Abstract
Weak mutators are common among clinical isolates of Escherichia coli. We show that the relative mutation rate and the “evolvability of fluoroquinolone resistance” are related by a power law slope of 1.2 over 3 orders of magnitude. Thus, even weak mutators can drive the evolution of fluoroquinolone resistance under selection pressure.
Fluoroquinolones are a widely used group of antimicrobial agents. The development of resistance to clinically achievable levels of fluoroquinolones in most organisms, including Escherichia coli, is a multistep mutational process. Although several plasmid-borne resistance determinants have been described (10, 13, 14, 20), the great majority of the genetic alterations associated with fluoroquinolone resistance are mutations of chromosomal genes, including gyrA, gyrB, parC, parE, marR, and acrR (11, 12). E. coli isolates with MICs above the CLSI (formerly NCCLS)-defined breakpoint for ciprofloxacin (4 μg/ml) usually have four or more mutations distributed among these genes (17). This implies that resistant lineages have gone through several cycles of mutation and selection. The relative mutation rate might be a significant factor that determines the probability that resistance will evolve in a lineage by increasing the rate of supply of rare new mutations.
The focus of interest regarding the influence of mutators on evolution has been on strong mutators such as mutS mutants (3, 7, 21, 24, 25). These increase mutation rates ∼500-fold and are found in up to 1% of natural E. coli isolates (2, 9, 17, 18, 21). They have strong selective benefits in experimental models (8, 19, 24, 27) but in vivo also lead to the accumulation of detrimental mutations that severely reduce fitness, measured as reduced competitiveness during transmission to and recolonization of eukaryotic hosts (8). In contrast, studies of hundreds of clinical E. coli isolates, also from a variety of sources, have found that about 25% are weakly hypermutable (up to 13-fold increase in the mutation rate) (2, 21). The relative preponderance of weak and moderate hypermutators among clinical isolates, including multiply resistant isolates (5), may reflect their better ability to evolve resistance, be transmitted to new hosts, and persist longer than strong mutators without incurring major fitness costs (4, 28).
We have previously noted an increased mutation rate correlated with fluoroquinolone resistance in clinical isolates of E. coli (17). The increases are modest, with the mutation rate in 80% of resistant isolates increasing by less than 20-fold the rate for the wild type. The influence of small increases in the mutation rate on the evolution of antibiotic resistance has not previously been tested experimentally. This raises the question of whether the link between fluoroquinolone resistance and a moderately increased mutation rate (17) is fortuitous or causal. We tested whether small and moderate increases in the mutation rate were sufficient to proportionately increase the ability of E. coli to evolve fluoroquinolone resistance.
Seven different mutator alleles were introduced into E. coli K12 MG1655 by P1 transduction (Table 1). These mutator alleles have been described in the literature, and their reported mutator phenotypes differ widely in magnitude (1, 15, 16, 22, 26, 29). Fifty independent lineages of each strain were evolved by serial passage in Luria-Bertani medium with successively higher concentrations of ciprofloxacin (Bayer AG, Wuppertal, Germany) in a BioscreenC machine (Oy Growth Curves Ab Ltd., Helsinki, Finland). The ciprofloxacin concentration steps were 0, 0.01, 0.02, 0.04, 0.08, 0.16, 0.32, 0.64, 1.25, 2.5, 5, 10, 20, 40, 80, 160, 320, 640, 1,280, and 2,560 μg/ml. Each cycle was 23 to 24 h of growth in a 200 μl, with the transfer of 5 μl at each step (initially 106 to 107 CFU). During the course of the evolution experiments, different lineages grew to different cell concentrations, reflecting their independent evolutionary trajectories; and thus, biological bottleneck sizes were lineage and step specific. The optical density at 600 nm (OD600) of each well was measured at 10-min intervals throughout the culture period. Extinction of a lineage was defined as the failure to obtain growth (OD600 < 0.03) after overnight incubation. The extinction coefficient (KE) is defined as the lowest drug concentration step at which ≥50% of the lineages were extinct.
TABLE 1.
Bacterial strains and mutator genotypes
| Strain | Genotypea | Source of mutator allele |
|---|---|---|
| MG1655 | Wild type | Coli Genetic Stock Center |
| CH188 | ΔmutS::FRT | This laboratory |
| CH286 | mutY::Tn10dTetr | Jeffrey H. Miller (23) |
| CH287 | damΔ16::Kanr | Martin Marinus (26) |
| CH289 | miaA::ΩCamr | Malcolm Winkler (29) |
| CH290 | ung::Spcr | Murat K. Saparbaev (15) |
| CH291 | mutM::Tn10dTetr | Ashok S. Bhagwat (16) |
| BS1511 | mutA∼ampC::Kanr | M. Zafri Humayun (1) |
“∼” means the markers are linked by P1 transduction.
The MIC of ciprofloxacin was determined by Etest, according to the instructions of the manufacturer (AB BIODISK, Solna, Sweden). The mutator mutations do not themselves significantly affect the MICs (Table 2).
TABLE 2.
Relationship between mutation rate and KE
| Genotype | MIC (μg/ml) | Mutation rate | Relative mutation rate | KE | Relative KE |
|---|---|---|---|---|---|
| Wild type | 0.016 | 1.8 × 10−8 | 1 | 0.04 | 1 |
| mutM | 0.016 | 3.3 × 10−8 | 2 | 0.64 | 16 |
| mutA | 0.023 | 6.4 × 10−8 | 4 | 0.64 | 16 |
| miaA | 0.023 | 1.4 × 10−7 | 8 | 0.04 | 1 |
| ung | 0.016 | 1.8 × 10−7 | 10 | 1.25 | 31 |
| mutY | 0.023 | 1.8 × 10−7 | 10 | 1.25 | 31 |
| dam | 0.016 | 7.2 × 10−7 | 41 | 10 | 250 |
| mutS | 0.016 | 9.2 × 10−6 | 525 | 160 | 4,000 |
Mutation rates were measured by fluctuation tests with 40 independent cultures of each strain, assaying for rifampin resistance as described previously (17). This is a standard assay and measures the occurrence of at least 69 different base substitutions in rpoB (6). The results (Table 2) are close to those expected on the basis of the measurements for the parental strains. The range of mutation rates covered by these strains is ∼500-fold, with an emphasis on the lower end of the range up to 10-fold the rate for the wild type.
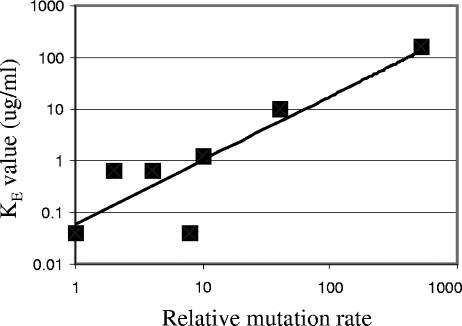
All lineages of each strain, with the exception of a few mutS lineages, went to extinction within the course of the experiment. At 1,280 and 2,560 μg/ml ciprofloxacin, 20% and 13% of the mutS lineages were still living, respectively. The KE values for each strain (Table 2) ranged over 3 orders of magnitude. When the KE values were plotted against the relative mutation rate, the data fit a straight line (linear regression R2 value, 0.77; P value, <0.001 at the 95% confidence level) to a power law with a slope of 1.2 (Fig. 1). Thus, “evolvability” (KE) increases as the 1.2 power of the mutation rate. The results for one miaA strain deviated notably from this regression line, possibly because it failed to generate an increased rate of some mutation required for the evolution of fluoroquinolone resistance, although pleiotropic side effects due to the absence of this tRNA modification cannot be ruled out. Removal of the data for the miaA strain from the analysis gives a slope of 1.2 for the remaining seven strains (linear regression R2 value, 0.95; P value, < 0.001). This particular power law relationship may reflect the average value of synergistic epistasis between different mutations and their effects on the resistance level. For example, a parC mutation does not show a resistance phenotype except in the presence of an appropriate gyrA mutation (30).
FIG. 1.
KE values for wild-type and mutator strains plotted as a function of relative mutation rate.
In conclusion, we found a good correlation between the mutation rate and KE that extended over a wide range of mutation rates. This shows that even small increases in the mutation rate have a positive and measurable effect on the evolution of fluoroquinolone resistance by mutation and that the effect is in proportion to the magnitude of the mutation rate increase. There is no minimum threshold of mutability for increasing the rate of evolution to drug resistance.
Acknowledgments
This work was supported by grants from the Swedish Research Council (Vetenskapsrådet) and the European Union Sixth Framework Programme (EAR project, contract LSHM-CT-2005-518152) to D. Hughes.
We thank the scientists listed in Table 1, Santanu Dasgupta (Uppsala University), and Monica Rydén Aulin (Stockholm University) for kindly sending us mutator strains.
REFERENCES
- 1.Balashov, S., and M. Z. Humayun. 2004. Specificity of spontaneous mutations induced in mutA mutator cells. Mutat. Res. 548:9-18. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, M. R., A. I. Nilsson, C. Turrientes Mdel, D. Sandvang, J. C. Galan, J. L. Martinez, N. Frimodt-Moller, F. Baquero, and D. I. Andersson. 2004. Polymorphic mutation frequencies in Escherichia coli: emergence of weak mutators in clinical isolates. J. Bacteriol. 186:5538-5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boe, L., M. Danielsen, S. Knudsen, J. B. Petersen, J. Maymann, and P. R. Jensen. 2000. The frequency of mutators in populations of Escherichia coli. Mutat. Res. 448:47-55. [DOI] [PubMed] [Google Scholar]
- 4.Denamur, E., and I. Matic. 2006. Evolution of mutation rates in bacteria. Mol. Microbiol. 60:820-827. [DOI] [PubMed] [Google Scholar]
- 5.Denamur, E., O. Tenaillon, C. Deschamps, D. Skurnik, E. Ronco, J. L. Gaillard, B. Picard, C. Branger, and I. Matic. 2005. Intermediate mutation frequencies favor evolution of multidrug resistance in Escherichia coli. Genetics 171:825-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garibyan, L., T. Huang, M. Kim, E. Wolff, A. Nguyen, T. Nguyen, A. Diep, K. Hu, A. Iverson, H. Yang, and J. H. Miller. 2003. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair (Amsterdam) 2:593-608. [DOI] [PubMed] [Google Scholar]
- 7.Giraud, A., I. Matic, M. Radman, M. Fons, and F. Taddei. 2002. Mutator bacteria as a risk factor in treatment of infectious diseases. Antimicrob. Agents Chemother. 46:863-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giraud, A., I. Matic, O. Tenaillon, A. Clara, M. Radman, M. Fons, and F. Taddei. 2001. Costs and benefits of high mutation rates: adaptive evolution of bacteria in the mouse gut. Science 291:2606-2608. [DOI] [PubMed] [Google Scholar]
- 9.Giraud, A., M. Radman, I. Matic, and F. Taddei. 2001. The rise and fall of mutator bacteria. Curr. Opin. Microbiol. 4:582-585. [DOI] [PubMed] [Google Scholar]
- 10.Hata, M., M. Suzuki, M. Matsumoto, M. Takahashi, K. Sato, S. Ibe, and K. Sakae. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 49:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper, D. C. 2001. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 7:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins, K. L., R. H. Davies, and E. J. Threlfall. 2005. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: recent developments. Int. J. Antimicrob. Agents 25:358-373. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby, G. A., N. Chow, and K. B. Waites. 2003. Prevalence of plasmid-mediated quinolone resistance. Antimicrob. Agents Chemother. 47:559-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurado, J., A. Maciejewska, J. Krwawicz, J. Laval, and M. K. Saparbaev. 2004. Role of mismatch-specific uracil-DNA glycosylase in repair of 3,N4-ethenocytosine in vivo. DNA Repair (Amsterdam) 3:1579-1590. [DOI] [PubMed] [Google Scholar]
- 16.Klapacz, J., and A. S. Bhagwat. 2005. Transcription promotes guanine to thymine mutations in the non-transcribed strand of an Escherichia coli gene. DNA Repair (Amsterdam) 4:806-813. [DOI] [PubMed] [Google Scholar]
- 17.Komp Lindgren, P., A. Karlsson, and D. Hughes. 2003. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob. Agents Chemother. 47:3222-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeClerc, J. E., B. Li, W. L. Payne, and T. A. Cebula. 1996. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274:1208-1211. [DOI] [PubMed] [Google Scholar]
- 19.Mao, E. F., L. Lane, J. Lee, and J. H. Miller. 1997. Proliferation of mutators in a cell population. J. Bacteriol. 179:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 21.Matic, I., M. Radman, F. Taddei, B. Picard, C. Doit, E. Bingen, E. Denamur, and J. Elion. 1997. Highly variable mutation rates in commensal and pathogenic Escherichia coli. Science 277:1833-1834. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1998. Mutators in Escherichia coli. Mutat. Res. 409:99-106. [DOI] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 24.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1254. [DOI] [PubMed] [Google Scholar]
- 25.Orencia, M. C., J. S. Yoon, J. E. Ness, W. P. Stemmer, and R. C. Stevens. 2001. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat. Struct. Biol. 8:238-242. [DOI] [PubMed] [Google Scholar]
- 26.Parker, B., and M. G. Marinus. 1988. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene 73:531-535. [DOI] [PubMed] [Google Scholar]
- 27.Sniegowski, P. D., P. J. Gerrish, and R. E. Lenski. 1997. Evolution of high mutation rates in experimental populations of E. coli. Nature 387:703-705. [DOI] [PubMed] [Google Scholar]
- 28.Taddei, F., M. Radman, J. Maynard-Smith, B. Toupance, P. H. Gouyon, and B. Godelle. 1997. Role of mutator alleles in adaptive evolution. Nature 387:700-702. [DOI] [PubMed] [Google Scholar]
- 29.Zhao, J., H. E. Leung, and M. E. Winkler. 2001. The miaA mutator phenotype of Escherichia coli K-12 requires recombination functions. J. Bacteriol. 183:1796-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]