Abstract
For two fungal strains to be vegetatively compatible and capable of forming a stable vegetative heterokaryon they must carry matching alleles at a series of loci variously termed het or vic genes. Cloned het/vic genes from Neurospora crassa and Podospora anserina have no obvious functional similarity and have various cellular functions. Our objective was to identify the homologue of the Neurospora het-c gene in Fusarium proliferatum and to determine if this gene has a vegetative compatibility function in this economically important and widely dispersed fungal pathogen. In F. proliferatum and five other closely related Fusarium species we found a few differences in the DNA sequence, but the changes were silent and did not alter the amino acid sequence of the resulting protein. Deleting the gene altered sexual fertility as the female parent, but it did not alter male fertility or existing vegetative compatibility interactions. Replacement of the allele-specific portion of the coding sequence with the sequence of an alternate allele in N. crassa did not result in a vegetative incompatibility response in transformed strains of F. proliferatum. Thus, the fphch gene in Fusarium appears unlikely to have the vegetative compatibility function associated with its homologue in N. crassa. These results suggest that the vegetative compatibility phenotype may result from convergent evolution. Thus, the genes involved in this process may need to be identified at the species level or at the level of a group of species and could prove to be attractive targets for the development of antifungal agents.
Fungal cells can interact with each other either vegetatively or sexually. In ascomycete fungi, sexual interactions are controlled by the alleles at the mating type locus (MAT), and asexual interactions are controlled by the alleles at the vic (vegetative incompatibility) or het (heterokaryon incompatibility) loci (20). (het and vic are used to indicate genes with similar functions and reflect differences in nomenclature, not function.) Vegetative incompatibility has been studied at least superficially in a large number of fungi, but in-depth studies have been limited to Neurospora crassa (12, 34, 35, 36), Podospora anserina (7, 10, 29, 30), Cryphonectria parasitica (6), Aspergillus nidulans (8), and several species of Fusarium (13, 17, 25, 26). The het/vic genes are important for the recognition process to occur, but there are genes that affect the physical ability of hyphae to fuse that act upstream of the het/vic interaction (5, 23) and other genes that are responsible for maintaining the stability of the heterokaryon that act following the het/vic interaction (23, 40).
Vegetative compatibility/incompatibility may result from interactions between alleles at the same locus (allelic vegetative compatibility) or at different loci (genic or nonallelic vegetative compatibility) (12, 20, 31). Most studies, including all previous studies with Fusarium, have examined allelic vegetative compatibility, in which vegetatively compatible strains have a common allele at each of the vic loci. A mismatch at one or more of these loci results in the death of any heterokaryotic cells formed, probably via an apoptotic process. Studies of het/vic genes at the molecular level have been performed with N. crassa and P. anserina, but the current study is the first study of this type for any Fusarium sp. The genes cloned thus far participate in various cellular activities and include genes that encode HMG-box proteins, glycine-rich signal peptide repeats, glycolipid transfer proteins, WD-40 repeats of putative GTP binding proteins, proteins with TOL domains, the large subunit of a type I ribonucleotide reductase, and a putative prion (31).
An important question has been whether the vic properties of vic genes are evolutionarily conserved or whether various genes have acquired this function in different fungi (2). If these genes and their associated functions are well conserved, then studies with model systems can be used to define such systems in less-studied systems (e.g., Fusarium spp.) in which these genes may define economically important groups. Triggering the death process associated with a vegetative incompatibility reaction could provide a novel means of limiting or eliminating fungal infections in both plants and animals. Thus, determining if vic genes in model ascomycetes have homologues with similar functions in other systems may have both basic and applied significance.
The het-c gene of N. crassa is one of the best studied of the het genes of N. crassa. This gene encodes a 966-amino-acid glycine-rich transmembrane protein that can act as part of a signaling pathway (34). There are at least three functionally distinct alleles at this locus (het-cOR, het-cGR, and het-cPA) (33), and strains that carry different alleles cannot form stable vegetative heterokaryons. These three alleles differ in a 34- to 48-amino-acid region that differs at multiple sites in the alleles (33). The het-c locus is polymorphic in 12 other species belonging to the Sordariaceae, and the three alleles known in N. crassa are also found in these other species (39).
The het-c homologue in P. anserina is hch (not het-C, which encodes a glycolipid transfer protein and participates in a genic/nonallelic vegetative incompatibility interaction) (32). hch lacks the polymorphic region found in the N. crassa alleles and may have no role in determining whether two strains of P. anserina are vegetatively compatible. Thus, while the structural protein appears to be conserved between N. crassa and P. anserina, the vegetative compatibility function appears to have been lost, if it was ever present.
Fusarium proliferatum (Gibberella intermedia) is a member of the Gibberella fujikuroi species complex, a set of fungal species that are important as plant pathogens and as producers of mycotoxins (9, 22). In other members of the G. fujikuroi species complex, 8 to 10 vic loci have been formally identified (23), and nearly isogenic lines that differ at individual vic loci have been constructed (26). Whether any of these loci are homologues of het-c or other het loci identified in N. crassa is unknown, as is whether the het-c homologue in F. proliferatum has a potential role in the vegetative incompatibility process in this fungus. We tested the hypothesis that an F. proliferatum homologue of the N. crassa het-c gene has a role in vegetative compatibility similar to that in N. crassa by cloning the gene, checking for polymorphisms in a diverse set of strains belonging to this and related species, and replacing the native allele with an allele whose sequence was altered in a manner similar to that observed in the naturally occurring alleles of N. crassa. These studies are important for determining the extent to which vic gene functions and polymorphisms are conserved across species within the ascomycetes.
MATERIALS AND METHODS
Strains and culture conditions.
The fungal strains assessed for hch polymorphisms, all members of the G. fujikuroi species complex, were F. proliferatum (G. intermedia [= G. fujikuroi mating population D]) FGSC 7614, FGSC 7615, ITEM 1475, ITEM 1477, ITEM 1478, ITEM 1479, ITEM 1480, ITEM 1724, ITEM 1725, ITEM 1726, ITEM 1727, ITEM 1748, ITEM 1749, ITEM 1764, ITEM 1799, ITEM 1800, ITEM 1802, ITEM 1808, ITEM 1916, ITEM 1918, ITEM 1920, ITEM 1921, ITEM 2111, ITEM 2112, ITEM 2113, ITEM 2114, ITEM 2115, ITEM 2116, ITEM 2287, ITEM 2336, ITEM 2337, ITEM 2339, ITEM 2341, ITEM 2343, ITEM 2365, ITEM 2366, ITEM 2367, ITEM 2368, ITEM 2369, ITEM 2383, ITEM 2386, ITEM 2387, ITEM 2401, ITEM 2402, ITEM 2408, ITEM 2409, ITEM 2620, ITEM 2631, ITEM 2802, ITEM 2811, ITEM 2819, ITEM 2823, ITEM 3268, ITEM 3269, ITEM 3270, ITEM 3271, ITEM 3272, ITEM 3273, ITEM 3274, ITEM 3275, ITEM 3276, ITEM 3277, ITEM 3307, ITEM 3308, ITEM 3798, ITEM 3945, ITEM 3948, ITEM 3951, ITEM 3956, ITEM 3960, ITEM 4078, ITEM 4080, ITEM 4081, ITEM 4083, ITEM 4084, and ITEM 4085; Fusarium verticillioides (Gibberella moniliformis [= G. fujikuroi mating population A]) FGSC 7600 and FGSC 7603; Fusarium sacchari (Gibberella sacchari [= G. fujikuroi mating population B]) FGSC 7610 and FGSC 7611; Fusarium fujikuroi (Gibberella fujikuroi [= G. fujikuroi mating population C]) FGSC 8931 and FGSC 8932; Fusarium subglutinans (Gibberella subglutinans [= G. fujikuroi mating population E]) FGSC 7616 and FGSC 7617; Fusarium thapsinum (Gibberella thapsina [= G. fujikuroi mating population F]) FGSC 7056 and FGSC 7057; and Fusarium nygamai (Gibberella nygamai [= G. fujikuroi mating population G]) FGSC 7491 and FGSC 7492. Strains of F. proliferatum were isolated from host plants as diverse as asparagus, date palm, fig, maize, reed, rice, and tomato, and their geographic origins were also very different and included Argentina, Italy, Slovakia, Saudi Arabia, and the United States (16). The FGSC and ITEM strains were obtained from Fungal Genetics Stock Center, Department of Biological Sciences, University of Missouri-Kansas City, Kansas City, MO, and Institute of Sciences of Food Production, CNR, Bari, Italy, respectively. Cultures were maintained on potato dextrose agar (Duchefa Biochemie, Haarlem, The Netherlands) and stored at 4°C.
Conidial suspensions were prepared from liquid cultures grown on CMC medium (3) with carboxymethyl cellulose as the sole carbon source for 3 days at 25°C with shaking (200 rpm) and with a diurnal cycle consisting of 12 h of light and 12 h of darkness. For nucleic acid extraction about 108 conidia were inoculated into 100 ml liquid complete medium (CM) (14) and grown for 3 days as a shake culture (200 rpm) at 25°C in the dark. Cultural characteristics of the Δfphch transformants were examined on CM agar.
Molecular techniques.
RNA and DNA were isolated as previously described (14, 18). A genomic library of F. proliferatum ITEM 2287 (MAT-2) was constructed by using Gigapack III Gold (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Isolation of fphch from the genomic library, ligation, bacterial transformation, plasmid isolation, restriction digestion, Southern hybridization, and Northern hybridization were performed by using standard procedures (28). DNA fragments were sequenced by the Sequencing Service of the Agricultural Biotechnology Center (Gödöllő, Hungary). Sequence data were analyzed with the Lasergene software package (DNAStar Inc., Madison, Wis.) and the FGENESH program (http://www.softberry.com). Blast searches (1) were done with the EMBL database and the BLAST program of GenomeNet (http://www.genome.jp).
PCR.
A conserved fragment of the putative hch sequence was amplified from F. proliferatum ITEM 2287 by using the degenerate primers Fs_hch_for (5′-ATCAGYGAGACTCTYACYGCYTT-3′) and Fs_hch_rev (5′-CCAGKGTTCTCCCARGCA-3′) designed on the basis of the j2f04fs.fl EST sequence of Fusarium sporotrichioides (http://www.genome.ou.edu/fsporo.html). Primers Fp_hchHVR_for (5′-CGTTTGCGAGGGCCCGTTGAGA-3′) and Fp_hchHVR_rev (5′-CACCGCCGGATCGATTGGAAGC-3′) were used to amplify the putative hypervariable domain of the hch gene from all strains listed above. These primers were designed based on the genomic sequence of fphch. A 1,160-bp fragment of the fphch gene was amplified from strain ITEM 2287 by using primers C1m_for (5′-GATATCGGCAGTTTGAAGGGTGTC-3′) and C1m_rev (5′-GGTCGGATGAAGGGAGCAAGAAGA-3′); this fragment was used in Southern and Northern hybridizations as a probe. Primers Hch-5′ (5′-TGCCTACCTATACATCGTAATCG-3′) and Hch-3′ (5′-CGTCCGTCTAGGTGGTTGG-3′) were used to amplify the entire fphch gene. The hph (hyromycin B phosphotransferase) gene was amplified from plasmid pAN7-1 (27) by using primers Hph_EcoRV_for (5′-CTTGGAAGCGGCGAGGAG-3′) and Hph_EcoRV_rev (5′-TATTGGGTGTTACGGAGCATTCA-3′). Primers C1iPCR1 (5′-GAGGGCCCGTTGAGAAGATAGAAA-3′) and C1m_rev were used to confirm replacement of the fphch gene by the hph cassette in the Δfphch transformants.
PCRs were performed with reaction mixtures containing 1× PCR buffer (MBI Fermentas, Vilnius, Lithuania), 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.5 mM, each primer at a concentration of 0.25 μM, 1 U of Taq polymerase (MBI Fermentas), and 20 ng of fungal DNA. The initial denaturation was done at 95°C for 2 min and was followed by 30 to 40 cycles consisting of 15 s at 94°C, 30 s at 55 to 65°C (depending on the melting temperature of the primers), and 30 s at 72°C and a final elongation step at 72°C for 5 min.
fphch and hph sequences used in control gene replacement events in Δfphch transformants were amplified by long PCRs. The reaction mixtures contained 1× long PCR buffer (MBI Fermentas), 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.2 mM, each primer at a concentration of 0.2 μM, 1 U of long PCR enzyme mix (MBI Fermentas), and 20 ng of fungal DNA. The initial denaturation was at 94°C for 2 min and was followed by 10 cycles consisting of 15 s at 94°C, 30 s at 55 to 58°C (depending on the melting temperature of the primers), and 3 min at 68°C, 25 cycles consisting of 15 s at 94°C, 30 s at 55 to 58°C (depending on the melting temperature of the primers), and 3 min plus 10 s/cycle at 68°C, and a final elongation step at 68°C for 10 min.
Restriction fragment length polymorphisms.
A ∼500-bp fragment, representing the putative hypervariable region of the hch sequence, was amplified from all strains by using primers Fp_hchHVR_for and Fp_hchHVR_rev. The amplicons were digested with NcoI, SalI, HpaII, and TaqI (all from MBI Fermentas) according to the manufacturer's recommendations. Restriction fragments were separated by electrophoresis.
Transformation of F. proliferatum ITEM 2287.
Protoplasts were isolated from exponentially growing mycelial cultures by digestion with a mixture of Novozyme 234, Driselase, and Chitinase (all from Sigma, St. Louis, MO). Polyethylene glycol-mediated transformation was performed as described by Proctor et al. (24). Transformants were selected on regeneration medium (0.1% yeast extract, 0.1% casein hydrolysate, 1% agar, 0.8 M sucrose) containing 200 μg ml−1 hygromycin B (Duchefa). Resistant transformants detected after 4 to 5 days of incubation were transferred to CM plates amended with hygromycin B (200 μg ml−1).
Sexual crosses.
Strain ITEM 2287 (MATD-2) and its Δfphch derivatives were crossed on carrot agar with strain FGSC 7615 (MATD-1) used as either the male parent or the female parent. Fertility was evaluated as described by Klittich and Leslie (19). When used as female parents, strains were grown on carrot agar, and a conidial suspension (obtained from water agar plates) of the opposite partner was sprinkled on each of them. The carrot agar plates were incubated for 4 weeks at 23 to 24°C with a diurnal cycle consisting of 12 h of light and 12 h of darkness until perithecia were observed.
Vegetative incompatibility test.
Non-nitrate-utilizing (nit) mutants were selected on complete medium containing 2% (wt/vol) potassium chlorate (17), were assigned to phenotypic classes (Nit1, Nit3, and NitM) based on growth on basal minimal medium with nitrate, nitrite, or hypoxanthine as the sole nitrogen source, and were tested for complementation as previously described (4).
Nucleotide sequence accession numbers.
The nucleotide sequences of fphch and the amplified hypervariable regions of the het-c homologues from F. proliferatum ITEM 2366 and ITEM 2823, F. sacchari FGSC 7610 and FGSC 7611, and F. subglutinans FGSC 7616 and FGSC 7617 have been deposited in the EMBL database under accession numbers DQ067618 and DQ329213 to DQ329218.
RESULTS
Cloning of fphch.
The F. sporotrichioides EST library (http://www.genome.ou.edu/fsporo.html) was screened for het-c homologous sequences with a DNA oligonucleotide based on the deduced amino acid sequence for the het-cOR gene from N. crassa as the query sequence. EST j2f04fs.f1 had the highest level of homology (Fig. 1) (72% identity and 84% similarity). The degenerate primers Fs_hch_for and Fs_hch_rev were used to amplify the homologous DNA fragment from F. proliferatum ITEM 2287. This putative hch fragment was 340 bp long and 85% identical to the F. sporotrichioides EST j2f04fs.f1 sequence.
FIG. 1.
Comparison of deduced amino acid sequences for N. crassa het-cOR (Nchet-cOR) and F. sporotrichioides EST j2f04fs.f1 (Fshet-cEST) using the tblastX (http://BLAST.genome.ad.jp) program. Arrows indicate sequences used to design primers (Fs_hch_for and Fs_hch_rev) for amplification of the het-c homologous sequence from F. proliferatum ITEM 2287.
The entire gene (fphch) was cloned from the genomic library of F. proliferatum ITEM 2287 by plaque hybridization with the amplified 340-bp DNA fragment as the probe. Phage inserts from positive plaques were restriction mapped, and a 5-kb XbaI fragment expected to contain the entire fphch gene was ligated into pBluescript KS to obtain plasmid pHC1. fphch contains a 2,536-bp open reading frame with four introns and five exons. The deduced FPHCH protein is 770 amino acids long and has high levels of homology to HCH (69% identity and 80% similarity) and HET-CGR (63% identity and 73% similarity) from P. anserina and N. crassa, respectively.
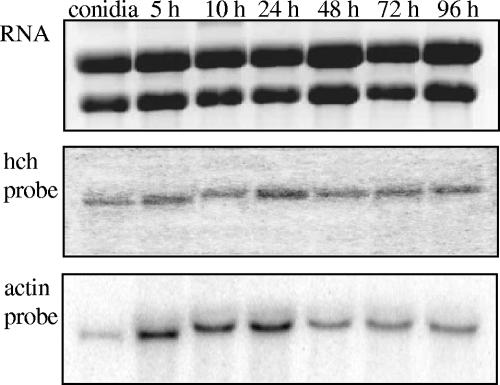
Based on Southern analysis of genomic DNA, fphch is a single-copy gene in F. proliferatum (data not shown). fphch expression after 5, 10, 24, 48, 72, and 96 h of vegetative growth was evaluated by performing Northern analyses with a 900-bp PCR-amplified fragment of fphch as the probe for total fungal RNA (Fig. 2). fphch is constitutively expressed as transcripts were present at all times sampled.
FIG. 2.
Northern analysis of fphch expression during germination of conidia. Samples were taken between zero time and 96 h during germination. The blot was hybridized with a 900-bp PCR-amplified fragment of the fphch gene (middle panel) and then reprobed with a 600-bp fragment of ACT1, an actin-encoding gene from F. proliferatum ITEM 2287 used as a loading control (bottom panel). The upper panel shows total RNA stained with ethidium bromide, demonstrating that there was equal RNA loading.
Analysis of the het-c variable region in G. fujikuroi.
A 34- to 48-amino-acid domain of the HET-C protein controls allele specificity in N. crassa (33). Based on nucleotide sequence similarity, the corresponding region in fphch is between nucleotides 703 and 1198. Oligonucleotide primers Fp_hchHVR_for and Fp_hchHVR_rev hybridized to the flanks of this region and were used to amplify homologous sequences from 76 strains of F. proliferatum and 10 additional strains of five other species in the G. fujikuroi species complex. All of the amplifications yielded a fragment that was ∼500 bp long. DNA fragments were digested with four restriction endonucleases that would yield restriction fragment length polymorphisms if they were used to digest similar fragments of the three N. crassa alleles. We found polymorphisms in six strains and sequenced the six mutant alleles, two from F. proliferatum (ITEM 2366 and ITEM 2823), two from F. sacchari (FGSC 7610 and FGSC 7611), and two from F. subglutinans (FGSC 7616 and FGSC 7617). The 495-bp fragments (nucleotides 627 to 1122 of the fphch sequence) contained a few nucleotide substitutions, but these point mutations did not change the deduced amino acid sequences of the resulting proteins.
Disruption of fphch.
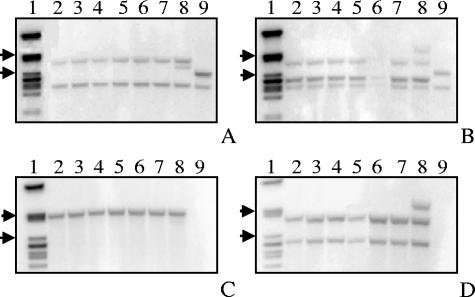
We replaced an EcoRV fragment of pHC1 containing a 2,299-bp region of fphch with a 3,800-bp hygromycin expression cassette (hph) containing the hygB (hygromycin B phosphotransferase) gene from Escherichia coli. Fragments resulting from PCR amplification with primers Hph_EcoRV_for, Hph_EcoRV_rev, Hch-5′, and Hch-3′ of genomic DNA from the transformed strains indicated that the hph cassette was inserted at a single site in the fphch sequence in the 3′→5′ direction. Ten micrograms of plasmid DNA was linearized by digestion with NotI and used to transform protoplasts of F. proliferatum ITEM 2287. Hygromycin-resistant colonies were detected after 4 to 5 days of growth and were transferred to CM plates containing 200 μg ml−1 hygromycin B. Fifty-four transformants were assayed by PCR with primers C1iPCR1 and C1m_rev, which detected sequence replacement events. Of the 54 transformants, 7 had PCR patterns consistent with disruption of fphch by a double recombination event. In six of these seven transformants the double recombination was confirmed by Southern hybridization with 4,400- and 3,800-bp fragments that carried the entire fphch gene and the hph cassette, respectively (Fig. 3). All Δfphch mutants grew normally on CM with no observable differences in morphology, sporulation, or spore germination. Thus, based on growth characteristics of vegetative cultures, Δfphch mutants were indistinguishable from the wild type.
FIG. 3.
Southern blot analysis of KpnI-digested (A and C) and PstI-digested (B and D) DNA samples of Δfphch transformants and wild-type strain F. proliferatum ITEM 2287 probed with fphch (A and B) and hph (C and D) fragments. Lane 1, λ PstI ladder (arrows indicate the positions of 5,080 and 2,840 bp); lanes 2 to 8, Δfphch transformant strains; lane 9, wild type. The expected fragments were as follows: in panel A, two fragments for the wild type (one of which was at 3,344 bp) and two fragments for the Δfphch transformants (one of which was at 4,826 bp); in panel B, three fragments for the wild type (one of which was at 2,795 bp) and three fragments for the Δfphch transformants (one of which was at 2,518 bp); in panel C with KpnI digestion and the hph probe, no fragment for the wild type and one fragment (4,826 bp) for the Δfphch transformants; in panel D with PstI digestion and the hph probe, no fragment for the wild type and two fragments for the Δfphch transformants (one of which was at 2,518 bp). (Lane 8 contained DNA from a transformant in which the gene was not properly replaced; this transformant was not included in further experiments.)
F. proliferatum strain ITEM 2287 has a heterokaryon self-incompatible phenotype. In heterokaryon self-incompatible strains, the number of hyphal fusions is reduced, and the practical result is that complementary nit mutants derived from such strains often do not form visibly noticeable heterokaryons when they are paired (5). Strains that are heterokaryon self-incompatible occasionally form weak heterokaryons with strains in the same vegetative compatibility group that are heterokaryon self-compatible, but nit mutants from the other F. proliferatum strains that we examined also failed to form heterokaryons with the nit mutant strains derived from ITEM 2287. nit mutants derived from the Δfphch mutants also failed to form heterokaryons with any of the other strains used in this study and did not form heterokaryons when they were paired with one another. Thus, there is no evidence that Δfphch plays a role in the heterokaryon self-incompatibility phenotype.
We evaluated the sexual fertility of three of the Δfphch mutants. When the Δfphch mutants were used as the male parent, all of the crosses were fertile, with mature perithecia oozing ascospores 3 to 4 weeks after fertilization. If the Δfphch mutants were used as the female parent, perithecia formed more slowly (4 weeks was required for the first appearance instead of 10 days), and the total number of perithecia declined as well (<0.6 perithecium cm−2 at the end of the fourth week for the Δfphch mutants and 5 to 7 perithecia cm−2 for the parental strain). The ITEM 2287 parental strain was female fertile, which is unusual since previously reported heterokaryon self-incompatible strains (5) have been female sterile.
Artificial hch allele.
We made a 6-amino-acid insertion (5′-GACACACGTAACAATGGA-3′) via site-directed mutagenesis at nucleotide 2260 of fphch. This artificial allele is similar to the het-cOR allele of N. crassa. We transformed protoplasts of ITEM 2287 with this allele and with the cloned wild-type allele. The foreign DNA in the transformants integrated ectopically, so the transformants had a copy of both the native allele and the artificial allele. In N. crassa, strains that carry copies of two different functional alleles at a het/vic locus are poorly viable, if they are viable at all. In F. proliferatum, the numbers of transformants detected were similar whether the wild-type allele or the artificial allele was used in the transformation process. In a similar experiment with N. crassa, transforming a wild-type strain with a construct containing the het-c allele present in the strain resulted in no loss of transformant viability, but transformation with a construct that carried a different allele resulted in a precipitous decrease in the number of viable transformants (33). Since the artificial allele of fphch did not reduce the number of viable transformants in F. proliferatum, we concluded that the artificial allele lacks het/vic activity and that fphch does not function as a vic locus in F. proliferatum.
DISCUSSION
We cloned and sequenced a homologue of the het-c gene of N. crassa from F. proliferatum, fphch. In Neurospora and several closely related species (39), there are at least three functional alleles whose protein products interact, resulting in the death of cells that express different alleles. The homologous protein in P. anserina does not function in vegetative incompatibility interactions (32).
Based on our results, fphch, does not have a vegetative incompatibility function in F. proliferatum. The DNA sequences of this gene from 76 strains of F. proliferatum and from the mating type tester strains of five other species in the G. fujikuroi species complex were all similar, and the observed sequence changes were all silent in terms of changes in the encoded amino acid sequence. Such homogeneity is expected if FPHCH has an important role in the cell but not if it has acquired a vegetative compatibility function.
The N. crassa het-c gene encodes a nonessential glycine-rich cell wall protein similar to structural cell envelope proteins known from a number of organisms (34). In F. proliferatum this protein is not required for normal vegetative growth, at least under laboratory conditions. It also does not affect the ability of a strain to function as a male parent in a sexual cross. As female parents in a cross, Δfphch mutants produce ∼10% of the perithecia produced by the wild-type strain from which they were derived, and they may be effectively even less fertile as the perithecia that are produced are produced later than those produced by the wild-type parent. FPHCH could affect membrane composition and/or the cytoskeletal organization that is required for formation and differentiation of sexual structures in this fungus. The lack of variation in the sequence of fphch among the various field isolates of the G. fujikuroi species complex suggests that mutations that inactivate this gene in field populations are not common. fphch could be one of the numerous genes at which mutations may occur in field populations of F. proliferatum that can reduce the overall female fertility of the population (21).
The HET-C proteins are thought to form a highly ordered complex structure in the cell wall of N. crassa. Vegetative incompatibility is thought to result when this precisely organized structure is disrupted by the presence of more than one type of HET-C protein within it. The resulting cellular abnormality then triggers the death process observed in heterokaryotic cells (33). We mimicked this reaction in F. proliferatum by constructing an artificial allele whose sequence was similar to the sequence of a second functional het-c allele known in N. crassa. Transformation of a wild-type strain with this artificial allele did not reduce the transformation frequency, as would be expected if a vegetative incompatibility interaction were occurring. This type of interaction is insensitive to the self-incompatibility phenotype of the transformed strain. Thus, the disturbance that results in vegetative compatibility in N. crassa does not occur in F. proliferatum.
Given the variety of proteins known to have a vegetative incompatibility function (10, 11, 29, 34, 36, 37, 38), the lack of a vegetative incompatibility response by cells carrying the native and artificial alleles of fphch is particularly important. This result suggests that there are additional proteins or other molecules involved in sending the vegetative incompatibility signal (e.g., pin-c [15]) and that something more than just a disturbance in cellular metabolism is necessary to send the signals that result in cell death. It also indicates that the signals that result in vegetative incompatibility may vary by species or groups of species and need not be evolutionarily conserved. Thus, different proteins may have acquired vegetative incompatibility functions at various stages of the evolutionary process. In a practical sense, vegetative compatibility must be evaluated for individual species or groups of species and cannot be assumed a priori to be functional across a broad range of species. This type of variation might be exploited to develop antifungal agents that could selectively trigger cell death in some fungi but not in others.
Vegetative incompatibility in fungi remains an interesting phenomenon whose molecular basis is not well understood. Its effect is to prevent the formation of a stable heterokaryon, but beyond that it is not clear what the genes that result in this phenotype share other than the phenotype itself. Vegetative compatibility and sexual mating are the two primary ways in which the fungi interact with members of their own species, and there is some evidence that functions downstream of the initial interaction have similar activities in both interactions (e.g., vib-1) (40, 41). From the genes already identified and cloned from Neurospora and Podospora it was clear that diverse proteins could acquire vegetative compatibility functions, which is quite different from the situation with respect to mating type, in which the same genes are broadly conserved across many ascomycete fungi (22). Our results from this study suggest that proteins with a vegetative compatibility function need not have this function in all settings even if different and putatively functional forms of the protein are present. The common theme that allows these various proteins to induce vegetative compatibility in different systems still must be identified, and both model organisms and organisms that have not been so intensively studied will have important roles to play in this identification process.
Acknowledgments
This research was supported in part by Hungarian State Research grants OTKA T 38423 and TS 44778 and by the Kansas Agricultural Experiment Station. Z.K. was the recipient of Bolyai Janos research fellowship BO/00166/02.
Footnotes
Contribution no. 06-324-J from the Kansas Agricultural Experiment Station, Manhattan.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Millwer, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bégueret, J., B. Turcq, and C. Clavé. 1994. Vegetative incompatibility in filamentous fungi: het genes begin to talk. Trends Genet. 10:441-446. [DOI] [PubMed] [Google Scholar]
- 3.Cappellini, R., and J. L. Peterson. 1965. Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 57:962-966. [Google Scholar]
- 4.Correll, J. C., C. J. R. Klittich, and J. F. Leslie. 1987. Nitrate nonutilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77:1640-1646. [Google Scholar]
- 5.Correll, J. C., C. J. R. Klittich, and J. F. Leslie. 1989. Heterokaryon self-incompatibility in Gibberella fujikuroi (Fusarium moniliforme). Mycol. Res. 93:21-27. [Google Scholar]
- 6.Cortesi, P., and M. G. Milgroom. 1998. Genetics of vegetative incompatibility in Cryphonectria parasitica. Appl. Environ. Microbiol. 64:2988-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coustou, V., C. Deleu, S. Saupe, and J. Bégueret. 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. USA 94:9773-9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft, J. H., and J. L. Jinks. 1977. Aspects of the population genetics of Aspergillus nidulans, p. 339-360. In J. E. Smith and J. A. Pateman (ed.), Genetics and physiology of Aspergillus. Academic Press, New York, N.Y.
- 9.Desjardins, A. E. 2006. Fusarium mycotoxins: chemistry, genetics and biology. APS Press, St. Paul, Minn.
- 10.Espagne, E., P. Balhadére, M. L. Penin, C. Barreau, and B. Turcq. 2002. HET-E and HET-D belong to a new subfamily of WD40 proteins involved in vegetative incompatibility specificity in the fungus Podospora anserina. Genetics 161:71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass, N. L., J. Grotelueschen, and R. L. Metzenberg. 1990. Neurospora crassa A mating type region. Proc. Natl. Acad. Sci. USA 87:4912-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass, N. L., and G. A. Kuldau. 1992. Mating type and vegetative incompatibility in filamentous ascomycetes. Annu. Rev. Phytopathol. 30:201-224. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson, D. J., and T. R. Gordon. 1988. Vegetative compatibility and self-incompatibility within Fusarium oxysporum f. sp. melonis. Phytopathology 78:668-672. [Google Scholar]
- 14.Jeney, A., E. Béki, J. Mulé, and L. Hornok. 2004. Identification of growth stage specific transcript profiles in Fusarium proliferatum (Gibberella fujikuroi, mating population D) by cDNA-AFLP analysis. Eur. J. Plant Pathol. 110:619-625. [Google Scholar]
- 15.Kaneko, I., K. Dementhon, Q. Xiang, and N. L. Glass. 2006. Nonallelic interactions between het-c and a polymorphic locus, pin-c, are essential for nonself recognition and programmed cell death in Neurospora crassa. Genetics 172:1545-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerényi, Z., G. Mulé, A. Moretti, C. Waalwijk, and L. Hornok. 2002. Fertility and mating type assessment within Fusarium proliferatum isolates from different host plants. J. Appl. Genet. 43:55-68.12084971 [Google Scholar]
- 17.Kerényi, Z., É. Táborhegyi, A. Pomázi, and L. Hornok. 1997. Variability amongst strains of Fusarium poae assessed by vegetative compatibility and RAPD polymorphism. Plant Pathol. 46:882-889. [Google Scholar]
- 18.Kerényi, Z., K. Zeller, L. Hornok, and J. F. Leslie. 1999. Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 65:4071-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klittich, C. J. R., and J. F. Leslie. 1988. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 118:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie, J. F. 1993. Fungal vegetative compatibility. Annu. Rev. Phytopathol. 31:127-150. [DOI] [PubMed] [Google Scholar]
- 21.Leslie, J. F., and K. K. Klein. 1996. Female fertility and mating-type effects on effective population size and evolution in filamentous fungi. Genetics 144:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie, J. F., and B. A. Summerell. 2006. The Fusarium laboratory manual. Blackwell Professional, Ames, Iowa.
- 23.Leslie, J. F., and K. Zeller. 1997. Mutants that blur the line between biological species and vegetative compatibility groups. Cereal Res. Commun. 25:539-542. [Google Scholar]
- 24.Proctor, R. H., T. M. Hohn, and S. P. McCormick. 1997. Restoration of wild-type virulence to Tri5 disruption mutants of Gibberella zeae via gene reversion and mutant complementation. Microbiology 143:2583-2591. [DOI] [PubMed] [Google Scholar]
- 25.Puhalla, J. E., and P. T. Spieth. 1983. Heterokaryosis in Fusarium moniliforme. Exp. Mycol. 7:328-335. [Google Scholar]
- 26.Puhalla, J. E., and P. T. Spieth. 1985. A comparison of heterokaryosis and vegetative incompatibility among varieties of Gibberella fujikuroi (Fusarium moniliforme). Exp. Mycol. 9:39-47. [Google Scholar]
- 27.Punt, P. J., R. P. Oliver, M. A. Dingemanse, P. H. Pouwels, and C. A. M. J. J. van den Hondel. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117-124. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, S., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Saupe, S., C. Descamps, B. Turcq, and J. Bégueret. 1994. Inactivation of the Podospora anserina vegetative incompatibility locus het-c, whose product resembles a glycolipid transfer protein, drastically impairs ascospore production. Proc. Natl. Acad. Sci. USA 91:5927-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saupe, S., B. Turcq, and J. Bégueret. 1995. A gene responsible for vegetative incompatibility in the fungus Podospora anserina encodes a protein with GTP-binding motif and Gβ homologous domain. Gene 162:135-139. [DOI] [PubMed] [Google Scholar]
- 31.Saupe, S. J., C. Clavé, and J. Bégueret. 2000. Vegetative incompatibility in filamentous fungi: Podospora and Neurospora provide some clues. Curr. Opin. Microbiol. 3:608-612. [DOI] [PubMed] [Google Scholar]
- 32.Saupe, S. J., C. Clavé, M. Sabourin, and J. Bégueret. 2000. Characterization of hch, the Podospora anserina homolog of the het-c heterokaryon incompatibility gene of Neurospora crassa. Curr. Genet. 38:39-47. [DOI] [PubMed] [Google Scholar]
- 33.Saupe, S. J., and N. L. Glass. 1997. Allelic specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa is determined by a highly variable domain. Genetics 146:1299-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saupe, S. J., G. A. Kuldau, M. L. Smith, and N. L. Glass. 1996. The product of the het-c heterokaryon incompatibility gene of Neurospora crassa has characteristics of a glycine-rich cell wall protein. Genetics 143:1589-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, M. L., S. P. Hubbard, D. J. Jacobson, O. C. Micali, and N. L. Glass. 2000. An osmotic-remedial, temperature-sensitive mutation in allosteric activity site of ribonucleotide reductase in Neurospora crassa. Mol. Gen. Genet. 262:1022-1035. [DOI] [PubMed] [Google Scholar]
- 36.Smith, M. L., O. C. Micali, S. P. Hubbard, N. Mir-Rashed, D. J. Jacobson, and N. L. Glass. 2000. Vegetative incompatibility activity in the het-6 region of Neurospora crassa is mediated by two linked genes. Genetics 155:1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staben, C., and C. Yanofsky. 1990. Neurospora crassa a mating type region. Proc. Natl. Acad. Sci. USA 87:4917-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turcq, B., M. Denayrolles, and J. Bégueret. 1990. Isolation of the two allelic incompatibility genes s and S of the fungus Podospora anserina. Curr. Genet. 17:297-303. [Google Scholar]
- 39.Wu, J., S. J. Saupe, and N. L. Glass. 1998. Evidence for balancing selection operating at the het-c heterokaryon incompatibility locus in a group of filamentous fungi. Proc. Natl. Acad. Sci. USA 95:12398-12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang, Q., and N. L. Glass. 2002. Identification of vib-1, a locus involved in vegetative incompatibility mediated by het-c in Neurospora crassa. Genetics 162:89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang, Q., and N. L. Glass. 2004. The control of mating type heterokaryon incompatibility by vib-1, a locus involved in het-c heterokaryon incompatibility in Neurospora crassa. Fungal Genet. Biol. 41:1063-1076. [DOI] [PubMed] [Google Scholar]