Abstract
We investigated the efficacies and durability of novel antimicrobial central venous catheters (CVCs) in preventing the adherence of microbial organisms to the surfaces of the CVCs. Novel antimicrobial CVCs investigated in this in vitro study were impregnated with antibiotics (minocycline and rifampin), with Oligon agent (silver, platinum, and carbon black), with approved antiseptics (chlorhexidine and silver sulfadiazine), or with a novel antiseptic agent, gendine, which contains gentian violet and chlorhexidine. When tested against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa, gendine-coated CVC segments provided protection against bacterial adherence significantly more than all other types of tested CVCs (P < 0.05). Gendine-coated CVCs also provided better protection against Candida albicans and Candida parapsilosis than CVCs impregnated with antibiotics or with silver, platinum, and carbon (P < 0.02). After 28 days of being soaked in serum, the CVCs impregnated with chlorhexidine and silver sulfadiazine and the CVCs impregnated with silver, platinum, and carbon had lost antimicrobial activity against MRSA, P. aeruginosa, and C. parapsilosis, and the CVCs impregnated with minocycline and rifampin had lost activity against P. aeruginosa and C. parapsilosis. The CVCs impregnated with gendine maintained antimicrobial activities against MRSA, P. aeruginosa, and C. parapsilosis after 28 days of being soaked in serum. Central venous catheters impregnated with the novel investigational antiseptic gendine showed in vitro efficacy and provided protection against bacterial adherence more than other approved novel antimicrobial-coated CVCs.
Central venous catheters (CVCs) are used for the monitoring and management of critically ill patients and those with cancer through the administration of antibiotics, blood products, total parenteral nutrition, chemotherapeutic agents, and other medications. It is estimated that, in the United States alone, over 5 million CVCs are inserted annually (18, 23). In intensive care units (ICUs) of the United States, about 48% of patients have CVCs, accounting for about 15 million CVC days/year (22).
Unfortunately, the use of these valuable devices is associated with a number of complications, the most important of which is infection. Short-term noncuffed polyurethane CVCs account for 90% of all catheter-related bloodstream infections (CR-BSI) (18). The burden and consequences of CR-BSI are both economic and clinical, due to their impact on morbidity and mortality as well as inconvenient and premature CVC removal (10, 14, 28, 29).
CVCs are responsible for 250,000 to 400,000 cases of nosocomial bacteremia annually in the United States (21, 30). In ICUs alone, it has been estimated that approximately 80,000 CR-BSI occur annually, with an estimated cost that ranges between $296 million and $2.3 billion and attributable mortalities of 4% to 20%, resulting in approximately 2,400 to 20,000 deaths annually (22, 28, 40).
The process of CVC insertion disrupts the integrity of the skin, rendering infection with bacteria or fungi possible. Catheter-related infections are often preceded by colonization of the catheter with microorganisms. Scanning electron microscopy proved that most CVCs become colonized with microorganisms during their dwell time inside blood vessels, whether catheter-related infection occurred or not (19, 31). Microbial organisms embed themselves in a layer of fibrous glycocalyx biofilm, making them less susceptible to conventional antibiotics (41).
Measures that prevent microbial access to the CVC insertion tract have been proven to help in preventing CR-BSI and therefore have been recommended by the guidelines for the prevention of intravascular-catheter-related infections (26). These measures include, among others, good hand hygiene, the use of maximal sterile barrier precautions during CVC insertion, the use of effective skin antiseptic agents, such as 2% chlorhexidine (CHX), and the use of antimicrobial CVCs, such as those coated with CHX and silver sulfadiazine (CHX/SS) or minocycline and rifampin (M/R) (6, 11, 13, 16, 20, 27, 35). Antimicrobial CVCs represent novel technologic innovations in that the catheter surfaces are coated with antimicrobial agents, rendering them more resistant to colonization by microorganisms.
This in vitro study investigated the comparative activities of novel antimicrobial CVCs in preventing the adherence of microbial organisms to the surfaces of the CVCs and the durability of the antimicrobial activities of such CVCs in serum. A novel antiseptic agent, gendine (GND), which contains gentian violet and chlorhexidine, was used as the impregnating agent of one of the novel antimicrobial catheters evaluated in this study.
MATERIALS AND METHODS
Investigated catheters.
We evaluated the efficacies of four types of novel antimicrobial polyurethane CVCs, in comparison with that of a control, uncoated polyurethane catheter. We investigated catheter segments that were coated with GND (GND-CVCs) (prepared at our laboratory), with the antibiotics minocycline and rifampin (M/R-CVCs) (Spectrum; Cook Critical Care, Bloomington, IN), with the antiseptics chlorhexidine and silver sulfadiazine (CHX/SS-CVCs) (Arrow Guard Plus; Arrow, Reading, PA), and with silver, platinum, and carbon black (SPC-CVCs) (Edwards Life Sciences, Irvine, CA).
Preparation of gendine-coated Segments.
Gendine was prepared according to a proprietary method described in a patent application, which is pending. One-centimeter segments of polyurethane CVCs made of polyurethane were dipped into the gendine solution to coat both internal and external surfaces. The pieces were left to dry overnight before being washed with a mild detergent and deionized water to remove any loosely attached antiseptic from the surfaces of the coated segments. The CVC segments were then left to dry for an additional 48 h.
Adherence testing.
We evaluated bacterial adherence to the surfaces of uncoated CVCs, GND-CVCs, M/R-CVCs, CHX/SS-CVCs, and SPC-CVCs. All tested polyurethane catheters had a diameter of 2 mm. Four 5-mm-long segments of each catheter type were tested per organism. We used a modification of a previously published method for testing adherence and biofilm formation on silicone disks to test microbial adherence to the surfaces of these CVCs (15). Sterile CVC segments were placed into sterile 24-well tissue culture plates containing 1 ml of plasma to enhance the formation and binding of blood proteins and biofilm to the surfaces of the catheter segments. The plates were then placed into the incubator for 24 h at 37°C. The plasma was then removed from the wells, with the catheter segments left inside, and was replaced with 1 ml of Mueller-Hinton broth (MHB) that had been inoculated with microorganisms. Organisms used were methicillin-resistant Staphylococcus aureus (MRSA) strain 4798, Pseudomonas aeruginosa strain 4689, Candida albicans strain 21, and Candida parapsilosis strain 091-18, all of which were clinical isolates that had previously caused CR-BSI in cancer patients. To prepare the inoculum, freshly grown microorganisms were prepared to a 0.5 McFarland standard (approximately 1.3 × 108 CFU/ml) and then diluted to 5.5 × 105 CFU/ml in MHB and immediately used. The plates, containing the catheter segments in the inoculated broth, were placed in the incubator for 24 h at 37°C. The MHB was then removed and replaced with 1 ml of 0.9% saline solution, and the plates were placed for 30 min in an incubator at 37°C as a washing step to simulate a physiological condition under which the inserted central venous catheter would be present. Catheter segments were then removed from the saline, placed into sterile 15-ml tubes containing 5 ml of sterile saline solution, and sonicated for 15 min. After sonication, the tubes containing the catheter segments were vortexed for 60 seconds. A 100-μl volume of the saline was pipetted and spread onto a Trypticase soy agar plate with 5% sheep blood. The plates were incubated for 24 h at 37°C, and the colonies were then counted. A value of 100 CFU was used for any plate that had at least 100 counted colonies. When the dilution factor was taken into consideration, the maximum number of CFU reported was 5,000. Data displayed in Table 1 show a maximum of 5,000 CFU.
TABLE 1.
Adherence of bacteria to polyurethane CVC surfaces
| Device | MRSA 4798
|
P. aeruginosa 4689
|
C. albicans 21
|
C. parapsilosis 091-18
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean no. of CFU | Median (range) | P valuea | Mean no. of CFU | Median (range) | P value | Mean no. of CFU | Median (range) | P value | Mean no. of CFU | Median (range) | P value | |
| Control CVC | 5,000 | 5,000 (5,000-5,000) | 5,000 | 5,000 (5,000-5,000) | 5,000 | 5,000 (5,000-5,000) | 5,000 | 5,000 (5,000-5,000) | ||||
| M/R-CVC | 1,912.5 | 100 (0-5,000) | 0.01 | 5,000 | 5,000 (5,000-5,000) | NS | 5,000 | 5,000 (5,000-5,000) | NS | 5,000 | 5,000 (5,000-5,000) | NS |
| CH/SS-CVC | 2,506.3 | 2,525 (0-5,000) | 0.03 | 4,443.8 | 5,000 (550-5,000) | NS | 937.5 | 1,000 (0-1,750) | 0.01 | 1,037.5 | 1,125 (0-1,900) | 0.01 |
| SPC-CVC | 5,000 | 5,000 (5,000-5,000) | NS | 5,000 | 5,000 (5,000-5,000) | NS | 5,000 | 5,000 (5,000-5,000) | NS | 5,000 | 5,000 (5,000-5,000) | NS |
| GND-CVC | 0 | 0 (0-0) | 0.0001 | 387.5 | 0 (0-3,100) | <0. 0001 | 25 | 0 (0-100) | 0.01 | 0 | 0 (0-0) | 0.008 |
P values indicate results of comparisons made with a control uncoated catheter (Wilcoxon rank sum test). NS, statistically not significant.
Zones of inhibition and antimicrobial durability.
We used a modified Kirby-Bauer method to evaluate baseline antimicrobial activities of catheter segments (39). Duplicates of catheter segments were vertically embedded in Mueller-Hinton agar plates coated with one of the following organisms that had previously caused CR-BSI in cancer patients: MRSA 4789, C. parapsilosis 091-18, and P. aeruginosa 4689. The plates were incubated overnight at 37°C, and the zones of inhibition produced around catheter segments were measured and recorded as the diameters, in millimeters, across the centers of the embedded catheter segments.
Furthermore, the antimicrobial durability of catheter segments was also assessed over time by testing zones of inhibition produced by segments after they were soaked in serum. The catheter segments were placed in sterile 50-ml polystyrene tubes (Falcon) containing 10 ml sterile serum as a suitable biological body fluid and were incubated at 37°C. The 10-ml volume was used to ensure the complete immersion of all the segments placed in the tube. At weekly intervals, the serum was changed and two segments per catheter type were tested to determine the antimicrobial durability after the segments were immersed in serum. Zones of inhibition were determined using the modified Kirby-Bauer method against the same organisms mentioned above.
Statistical methods.
The numbers of CFU for all tested organisms retrieved from each type of tested catheter (representing adherence of organisms to the surfaces of catheter segments) were compared by Kruskal-Wallis test, and a P of ≤0.05 was considered statistically significant. A value of 100 CFU was used for any plate that had 100 or more counted colonies.
When a significant result was detected for the test, a series of Wilcoxon rank sum tests were used for pairwise comparisons to identify the significant differences. A series of pairwise comparisons were conducted, and bacterial adherence to the surfaces of GND-CVC segments was compared with that of each type of other tested catheter. The alpha levels of the pairwise comparisons, the chance to make a type one error, were adjusted using a sequential Bonferroni adjustment to control type one error. Data analyses were performed using SAS version 9 (SAS Institute, Cary, NC).
RESULTS
Adherence testing.
Gendine-coated catheter segments demonstrated an antiadherence ability against all tested organisms, namely, MRSA, P. aeruginosa, C. albicans, and C. parapsilosis. Table 1 shows the microbial adherence to the surfaces of all tested catheter segments in comparison with adherence to control, uncoated catheters. When tested against MRSA, the M/R-CVCs, CHX/SS-CVCs, and GND-CVCs were superior to the control CVCs and only CHX/SS-CVCs and GND-CVCs were superior against C. albicans and C. parapsilosis. No organisms were retrieved from the surfaces of GND-CVC segments when the segments were tested against MRSA and C. parapsilosis, which number was significantly less than the numbers of organisms adhering to the surfaces of all other tested catheters (P < 0.05). In tests with P. aeruginosa and C. albicans, the median numbers (ranges) of CFU retrieved from GND-CVC segments were 0 (0 to 3,100) and 0 (0 to 100), significantly less than the numbers of CFU adhering to the surfaces of the uncoated control, the M/R-CVCs, and the SPC-CVCs (P < 0.01). GND-CVC segments were the only CVCs among all tested catheters to demonstrate activity against P. aeruginosa (Table 1).
Efficacy and antimicrobial durability.
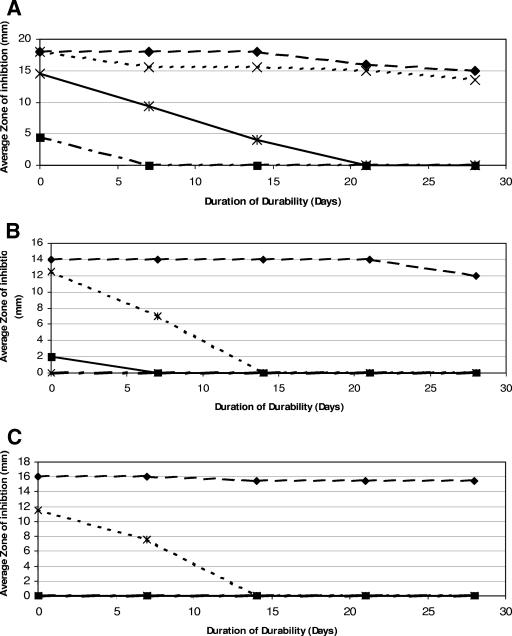
Figure 1A to C shows the results of the baseline efficacy of the GND-CVCs in comparison with those of the other three tested antimicrobial catheter segments, M/R-CVCs, CHX/SS-CVCs, and SPC-CVCs, against MRSA, P. aeruginosa, and C. parapsilosis. The figure also shows the results of antimicrobial durability for up to 28 days, comparing the diameters of the zones of inhibition, measured in millimeters, produced around different catheter segments embedded in the agar. A measured zone of inhibition included the diameter of the catheter segment; all catheter segments were 2 mm in diameter. At baseline, the mean zones of inhibition produced by GND-CVCs were 18, 14, and 16 mm in diameter when tested against MRSA, P. aeruginosa, and C. parapsilosis, respectively. Gendine-coated segments continued to maintain over time antimicrobial durability against these organisms. After being immersed in serum that was changed weekly, at day 28 of testing the mean zones of inhibition produced by the GND-CVCs against the aforementioned organisms were 15, 12, and 15.5 mm, respectively. By day 28 of testing, the CHX/SS-CVC segments and the SPC-CVC segments had lost all activity against all three organisms and the M/R-CVC segments had lost activity against P. aeruginosa and C. parapsilosis.
FIG. 1.
(A to C) Efficacies and durabilities of different coatings on polyurethane catheters against MRSA (A), P. aeruginosa (B), and C. parapsilosis (C) as determined by the sizes of the zones of inhibition over 28 days. Symbols: ♦, GND-CVCs; ×, M/R-CVCs; ✠, CHX/SS-CVCs; ▪, SPC-CVCs.
DISCUSSION
This study demonstrates the antimicrobial activities and durabilities of various anti-infective CVCs against MRSA, Pseudomonas aeruginosa, Candida albicans, and Candida parapsilosis. The investigational gendine-coated CVCs were the most effective in preventing the adherence of bacteria and fungi compared to other available antimicrobial CVCs and also demonstrated the most prolonged antimicrobial activity in serum, particularly against multidrug-resistant P. aeruginosa and C. parapsilosis.
CVCs are the leading source of bloodstream infections in critically ill patients. In a study by the CDC that included more than 14,000 nosocomial infections in critically ill patients, 87% of bloodstream infections were attributed to central venous catheters (37). Novel antimicrobial catheters have been shown to be effective in preventing catheter-related bloodstream infections (42). Recent CDC guidelines have suggested the use of antimicrobial CVCs in adults whose catheters are expected to remain in place for more than 5 days if the implementation of other antiseptic techniques did not control the rate of CR-BSI (26).
Maki et al. have demonstrated that narrow-spectrum antiseptic catheters, only the external surfaces of which are coated with chlorhexidine and silver sulfadiazine, were effective in decreasing colonization and CR-BSI (21). When catheters coated with the antibiotics minocycline and rifampin were compared with the narrow-spectrum antiseptic catheters coated with chlorhexidine and silver sulfadiazine in a multicenter prospective randomized trial, they were shown to be more efficacious in preventing bloodstream infections (9). The difference between these two antimicrobial catheters in preventing CR-BSI was most pronounced after 7 days of CVC placement, which could be related to the difference in antimicrobial durability between the two catheters, as shown by this study (Fig. 1A [antimicrobial durability against MRSA]) and which was also described in a previous study (33). Logghe et al. have demonstrated the lack of efficacy of chlorhexidine-silver sulfadiazine antiseptic catheters in preventing CR-BSI in leukemia and lymphoma patients who had CVCs with an average dwell time of 20 days, a finding that is consistent with the limited antimicrobial durability of the CHX/SS-CVCs observed in this study (17).
A broad-spectrum antiseptic catheter coated with chlorhexidine and silver sulfadiazine on the external surface and with chlorhexidine on the internal surface has recently been developed. However, two separate studies have shown that such a catheter, compared to uncoated catheters, significantly decreased colonization but that it did not decrease CR-BSI rates significantly, which may have been due to the lack of power in the studies’ designs to detect a difference if it existed (2, 38).
Prospective randomized studies have demonstrated the efficacy of long-term silicone M/R-CVCs in preventing CR-BSI. These CVCs were used in cancer patients and in other immunocompromised patients or critically ill patients, for whom the average dwell time ranged from 30 to 66 days (8, 12). This finding is also consistent with the prolonged antimicrobial durability of the antibiotic-coated catheter against MRSA in this study.
Although the antibiotic-coated M/R-CVCs have been shown through a number of prospective randomized clinical trials to be highly efficacious in preventing CR-BSI in short-term and long-term CVCs, there are several concerns associated with their use (4, 8, 9, 12, 32). The first is their limited antimicrobial durability and activity against the adherence of resistant gram-negative bacteria, such as P. aeruginosa, or Candida species, e.g., C. albicans and C. parapsilosis (Fig. 1A and B). Although P. aeruginosa and Candida spp. are the causes of 3% and 10% of all CR-BSI, respectively, they are, however, associated with high morbidity and mortality (1, 34). Furthermore, although antibiotic-coated CVCs are highly effective in the clinical setting, there is a potential for these devices to select for resistant gram-negative bacteria and Candida organisms, leading to breakthrough bacteremias and fungemias. Another concern is the emergence of resistant bacteria with decreased susceptibility to either minocycline or rifampin. In a prospective cohort of medical/surgical ICU patients, antibiotic-coated CVCs were found to be associated with increased colonization with Candida and with the emergence of rifampin-resistant Staphylococcus epidermidis (43).
Although several other studies have demonstrated that such a possibility is low, the use of an antiseptic catheter coating, especially one which contains two separate antiseptics, is probably associated with less risk of emergence of organisms that could be resistant to those antibiotics that are commonly used therapeutically (5, 12, 25).
The Vantex CVCs with the Oligon agent (Edwards Life Sciences, Irvine, CA) are made of polyurethane that is impregnated with silver, platinum, and carbon black and has been designed to reduce the risk of bacterial adherence to the surface of the catheter, as suggested by several in vitro studies (44, 45). In prospective randomized clinical trails, the Oligon Vantex CVCs (SPC-CVCs) significantly reduced the incidence of catheter colonization (7, 36). However, the study by Ranucci et al. lacked the power to detect differences in rates of catheter-related bloodstream infection (36). Another recent prospective, randomized, controlled multicenter clinical trial showed that the Oligon Vantex CVCs failed to prevent CR-BSI and catheter colonization (24). In our study, the SPC-CVCs failed to show in vitro efficacy in preventing the adherence of resistant bacteria or Candida species to the CVC surfaces and had no antimicrobial activity against MRSA, P. aeruginosa, or C. parapsilosis. The high efficacy of GND-CVCs in preventing the adherence of resistant gram-positive and gram-negative bacteria as well as Candida species and their prolonged antimicrobial durability against these organisms suggest that this novel antiseptic may prove to be efficacious in vivo and useful in the clinical setting. Zone-of-inhibition testing is a crude measure of antimicrobial activity against the free-floating form of microorganisms, while bacterial-adherence testing measures the antimicrobial activities against organisms in biofilm. Therefore, some antimicrobial agents may be less active against microorganisms in biofilm than against organisms in planktonic free-floating form. Gendine-coated catheter segments showed efficacy when tested by both methods. The antiseptic coating with gendine has several favorable characteristics. First, gendine coats the internal and external surfaces of the catheter and has the ability to coat various CVC polymers, including silicone and polyurethane. Second, the coating provides broad-spectrum activity against gram-positive and gram-negative bacteria as well as yeasts. Third, the gendine coating maintains prolonged antimicrobial durability in serum. Fourth, previous in vitro testing has demonstrated that catheters and endotracheal tubes coated with gendine were not associated with cytotoxicity in mouse fibroblast cell cultures (3). However, future in vivo testing and clinical trials are needed to determine whether gendine-coated CVCs are safe and able to reduce the risk of bloodstream infections associated with the use of CVCs without a risk of emergence of resistance.
Acknowledgments
Issam Raad is a coinventor of the approved catheters coated with minocycline-rifampin. This technology is the property of the University of Texas M. D. Anderson Cancer Center and Baylor College of Medicine and is licensed to Cook, Inc.
REFERENCES
- 1.Benezra, D., T. E. Kiehn, J. W. Gold, A. E. Brown, A. D. Turnbull, and D. Armstrong. 1988. Prospective study of infections in indwelling central venous catheters using quantitative blood cultures. Am. J. Med. 85:495-498. [DOI] [PubMed] [Google Scholar]
- 2.Brun-Buisson, C., F. Doyon, J. P. Sollet, J. F. Cochard, Y. Cohen, and G. Nitenberg. 2004. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 30:837-843. [DOI] [PubMed] [Google Scholar]
- 3.Chaiban, G., H. Hanna, T. Dvorak, and I. Raad. 2005. A rapid method of impregnating endotracheal tubes and urinary catheters with gendine: a novel antiseptic agent. J. Antimicrob. Chemother. 55:51-56. [DOI] [PubMed] [Google Scholar]
- 4.Chatzinikolaou, I., K. Finkel, H. Hanna, M. Boktour, J. Foringer, T. Ho, and I. Raad. 2003. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am. J. Med. 115:352-357. [DOI] [PubMed] [Google Scholar]
- 5.Chatzinikolaou, I., H. Hanna, L. Graviss, G. Chaiban, C. Perego, R. Arbuckle, R. Champlin, R. Darouiche, G. Samonis, and I. Raad. 2003. Clinical experience with minocycline and rifampin-impregnated central venous catheters in bone marrow transplantation recipients: efficacy and low risk of developing staphylococcal resistance. Infect. Control Hosp. Epidemiol. 24:961-963. [DOI] [PubMed] [Google Scholar]
- 6.Clemence, M. A., D. Walker, and B. M. Farr. 1995. Central venous catheter practices: results of a survey. Am. J. Infect. Control 23:5-12. [DOI] [PubMed] [Google Scholar]
- 7.Corral, L., M. Nolla-Salas, J. Ibanez-Nolla, M. A. Leon, R. M. Diaz, M. Cruz Martin, R. Iglesia, and R. Catalan. 2003. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J. Hosp. Infect. 55:212-219. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche, R. O., D. H. Berger, N. Khardori, C. S. Robertson, M. J. Wall, Jr., M. H. Metzler, S. Shah, M. D. Mansouri, C. Cerra-Stewart, J. Versalovic, M. J. Reardon, and I. I. Raad. 2005. Comparison of antimicrobial impregnation with tunneling of long-term central venous catheters: a randomized controlled trial. Ann. Surg. 24:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darouiche, R. O., I. I. Raad, S. O. Heard, J. I. Thornby, O. C. Wenker, A. Gabrielli, J. Berg, N. Khardouri, H. Hanna, R. Hachem, R. L. Harris, G. Mayhall, et al. 1999. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 340:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Dimick, J. B., R. K. Pelz, R. Consunji, S. M. Swoboda, C. W. Hendrix, and P. A. Lipsett. 2001. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch. Surg. 36:229-234. [DOI] [PubMed] [Google Scholar]
- 11.Garland, J. S., R. K. Buck, P. Maloney, D. M. Durkin, S. Toth-Lloyd, M. Duffy, P. Szocik, T. L. McAuliffe, and D. Goldmann. 1995. Comparison of 10% povidone-iodine and 0.5% chlorhexidine gluconate for the prevention of peripheral intravenous catheter colonization in neonates: a prospective trial. Pediatr. Infect. Dis. J. 14:510-516. [DOI] [PubMed] [Google Scholar]
- 12.Hanna, H., R. Benjamin, I. Chatzinikolaou, B. Alakech, D. Richardson, P. Mansfield, T. Dvorak, M. F. Munsell, R. Darouiche, H. Kantarjian, and I. Raad. 2004. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J. Clin. Oncol. 22:3163-3171. (Erratum, 23:3652, 2005.) [DOI] [PubMed] [Google Scholar]
- 13.Humar, A., A. Ostromecki, J. Direnfeld, J. C. Marshall, N. Lazar, P. C. Houston, P. Boiteau, and J. M. Conly. 2000. Prospective randomized trial of 10% povidone-iodine versus 0.5% tincture of chlorhexidine as cutaneous antisepsis for prevention of central venous catheter infection. Clin. Infect. Dis. 31:1001-1007. [DOI] [PubMed] [Google Scholar]
- 14.Jarvis, W. R. 1996. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect. Control Hosp. Epidemiol. 17:552-557. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson, E. L. 1995. APIC guideline for handwashing and hand antisepsis in health care settings. Am. J. Infect. Control 23:251-269. [DOI] [PubMed] [Google Scholar]
- 17.Logghe, C., C. Van Ossel, W. D'Hoore, H. Ezzedine, G. Wauters, and J. J. Haxhe. 1997. Evaluation of chlorhexidine and silver-sulfadiazine impregnated central venous catheters for the prevention of bloodstream infection in leukaemic patients: a randomized controlled trial. J. Hosp. Infect. 37:145-156. [DOI] [PubMed] [Google Scholar]
- 18.Maki, D. G. 1992. Infections due to infusion therapy, p. 849-898. In J. V. Bennett and P. S. Brachman (ed.), Hospital infections, 3rd ed. Little, Brown, Boston, Mass.
- 19.Maki, D. G. 1989. Pathogenesis, prevention, and management of infections due to intravascular devices for infusion therapy, p. 161-177. In S. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices. ASM Press, Washington, D.C.
- 20.Maki, D. G., M. Ringer, and C. J. Alvarado. 1991. Prospective randomized trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338:339-343. [DOI] [PubMed] [Google Scholar]
- 21.Maki, D. G., S. M. Stolz, S. Wheeler, and L. A. Mermel. 1997. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann. Intern. Med. 127:257-266. [DOI] [PubMed] [Google Scholar]
- 22.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402. (Erratum, 133:5.) [DOI] [PubMed] [Google Scholar]
- 23.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 24.Moretti, E. W., C. L. Ofstead, R. M. Kristy, and H. P. Wetzler. 2005. Impact of central venous catheter type and methods on catheter-related colonization and bacteraemia. J. Hosp. Infect. 61:139-145. [DOI] [PubMed] [Google Scholar]
- 25.Munson, E. L., S. O. Heard, and G. V. Doern. 2004. In vitro exposure of bacteria to antimicrobial impregnated-central venous catheters does not directly lead to the emergence of antimicrobial resistance. Chest 126:1628-1635. [DOI] [PubMed] [Google Scholar]
- 26.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, and R. A. Weinstein. 2002. Guidelines for the prevention of intravascular catheter-related infections. Morb. Mortal. Wkly. Rep. Recomm. Rep. 51(RR10):1-29. [PubMed] [Google Scholar]
- 27.Pittet, D., S. Hugonnet, S. Harbarth, P. Mourouga, V. Sauvan, S. Touyenau, T. V. Perneger, and members of the Infection Control Programme. 2000. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet 356:1307-1312. [DOI] [PubMed] [Google Scholar]
- 28.Pittet, D., D. Tarara, and R. P. Wenzel. 1994. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271:1598-1601. [DOI] [PubMed] [Google Scholar]
- 29.Pittet, D., and R. P. Wenzel. 1995. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch. Intern. Med. 155:1177-1184. [DOI] [PubMed] [Google Scholar]
- 30.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 31.Raad, I., W. Costerton, U. Sabharwal, M. Sacilowski, E. Anaissie, and G. P. Bodey. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 168:400-407. [DOI] [PubMed] [Google Scholar]
- 32.Raad, I., R. Darouiche, J. Dupuis, D. Abi-Said, A. Gabriel, R. Hachem, M. Wall, R. Harris, J. Jones, A. Buzaid, C. Robertson, S. Shenaq, P. Curling, T. Burke, C. Ericsson, and the Texas Medical Center Catheter Study Group. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double blind trial. Ann. Intern. Med. 127:267-274. [DOI] [PubMed] [Google Scholar]
- 33.Raad, I., R. Darouiche, R. Hachem, M. Mansour, and G. P. Bodey. 1996. The broad-spectrum activity and efficacy of catheters coated with minocycline and rifampin. J. Infect. Dis. 173:418-428. [DOI] [PubMed] [Google Scholar]
- 34.Raad, I., H. Hanna, M. Boktour, E. Girgawy, H. Danawi, M. Mardani, D. Kontoyiannis, R. Darouiche, R. Hachem, and G. P. Bodey. 2004. Management of central venous catheters in patients with cancer and candidemia. Clin. Infect. Dis. 38:1119-1127. [DOI] [PubMed] [Google Scholar]
- 35.Raad, I. I., D. C. Hohn, B. J. Gilbreath, N. Suleiman, L. A. Hill, P. A. Bruso, K. Marts, P. F. Mansfield, and G. P. Bodey. 1994. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect. Control Hosp. Epidemiol. 15:231-238. [PubMed] [Google Scholar]
- 36.Ranucci, M., G. Isgro, P. P. Giomarelli, M. Pavesi, A. Luzzani, I. Cattabriga, M. Carli, P. Giomi, A. Compostella, A. Digito, V. Mangani, V. Silvestri, E. Mondelli, and the Catheter Related Infection Trial (CRIT) Group. 2003. Impact of oligon central venous catheters on catheter colonization and catheter-related bloodstream infection. Crit. Care Med. 31:52-59. [DOI] [PubMed] [Google Scholar]
- 37.Richards, M. J., J. R. Edwars, D. H. Culver, R. P. Gaynes, et al. 1999. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 38.Rupp, M. E., S. J. Lisco, P. A. Lipsett, T. M. Pearl, K. Keating, J. M. Civetta, L. A. Mermel, D. Lee, E. P. Dellinger, M. Donahoe, D. Giles, M. A. Pfaller, D. G. Maki, and R. Sherertz. 2005. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann. Intern. Med. 143:570-580. [DOI] [PubMed] [Google Scholar]
- 39.Sherertz, R. J., W. A. Carruth, A. A. Hampton, M. P. Byron, and D. D. Solomon. 1993. Efficacy of antibiotic-coated catheters in preventing subcutaneous Staphylococcus aureus infections in rabbits. J. Infect. Dis. 167:98-106. [DOI] [PubMed] [Google Scholar]
- 40.Soufir, L., J. F. Timset, C. Mahe, J. Carlet, B. Regnier, and S. Chevret. 1999. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect. Control Hosp. Epidemiol. 20:396-401. [DOI] [PubMed] [Google Scholar]
- 41.Watnick, R., and R. Kolter. 2000. Biofilm: city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel, R. P., and M. B. Edmond. 1999. The evolving technology of venous access. N. Engl. J. Med. 340:48-50. [DOI] [PubMed] [Google Scholar]
- 43.Wright, F., D. K. Heyland, J. W. Drover, S. McDonald, and D. Zoutman. 2001. Antibiotic-coated central lines: do they work in the critical care setting? Clin. Intensive Care 12:21-28. [Google Scholar]
- 44.Yorganci, K., C. Krepel, J. A. Weigelt, and C. E. Edmiston. 2002. Activity of antibacterial impregnated central venous catheters against Klebsiella pneumoniae. Intensive Care Med. 28:438-442. [DOI] [PubMed] [Google Scholar]
- 45.Yorganci, K., C. Krepel, J. A. Weigelt, and C. E. Edmiston. 2002. In vitro evaluation of the antibacterial activity of three different central venous catheters against gram-positive bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 21:379-384. [DOI] [PubMed] [Google Scholar]