were harvested and the acid-fast bacteria were enumerated by direct microscopic count (23).
Sequence analysis.
The primary sequences of the M. leprae and M. tuberculosis genes were obtained from Leproma (http://genolist.pasteur.fr/Leproma/) and Tuberculist (http://genolist.pasteur.fr/TubercuList).
RESULTS
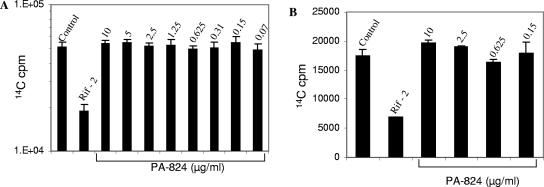
As a first attempt to determine an effect of PA-824 on M. leprae, we examined the radiorespirometric activity of M. leprae, as measured by 14CO2 production, in the presence of PA-824 in axenic cultures. While rifampin, as expected, significantly reduced the metabolic activity, PA-824 had no effect at concentrations up to 10 μg/ml (Fig. 1A). While the axenic suspension data offer clues as to the direct effect of the agents on the metabolism of M. leprae, it is possible that an agent could have a killing effect without inhibiting the production of CO2 from lipid substrates. However, BACTEC data for M. tuberculosis, which include measurements of oxidation of [14C]palmitic acid, showed a profound effect of PA-824, where the MIC99 was 0.15 μg/ml (data not shown). Thus, the lack of activity of PA-824 against M. leprae at 10 μg/ml suggested that M. leprae has at least 60-fold-higher inherent resistance.
FIG. 1.
Effect of PA-824 on the viability of extracellular (A) and intracellular (B) M. leprae. Viability was measured by the radiorespirometry method (n = 4 replicates for each concentration). The bar graphs show the means ± standard deviations of cumulative radiorespirometry counts. cpm, counts per minute.
Radiorespirometric analysis of organism viability following exposure of M. leprae to PA-824 in the context of an infection of peritoneal macrophages revealed that PA-824 at concentrations up to 10 μg/ml had no effect on intracellular M. leprae (Fig. 1B). A similar evaluation of M. tuberculosis-infected macrophages treated with PA-824 revealed at least a 2-log reduction in numbers of CFU with 2.5 μg/ml PA-824 for 7 days (data not shown).
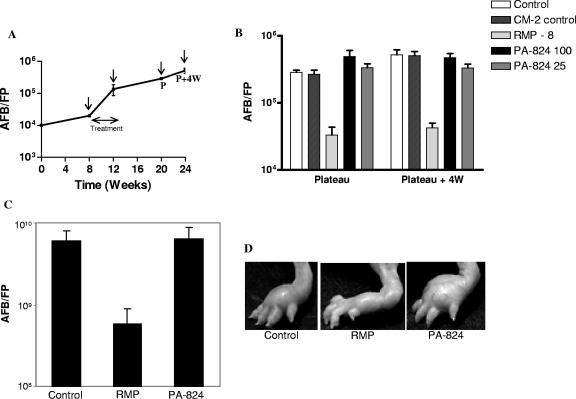
To establish conclusively the activity of PA-824 on the viability of M. leprae, we performed mouse footpad experiments with both BALB/c and immunocompromised nude mice, using a kinetic method to determine drug efficacy (22). Normal multiplication of M. leprae was observed in the footpads of BALB/c mouse control groups (Fig. 2A), where the bacillary counts reached up to 106/footpad (from 104) in approximately 6 months. As shown in Fig. 2B, both 25 mg/kg and 100 mg/kg of PA-824 had no effect on bacillary counts. The counts in the PA-824-treated animals were indistinguishable from those of the untreated control animals 2 and 3 months after discontinuation of the treatment. However, animals treated with 8 mg/kg of rifampin had at least 1 log fewer AFB than did the control animals. Furthermore, in athymic immunocompromised animals, while rifampin significantly prevented the multiplication of M. leprae (Fig. 2C) and the development of swelling footpads (Fig. 2D), PA-824 had no effect on either event.
FIG. 2.
Effects of PA-824 and rifampin on the multiplication of M. leprae in BALB/c and nude mice. The drugs were given orally in a CM-2 formulation for 4 weeks during the third month. (A) Growth kinetics of M. leprae in BALB/c mice. The drug treatment and harvest time points are indicated by arrows. (B) AFB counts were measured at the plateau and 4 weeks later in all groups (n = 5) of BALB/c mice. Drugs were administered at 8, 100, and 25 mg/kg as indicated in the key. (C) AFB counts measured 6 months postinfection in the nude mouse model (n = 5). (D) Photographs of drug-treated nude mouse footpads before harvest. FP, footpad; RMP, rifampin; P, plateau in AFB growth; W, weeks.
DISCUSSION
Nitroimidazoles are an interesting class of compounds that have only recently been explored for antimycobacterial activity (2). Unlike metronidazole (a 5-nitroimidazole), bicyclic 4-nitroimidazo-oxazines, such as PA-824 and OPC-67683, have inhibitory activity against both actively growing and nonreplicating M. tuberculosis (19, 24). In this study, we have explored the possibility of broadening the utility of nitroimidazoles for leprosy treatment by analyzing the effect of PA-824 on the viability of M. leprae.
Because M. leprae cannot be cultured in vitro, its viability cannot be addressed directly; thus, it is difficult to conclusively demonstrate either drug efficacy or the lack thereof. In axenic and intracellular systems, M. leprae bacilli are not actively dividing but are viable. In both models, the presence of PA-824 did not affect the metabolic activity (Fig. 1A and B). It has been shown previously that the metabolic activity correlates well with actual growth in the mouse footpads (25). To confirm that PA-824 might reasonably be expected to have an effect on lipid catabolism as measured by radiorespirometry, we also confirmed the activity of PA-824 on M. tuberculosis by using radiorespirometry as a measure of viability (BACTEC). The radiorespirometric method has been used extensively to evaluate the in vitro activities of various antileprosy compounds, including macrolides (11), fluoroquinolones (13), phenazines (12), and fusidic acid (10).
In addition, we have also employed two in vivo models of M. leprae growth in mouse footpads, including both immunocompetent (Fig. 2B) and immunocompromised (Fig. 2C) mice, using Shepard's kinetic method, which distinguishes between bacteriostatic effects, i.e., indicated by a delay in the growth of bacilli corresponding to the duration of drug treatment, and bactericidal effects, which are revealed by a much more prolonged delay. Our findings establish that PA-824 has no effect on M. leprae in both BALB/c and nude mouse models. PA-824 has also been shown recently, by a proportional bactericidal method, to have only a very modest bactericidal activity against M. leprae 17543 in mice (14).
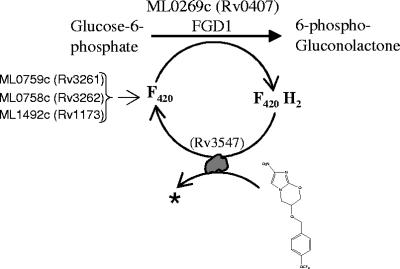
PA-824 is a prodrug, and its activation requires an F420-dependent glucose-6-phosphate dehydrogenase (FGD1) activity (24). FGD1 is a glucose-6-phosphate dehydrogenase (G6PD) that carries out enzymatic dehydrogenation of glucose-6-P to 6-P-gluconolactone while reducing the cofactor F420. F420 is a specialized deazaflavin cofactor with a low redox potential (−350 mV) that participates in two-electron redox reactions. PA-824-resistant M. tuberculosis (18) and M. bovis (5, 6, 24) mutants have been determined to have lesions in FGD1 and the F420 biosynthesis pathway. Mutants lacking F420 or the ability to reduce this cofactor are unable to activate PA-824 and other related bicyclic nitroimidazoles to more-polar metabolites (18).
M. leprae has undergone reductive evolution during adaptation as a human parasite and contains 1,133 pseudogenes, leaving only 1,614 protein-encoding genes in its genome (7), compared to 3,995 in M. tuberculosis (3). Interestingly, M. leprae encodes an FGD1 homologue (ML0269c) and also the proteins involved in F420 biosynthesis (ML0759c, ML0758c, and ML1492c) (Fig. 3). The conversion of glucose-6-P to 6-P-gluconolactone is the first committed reaction for the pentose phosphate pathway. The M. tuberculosis genome has four annotated G6PD genes: two are classical NADP+-dependent genes (Rv1121 and Rv1447c), and the other two are F420-dependent genes (Rv0407 and Rv0132c), of which Rv0407 has been shown to catalyze G6PD activity (18). In contrast, M. leprae has only one functional G6PD which is F420 dependent (ML0269c). It has been shown earlier that armadillo-grown M. leprae has F420-dependent G6PD activity but no detectable NADP-G6PD activity (20). In addition, all of the genes involved in the pentose phosphate pathway are present in the M. leprae genome, in spite of reductive evolution (7). This implies that in M. leprae, FGD1 and F420 biosynthesis may be essential for a functional pentose phosphate pathway, and thus we expected that M. leprae would be sensitive to PA-824. However, in contrast to our hypothesis, in the present study we have shown that in all three different models of M. leprae, i.e., an extracellular model (Fig. 1A), an intracellular model (Fig. 1B), and two animal models (Fig. 2B and C), PA-824 had no effect on the viability of M. leprae cells.
FIG. 3.
A proposed pathway for activation of PA-824. M. leprae and M. tuberculosis genes involved in nitro-reductive activation of PA-824 are shown.
More recently, we have characterized FGD1+ and F420+ M. tuberculosis PA-824-resistant mutants and identified mutations in the putative F420-dependent nitroreductase encoded by Rv3547 (18). Similar to FGD1− and F420− mutants, Rv3547 mutants of M. tuberculosis are also defective in activation of PA-824 (18). The Rv3547 product is a conserved hypothetical protein, with no considerable sequence homology to any other proteins of known function. Rv3547 is most likely to donate electrons to PA-824 (Fig. 3). M. tuberculosis has three homologues of Rv3547 with around 55% sequence similarity, namely, Rv1261c, Rv1558, and Rv3178. The homologues of Rv3547 have been implicated in playing a role in the activation of nitroimidazo-oxazoles (18). Sequence analysis revealed that the M. leprae genome does not carry Rv3547 or its homologues. Consequently, although the leprosy bacilli may produce reduced F420, this is not sufficient to reduce PA-824 in the absence of this specific nitroreductase. Hence, M. leprae is naturally resistant to PA-824 and most likely resistant to other promising antituberculosis bicyclic 4-nitroimidazoles.
Acknowledgments
Part of this work was supported by the Intramural Research Program of the National Institutes of Health, NIAID (C.E.B.). We acknowledge the Tuberculosis Antimicrobial Acquisition and Coordinating Facility NIAID contract Y1-A1-2004 to J.L.K. and a fellowship from the American Leprosy Mission to R.L.
REFERENCES
- 1.Baker, W. R., C. Shaopei, and E. L. Keeler. September. 1997. Nitroimidazole antibacterial compounds and methods of use thereof. U.S. patent 5,668,127.
- 2.Barry, C. E., III, H. I. Boshoff, and C. S. Dowd. 2004. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 10:3239-3262. [DOI] [PubMed] [Google Scholar]
- 3.Camus, J. C., M. J. Pryor, C. Medigue, and S. T. Cole. 2002. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 148:2967-2973. [DOI] [PubMed] [Google Scholar]
- 4.Cellona, R. V., M. F. Balagon, E. C. dela Cruz, J. A. Burgos, R. M. Abalos, G. P. Walsh, R. Topolski, R. H. Gelber, and D. S. Walsh. 2003. Long-term efficacy of 2 year WHO multiple drug therapy (MDT) in multibacillary (MB) leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 71:308-319. [DOI] [PubMed] [Google Scholar]
- 5.Choi, K.-P., T. B. Bair, Y.-M. Bae, and L. Daniels. 2001. Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F420 biosynthesis by Mycobacterium bovis BCG. J. Bacteriol. 183:7058-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, K.-P., N. Kendrick, and L. Daniels. 2002. Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F420 and FO biosynthesis. J. Bacteriol. 184:2420-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 8.Collins, L. A., and S. G. Franzblau. 1997. Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzblau, S. G. 1988. Oxidation of palmitic acid by Mycobacterium leprae in an axenic medium. J. Clin. Microbiol. 26:18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzblau, S. G., A. N. Biswas, and E. B. Harris. 1992. Fusidic acid is highly active against extracellular and intracellular Mycobacterium leprae. Antimicrob. Agents Chemother. 36:92-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franzblau, S. G., and R. C. Hastings. 1988. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32:1758-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franzblau, S. G., and J. F. O'Sullivan. 1988. Structure-activity relationships of selected phenazines against Mycobacterium leprae in vitro. Antimicrob. Agents Chemother. 32:1583-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzblau, S. G., and K. E. White. 1990. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34:229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji, B., A. Chauffour, K. Andries, and V. Jarlier. 2006. Bactericidal activities of R207910 and other newer antimicrobial agents against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 50:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji, B., S. Lefrançois, J. Robert, A. Chauffour, C. Truffot, and V. Jarlier. 2006. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob. Agents Chemother. 50:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahiri, R., B. Randhawa, and J. Krahenbuhl. 2005. Application of a viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J. Med. Microbiol. 54:235-242. [DOI] [PubMed] [Google Scholar]
- 17.Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manjunatha, U. H., H. Boshoff, C. S. Dowd, L. Zhang, T. J. Albert, J. E. Norton, L. Daniels, T. Dick, S. S. Pang, and C. E. Barry III. 2006. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto, M., H. Hashizume, T. Tomishige, and M. Kawasaki. 2005. In vitro and in vivo efficacy of novel antituberculous candidate OPC-67683, abstr. F-1462, p. 204. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 20.Purwantini, E., T. P. Gillis, and L. Daniels. 1997. Presence of F420-dependent glucose-6-phosphate dehydrogenase in Mycobacterium and Nocardia species, but absence from Streptomyces and Corynebacterium species and methanogenic Archaea. FEMS Microbiol. Lett. 146:129-134. [DOI] [PubMed] [Google Scholar]
- 21.Ramasesh, N., L. B. Adams, S. G. Franzblau, and J. L. Krahenbuhl. 1991. Effects of activated macrophages on Mycobacterium leprae. Infect. Immun. 59:2864-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepard, C. C. 1969. Further experience with the kinetic method for the study of drugs against Mycobacterium leprae in mice. Activities of DDS, DFD, ethionamide, capreomycin and PAM 1392. Int. J. Lepr. Other Mycobact. Dis. 37:389-397. [PubMed] [Google Scholar]
- 23.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 24.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 25.Truman, R. W., and J. L. Krahenbuhl. 2001. Viable M. leprae as a research reagent. Int. J. Lepr. Other Mycobact. Dis. 69:1-12. [PubMed] [Google Scholar]
- 26.Ustianowski, A. P., and D. N. Lockwood. 2003. Leprosy: current diagnostic and treatment approaches. Curr. Opin. Infect. Dis. 16:421-427. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 1998. WHO expert committee on leprosy: seventh report. WHO Tech. Rep. Ser. 874:1-43. [PubMed] [Google Scholar]