Abstract
Male-killing phenotypes are found in a variety of insects and are often associated with maternally inherited endosymbiotic bacteria. In several species of Drosophila, male-killing endosymbionts of the genus Spiroplasma have been found at low frequencies (0.1 to 3%). In this study, spiroplasma infection without causing male-killing was shown to be prevalent (23 to 66%) in Japanese populations of Drosophila hydei. Molecular phylogenetic analyses showed that D. hydei was infected with a single strain of spiroplasma, which was closely related to male-killing spiroplasmas from other Drosophila species. Artificial-transfer experiments suggested that the spiroplasma genotype rather than the host genotype was responsible for the absence of the male-killing phenotype. Infection densities of the spiroplasma in the natural host, D. hydei, and in the artificial host, Drosophila melanogaster, were significantly lower than those of the male-killing spiroplasma NSRO, which was in accordance with the hypothesis that a threshold infection density is needed for the spiroplasma-induced male-killing expression.
Incidences of male-specific death at early developmental stages, called male killing, have been found in a variety of insects, such as ladybird beetles, fruit flies, butterflies, and moths (16). In many of these cases, the causal agents have been identified as maternally inherited endosymbiotic bacteria, such as Spiroplasma, Rickettsia, Wolbachia, and Arsenophonus (18).
Members of the genus Spiroplasma, belonging to the class Mollicutes, are spiral, wall-less, actively motile, very small (0.1 to 0.2 μm thick and 4 to 5 μm long), and associated with plants and arthropods. For instance, Spiroplasma citri, Spiroplasma phoeniceum, and Spiroplasma kunkelii are known as plant pathogens transmitted by insect vectors; Spiroplasma apis and Spiroplasma melliferum as pathogens of the honeybee; and Spiroplasma poulsonii as a maternally inherited male killer in Drosophila fruit flies (44).
In Central and South American countries, S. poulsonii and its relatives with a male-killing phenotype have been found in the Drosophila willistoni species group, including D. willistoni, Drosophila nebulosa, Drosophila equinoxialis, and Drosophila paulistorum, and have been called by the acronyms WSRO (from willistoni sex ratio organism), NSRO, ESRO, and PSRO, respectively (45). Recently, a male-killing spiroplasma that was indistinguishable from NSRO on the basis of several gene sequences was found in Drosophila melanogaster in Brazil and designated MSRO (27, 28). Infection frequencies of the male-killing spiroplasmas are generally low: 0.1 to 3% in the D. willistoni species group (45) and 2.3% in D. melanogaster (28).
In a Japanese population of Drosophila hydei, Ota et al. (31) microscopically observed a high frequency (46%) of spiroplasma infection without the male-killing phenotype. To date, this case has been the only report of a non-male-killing spiroplasma occurring in natural Drosophila populations. It was of great interest to discover whether the high frequency of spiroplasma infection had been maintained in the D. hydei population, what spiroplasma strain is associated with D. hydei, and whether the non-male-killing spiroplasma is phylogenetically related to the male-killing spiroplasmas, like WSRO, NSRO, and MSRO.
In this study, we found that the non-male-killing spiroplasma is still prevalent in Japanese populations of D. hydei, even after 27 years. By using diagnostic PCR detection, molecular phylogenetic analysis, artificial symbiont transfer, and quantitative PCR techniques, we investigated the following aspects of the non-male-killing spiroplasma associated with D. hydei: (i) infection frequencies among host populations; (ii) phylogenetic position in the genus Spiroplasma; (iii) relationship to the male-killing spiroplasmas from other Drosophila species; (iv) inability to cause male killing, not only in D. hydei but also in D. melanogaster; and (v) infection dynamics during host development and aging.
MATERIALS AND METHODS
Fly collection.
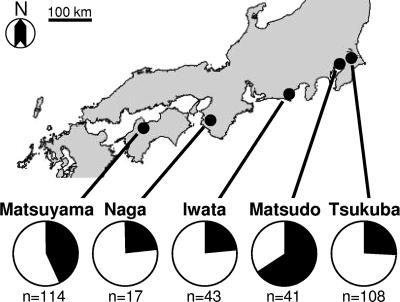
Adult D. hydei insects were collected by standard bait traps with fresh bananas and dried yeast at Tsukuba (Ibaraki Prefecture), Matsudo (Chiba Prefecture), Iwata (Shizuoka Prefecture), Naga (Wakayama Prefecture), and Matsuyama (Ehime Prefecture) from May to July 2005 (Fig. 1). The wild-caught females were individually allowed to lay eggs on a standard cornmeal medium. After a week, the ovaries were dissected from each of the females and used for DNA extraction. The flies were reared at 25°C under a long-day regimen (16 h of light and 8 h of darkness).
FIG. 1.
Collection sites of D. hydei in Japan and the frequencies of spiroplasma-infected individuals among collected females. In the pie graphs, black indicates the proportions of infected individuals, and white indicates those of uninfected individuals. The numbers of females collected are shown under each of the pie graphs.
Diagnostic PCR detection of endosymbiotic bacteria.
DNA was extracted from the dissected ovaries by macerating them in 200 μl of a squishing buffer (10 mM Tris-HCl [pH 8.2], 1 mM EDTA, 25 mM NaCl). To this was added 200 μg of proteinase K, and the samples were incubated at 55°C for 30 min. The samples were then heated to 95°C for 10 min to inactivate the proteinase K and centrifuged for 2 min at 10,000 × g. The supernatants were subjected to diagnostic PCR detection of Spiroplasma, Wolbachia, Cardinium, and Rickettsia. The following specific primers were used: SpoulF (5′-GCTTAACTCCAGTTCGCC-3′) and SpoulR (5′-CCTGTCTCAATGTTAACCTC-3′) for the 16S rRNA gene of Spiroplasma (27), wsp81F (5′-TGGTCCAATAAGTGATGAAGAAAC-3′) and wsp691R (5′-AAAAATTAAACGCTACTCCA-3′) for the wsp gene of Wolbachia (49), Ch-F (5′-TACTGTAAGAATAAGCACCGGC-3′) and Ch-R (5′-GTGGATCACTTAACGCTTTCG-3′) for the 16S rRNA gene of Cardinium (48), and Rb-F (5′-GCTCAGAACGAACGCTATC-3′) and Rb-R (5′-GAAGGAAAGCATCTCTGC-3′) for the 16S rRNA gene of Rickettsia (designed by E. Zchori-Fein). The following DNA samples were used as positive and negative controls for specific PCR detection: the fruit fly D. melanogaster infected with Spiroplasma (1), the fruit fly Drosophila simulans infected with Wolbachia (collected in Tsukuba by D.K.), the false spider mite Brevipalpus californicus infected with Cardinium (6), and the pea aphid Acyrthosiphon pisum infected with Rickettsia (37).
Cloning and sequencing.
From the whole-insect DNA of D. hydei, almost the entire length of the bacterial 16S rRNA gene (about 1.5 kb) was amplified by PCR with the primers 16SA1 (5′-AGAGTTTGATCMTGGCTCAG-3′) and 16SB1 (5′-TACGGYTACCTTGTTACGACTT-3′) (14) under the temperature profile of 94°C for 10 min, followed by 35 cycles consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The PCR product was electrophoresed in an agarose gel, excised, purified by using a Gel Extraction Kit (QIAGEN, Japan), and cloned with the TA cloning vector pT7Blue (Novagen, Darmstadt, Germany) and Escherichia coli DH5α competent cells (Takara, Japan), in which ampicillin and X-Gal (5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside) were used for the blue-white selection system. Plasmids were purified by using a MiniElute kit (QIAGEN). Sequencing reactions were performed by using the plasmids as template DNA with a GenomeLab sequencing kit (Beckman Coulter, Japan). Sequencing analyses were performed with a CEQ2000XL Sequencer (Beckman Coulter). The following sequencing primers were used: M13M4 (5′-GTTTTCCCAGTCACGAC-3′) and M13RV (5′-CAGGAAACAGCTATGAC-3′) in the flanking regions of the vector and internal primers 16SA2 (5′-GTGCCAGCAGCCGCGGTAATAC-3′), 16SA3 (5′-TGCATGGYTGTCGTCAGCTCG-3′), 16SB2 (5′-CGAGCTGACGACARCCATGCA-3′), and 16SB3 (5′-GTATTACCGCGGCTGCTGGCAC-3′) (14). The 16S rRNA gene from the male-killing spiroplasma NSRO was also sequenced for reconstruction of the phylogenetic tree. In addition, the following protein-coding genes were amplified by PCR, cloned, and sequenced: the P58 gene, a gene sequence similar to the putative adhesin P58 gene (47), was amplified by the primers p58-f (5′-GTTGGTTGAATAATATCTGTTG-3′) and p58-r (5′-GATGGTGCTAAATTATATTGAC-3′) (28); spoT, a gene sequence similar to that of the (p)ppGpp 39-pyrophosphohydrolase spoT gene (20), was amplified by the primers spoT-f (5′-CAAACAAAAGGACAAATTGAAG-3′) and spoT-r (5′-CACTGAAGCGTTTAAATGAC-3′) (28); and a partial sequence of the dnaA gene was amplified by the primers SRdnaAF1 (5′-GGAGAYTCTGGAYTAGGAAA-3′) and SRdnaAR1 (5′-CCYTCTAWYTTTCTRACATCA-3′) (1).
Phylogenetic analysis.
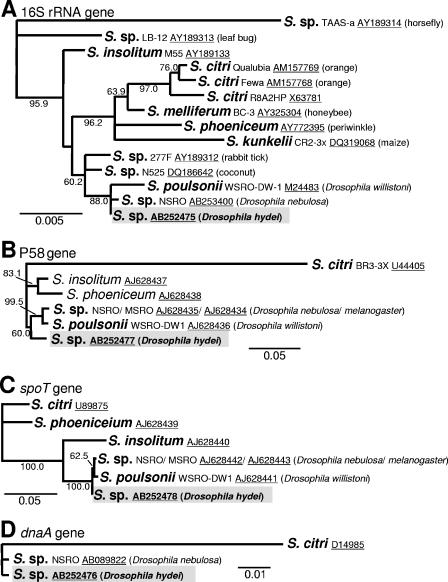
Nucleotide sequences were aligned by using the CLUSTAL W version 1.83 software (39). Maximum-parsimony trees and neighbor-joining trees were constructed by using the program package PHYLIP version 3.63 (11). Bootstrap resampling was performed with 1,000 replicates. DDBJ/EMBL/GenBank accession numbers of Spiroplasma genes used for phylogenetic reconstruction are indicated in Fig. 2.
FIG. 2.
Phylogenetic position of the spiroplasma of D. hydei (shaded) in the genus Spiroplasma. (A) One of the four most parsimonious trees inferred from 16S rRNA gene sequences (1,447 aligned nucleotide sites), which has the same topology as the neighbor-joining tree. S., Spiroplasma. (B) The most parsimonious tree inferred from P58 gene sequences (787 sites). (C) The most parsimonious tree inferred from spoT gene sequences (513 sites). (D) The most parsimonious tree inferred from dnaA gene sequences (474 sites). Bootstrap probabilities are shown at the nodes. Branches supported by bootstrap probabilities of less than 50% were collapsed.
Hemolymph transfer.
The non-male-killing spiroplasma of D. hydei (line TKB163) was transferred to D. melanogaster (strain Oregon-R) by hemolymph injection. Collection and injection of spiroplasma-laden hemolymph were conducted under a dissecting microscope with thin glass capillary tubes made by a microelectrode maker (PN-3; Narishige, Tokyo, Japan). For capillary collection of hemolymph, the tip was inserted into the thoraxes of adult donor flies anesthetized on ice. Approximately 0.1 μl (each) of the spiroplasma-laden hemolymph was injected by using an air pump into the thoraxes of adult recipient flies anesthetized on ice. The offspring of the injected flies were examined for spiroplasma infection by diagnostic PCR. To estimate the density of spiroplasma, G2 progeny after transfection were subjected to quantitative PCR.
Quantitative PCR.
To collect insect samples at different developmental stages, 20 adult females and 10 adult males were reared in each plastic vial with the cornmeal medium, from which first-, second-, and third-instar larvae and pupae were harvested. Newly emerged adult flies were transferred daily to new plastic vials with the cornmeal medium and harvested 0, 7, 14, 21, 28, and 35 days after emergence. These samples were individually subjected to DNA extraction by using a NucleoSpin Tissue kit (Macherey-Nagel, Düren, Germany). Real-time fluorescence detection quantitative PCR was performed by using SYBR Green and the Mx3000P QPCR System (Stratagene, La Jolla, CA). As an index of the titer of the spiroplasmas, the copy number of the dnaA gene was determined by using the primers dnaA109F (5′-TTAAGAGCAGTTTCAAAATCGGG-3′) and dnaA246R (5′-TGAAAAAAACAAACAAATTGTTATTACTTC-3′) under the temperature profile of 35 cycles of 95°C for 30 s, 55°C for 1 min, and 72°C for 30 s. To estimate the density of the spiroplasmas, the copy number of the host mitochondrial cytochrome c oxidase subunit II (COII) gene was determined for the same samples by using the primers COII-489f (5′-CATGAACAATTCCCGCTTTAGG-3′) and COII-590r (5′-ATTGTCCATAAAATAATCCTGGGC-3′) under the same temperature profile.
To compare the densities of spiroplasmas in different host species during adult aging, the numbers of spiroplasma dnaA copies were divided by the average body weight of adult flies. The body weight values of 10 individuals (mean ± standard deviation) measured with five replicates were 30 ± 0.07 mg for D. hydei females, 22.8 ± 0.84 mg for D. hydei males, 13.2 ± 0.48 mg for D. melanogaster females, and 8.6 ± 0.32 mg for D. melanogaster males. For standardization, we adopted the body weight values of 3.0 mg and 2.3 mg for D. hydei females and males and 1.3 mg and 0.86 mg for D. melanogaster females and males. Note that the body weights of adult flies vary between individuals and during aging, so standardization on the basis of the mean body weight values is approximate.
Statistical analysis.
The spiroplasma density data were subjected to statistical analyses by using the software R version 2.2.0 (33). Multiple comparison was performed with Bonferroni corrections. Since some of the data sets did not exhibit normal distribution and/or homogeneous variance, we adopted the generalized linear model (25) with a normal, gamma, or negative binomial distribution, which was selected according to the Akaike information criterion.
RESULTS
Infection frequencies of Spiroplasma sp. in natural populations of D. hydei.
In the wild-caught D. hydei individuals examined in this study, neither Wolbachia, Cardinium, nor Rickettsia was detected by diagnostic PCR, while Spiroplasma was detected at high frequencies: 28/108 (26%) in Tsukuba, 27/41 (66%) in Matsudo, 10/43 (23%) in Iwata, 4/17 (24%) in Naga, and 49/114 (43%) in Matsuyama, Japan (Fig. 1).
Removal of spiroplasma infection from D. hydei by antibiotic treatment.
Five spiroplasma-positive lines of D. hydei (i.e., one line from each of the five populations) were reared with the cornmeal medium containing tetracycline (0.2 mg/ml) for two generations and subsequently checked by diagnostic PCR. All the treated lines were diagnosed as spiroplasma negative, confirming that the PCR-based detection of spiroplasma reflected bacterial infection.
Infection with a single strain of spiroplasma in D. hydei.
The nucleotide sequences of the bacterial dnaA genes (474 bp) from 10 D. hydei individuals (i.e., 2 individuals from each of the five populations) were completely identical to each other. Likewise, the nucleotide sequences of the P58 genes (946 bp) and the spoT genes (555 bp) from five individuals (i.e., one individual from each of the five populations) were identical to each other. These results strongly suggested that a single strain of spiroplasma prevails in the Japanese populations of D. hydei.
Phylogenetic position of the spiroplasma from D. hydei.
Molecular phylogenetic trees based on 16S rRNA (1,447 bp), P58 (787 bp), spoT (513 bp), and dnaA (474 bp) gene sequences consistently indicated that the spiroplasma from D. hydei is closely related to the male-killing spiroplasmas from D. willistoni (WSRO), D. nebulosa (NSRO), and D. melanogaster (MSRO) (Fig. 2). Monophyly of the male-killing spiroplasmas was strongly supported by the P58 gene sequences but only weakly supported by the spoT sequences. On the other hand, monophyly of the male-killing spiroplasmas plus the non-male-killing spiroplasma from D. hydei was strongly supported by the 16S rRNA and spoT sequences but only weakly supported by the P58 gene sequences (Fig. 2).
Artificial transfer of the spiroplasma into D. melanogaster.
Interspecific transfer of the non-male-killing spiroplasma from D. hydei to D. melanogaster reproducibly established a heritable infection in the recipient flies, in which the spiroplasma infection did not cause male killing (Table 1). The infection in D. melanogaster was stably maintained for two generations after injection, but although the reason was obscure, the fidelity of vertical transmission drastically dropped at the third generation (Table 1). In each of the generations, both the infected males and females had a remarkably shorter adult life span, and about half of them died within a week after emergence (data not shown). The infected females also suffered remarkably reduced fecundity in comparison with uninfected females (data not shown).
TABLE 1.
Interspecific transfer of the non-male-killing spiroplasma from D. hydei to D. melanogastera
| Injection set | No. emerged (no. positive)b in generation:
|
|||||
|---|---|---|---|---|---|---|
| G1
|
G2
|
G3
|
||||
| Females | Males | Females | Males | Females | Males | |
| 1 | 9 (3/3) | 5 | ||||
| 2 | 6 (3/3) | 10 | 16 (2/2) | 28 | 4 (0/2) | 5 |
| 11 (0/8) | 9 (0/1) | |||||
| 3 | 17 (3/3) | 21 | 5 | 12 | ||
| 17 | 12 | |||||
| 4 | 33 (3/3) | 28 | 14 | 25 | ||
| 5 | 18 (3/3) | 23 | 16 (1/1) | 27 | ||
| 24 (9/9) | 29 | |||||
| 49 (8/8) | 43 | 11 (0/4) | 14 (5/5) | |||
| 18 (1/5) | 11 (3/5) | |||||
| 6 | 13 (3/3) | 15 | 11 | 14 | ||
| 20 | 31 | |||||
Hemolymph of the spiroplasma-infected strain of D. hydei (TKB163) was injected into adult females of the uninfected strain of D. melanogaster (Oregon-R), and their offspring were examined. In each of the generation columns, a brood produced by a single female is indicated in a single line.
For example, 9 (3/3) indicates that nine female adults emerged in the isofemale line and three of three adult females examined by diagnostic PCR were spiroplasma positive.
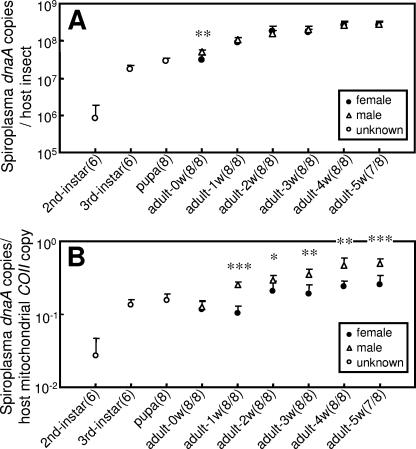
Infection dynamics of the non-male-killing spiroplasma during the development of D. hydei.
The infection titers of the spiroplasma in terms of dnaA copies per insect steadily increased during larval development and adult aging. The infection titers were at similar levels in adult males and females (Fig. 3A). Infection densities of the spiroplasma in terms of dnaA copies per COII copy rapidly increased during larval development, reached a plateau at the third-instar larval and pupal stages, and gradually increased during adult aging. In adult insects, except those newly emerged, the infection densities were significantly higher in males than in females (Fig. 3B), which was probably due to the larger body size of mature females.
FIG. 3.
Infection dynamics of the spiroplasma during the developmental course of D. hydei strain TKB163. (A) Infection titers of the spiroplasma in terms of symbiont dnaA gene copies per insect. (B) Infection densities of the spiroplasma in terms of symbiont dnaA copies per host COII gene copy. Filled circles, adult females; open triangles, adult males; open circles, larvae and pupae without sexing. Mean values and standard deviations are shown. Sample sizes (female/male) are given in parentheses. The asterisks indicate statistically significant differences between males and females (Mann-Whitney U test: *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Infection dynamics of the non-male-killing spiroplasma in the artificial host, D. melanogaster.
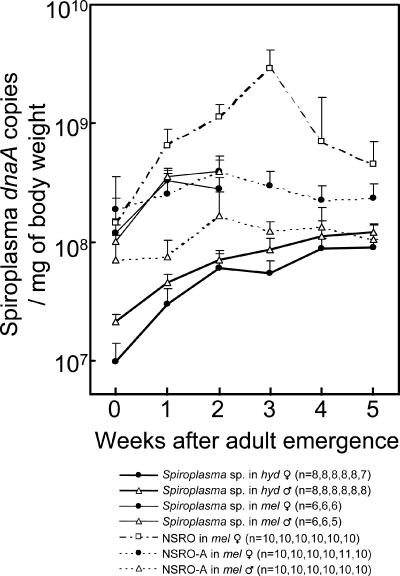
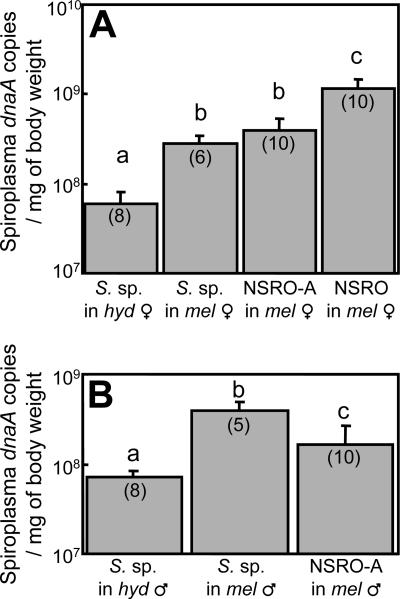
Figure 4 shows the infection densities of the spiroplasmas during host aging. In the artificially transfected strain of D. melanogaster, infection densities of the spiroplasma were consistently higher than those in the original strain of D. hydei. In the same Oregon-R genetic background of D. melanogaster, the infection densities of the spiroplasma were lower than those of the male-killing spiroplasma NSRO and were at levels similar to those of the non-male-killing spiroplasma NSRO-A (1). These differences in infection densities were statistically significant among 2-week-old flies (Fig. 5) and also among 1-week-old flies (data not shown).
FIG. 4.
Infection dynamics of the spiroplasma during adult host aging before and after transfection from the D. hydei (hyd) strain TKB163 into the D. melanogaster (mel) strain Oregon-R (solid lines). In addition, the infection dynamics data of the male-killing spiroplasma NSRO and the non-male-killing spiroplasma NSRO-A in the same Oregon-R genetic background, taken from Anbutsu and Fukatsu (1), are superimposed (dashed lines). Infection titers of the spiroplasmas are shown by mean values and standard deviations in terms of symbiont dnaA gene copies per mg of host body weight. Sample sizes are given in parentheses. Note that the spiroplasma densities in the transfected D. melanogaster strain are shown up to 2 weeks after adult emergence because of the high mortality of the fly strain.
FIG. 5.
Comparison of the infection densities of the male-killing and non-male-killing spiroplasmas in 2-week-old adult D. hydei (hyd) and D. melanogaster (mel) insects. (A) Females. (B) Males. Sample sizes are given in parentheses. Different letters indicate statistically significant differences (generalized linear model; P < 0.05 after Bonferroni correction). The error bars represent standard deviations.
DISCUSSION
In 1978, infection with the non-male-killing spiroplasma was detected in 51 of 111 individuals (45.9%) in a population of D. hydei (31). After 27 years, we observed similar levels of spiroplasma infection (23.3 to 65.9%) in five different populations of D. hydei across mainland Japan, suggesting that the spiroplasma infection is ubiquitous and stably maintained in Japanese populations of D. hydei (Fig. 1). Male-killing spiroplasmas have been reported in many Drosophila species (45), but to date, the infection in D. hydei is the only case of a naturally occurring spiroplasma that does not cause male killing in the Drosophila host. However, it is unclear whether male-killing spiroplasmas are more common in Drosophila species than non-male-killing spiroplasmas. It appears plausible that, without an apparent phenotype, non-male-killing endosymbionts are detected much less frequently than male-killing ones.
Male killing has been considered an evolutionarily adaptive trait for maternally transmitted endosymbionts on the ground that infected females can gain extra fitness due to reallocated resources from their killed brothers (16). How, then, can the non-male-killing spiroplasma be prevalent in natural populations of D. hydei? As argued by Ebbert (10), the high prevalence of the non-male-killing spiroplasma may call into question the advantage of the male-killing trait. There are several possible mechanisms that may be relevant to the prevalence of the symbiont. Instead of causing male killing, the spiroplasma might cause different types of reproductive phenotypes, such as the cytoplasmic incompatibility known from Wolbachia and Cardinium (3, 15). Alternatively, the spiroplasma might contribute to host fitness, acting as a mutualist of D. hydei. However, since D. hydei lives on nutrient-rich food sources, it seems unlikely that the spiroplasma provides the host with essential nutrients, as many microbial mutualists do (9). Actually, it was observed that, even when the spiroplasma infection was cured, D. hydei exhibited no remarkable changes in fecundity and survival (unpublished data). It was also observed that, among wild-caught D. hydei adults, spiroplasma-infected and uninfected insects exhibited no significant differences in wing size (unpublished data). These observations suggest that positive fitness effects of the spiroplasma infection, if they exist, are undetectable, at least under standard conditions. The stable cooccurrence of infected and uninfected individuals in sympatric populations could be realized through balancing selection for/against infected and uninfected individuals, leakage of infection due to imperfect vertical transmission, and several other processes. In the pea aphid Acyrthosiphon pisum, context-dependent positive fitness effects in association with facultative endosymbionts have been reported, including tolerance of high temperature (29), resistance to parasitoid wasps (30) and fungal parasites (38), and broadening of host plant range (40). It appears plausible that the spiroplasma infection might contribute to host fitness in such specific ecological contexts.
The spiroplasma of D. hydei caused no male killing when transfected into D. melanogaster (Table 1), which was in agreement with the results of Ota et al. (31). Williamson and Poulson (45) reported that when a male-killing spiroplasma, WSRO, was transfected from D. willistoni into D. hydei, male-killing did occur. Taking these data together, the symbiont genotype, rather than the host genotype, is responsible for the non-male-killing phenotype of the spiroplasma in D. hydei. Vertical transmission of the spiroplasma in D. melanogaster was not stable for generations (Table 1), and the transfected host insects suffered a shorter life span and reduced fecundity. Unstable vertical transmission and/or adverse effects on host fitness in association with symbiont transfection into novel hosts have also been reported in other insect-endosymbiotic systems (22, 26, 35, 41).
The infection density of the spiroplasma of D. hydei was significantly elevated when it was transfected into D. melanogaster (Fig. 4). This finding suggested that the density of the spiroplasma is regulated by the host genotype, as has been reported for Wolbachia and other insect endosymbionts (4, 19, 23, 32). The elevated infection density in the novel host may be relevant to the adverse fitness effects described above and could be interpreted in the context of the theories of host-parasite associations and the evolution of virulence (7).
The infection density of a symbiont is expected to affect the phenotypes caused by the symbiont infection, including the level of positive/negative fitness effects (2, 8, 12, 13, 34) and the intensity of reproductive manipulations (4, 5, 19, 36, 42, 43). On the basis of quantitative analyses of a male-killing spiroplasma, NSRO, and a non-male-killing spiroplasma, NSRO-A, Anbutsu and Fukatsu (1) proposed a threshold density hypothesis for the expression of male killing, which claims that NSRO can proliferate above the threshold density in the host body and thus causes male killing, whereas NSRO-A remains at a lower infection level and thus does not kill males. In this context, it is conceivable that the spiroplasma of D. hydei is potentially capable of male killing but does not kill males because its infection density is below the threshold level. In accordance with this idea, the infection densities of the non-male-killing spiroplasma in D. melanogaster were much lower than those of NSRO and almost equivalent to those of NSRO-A (Fig. 4), although it is also likely that the spiroplasma of D. hydei is unable to kill males because it is inherently incapable of male killing.
Molecular phylogenetic analyses revealed that the non-male-killing spiroplasma of D. hydei is closely related to the male-killing spiroplasmas from D. willistoni, D. nebulosa, and D. melanogaster, forming a monophyletic group in the genus Spiroplasma (Fig. 2). The phylogenetic trees on the bases of different genes consistently indicated that the spiroplasma of D. hydei is the most basal taxon of the clade (Fig. 2). As for the origin of male killing, two parsimonious scenarios can be inferred from the phylogenetic relationship. One possibility is that the common ancestor of the clade had already evolved the male-killing ability and the trait has been lost in the lineage of the spiroplasma of D. hydei. Another possibility is that the common ancestor of the clade caused no male killing and the male-killing ability evolved in the clade after the divergence of the spiroplasma of D. hydei. The observation that a non-male-killing spiroplasma (NSRO-A) spontaneously emerged from a male-killing spiroplasma (NSRO) in the laboratory (46) indicates that the male-killing trait can be lost easily, which may favor the former scenario. On the other hand, the male-killing trait has evolved independently at least twice in the genus Spiroplasma, once in group II with male killers of Drosophila flies and once in group IV with male killers of ladybird beetles and butterflies (17, 21, 24), which may favor the latter scenario. A wider survey of male-killing and non-male-killing spiroplasmas in natural populations of D. hydei and other Drosophila species would provide insights into the origin and evolution of the symbiont-induced reproductive phenotype.
Acknowledgments
We thank Brooke A. LaFlamme, Yohsuke Tagami, Satoko Narita, and Makiko Sakurai for sampling of D. hydei; Atsushi Chigira for the DNA sample of the Cardinium-infected mite Brevipalpus californicus; Wakana Kikuchi for secretarial assistance; and Satoko Tatsuno, Sachie Suo, and Noriko Totsuka for maintenance of fly strains.
This study was supported by the 21st COE Program, University of Tokyo at Komaba, Japan, and also by the Program for Promotion of Basic Research Activities for Innovation Biosciences (ProBRAIN) of the Bio-Oriented Technology Research Advancement Institution, Japan.
REFERENCES
- 1.Anbutsu, H., and T. Fukatsu. 2003. Population dynamics of male-killing and non-male-killing spiroplasmas in Drosophila melanogaster. Appl. Environ. Microbiol. 69:1428-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordenstein, S., and J. H. Werren. 2000. Do Wolbachia influence fecundity in Nasonia vitripennis? Heredity 84:54-62. [DOI] [PubMed] [Google Scholar]
- 3.Bourtzis, K., H. R. Braig, and T. L. Karr. 2003. Cytoplasmic incompatibility, p. 217-246. In K. Bourtzis and T. A. Miller (ed.), Insect symbiosis. CRC Press, New York, N.Y.
- 4.Boyle, L., S. L. O'Neill, H. M. Robertson, and T. L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796-1799. [DOI] [PubMed] [Google Scholar]
- 5.Breeuwer, J. A., and J. H. Werren. 1993. Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chigira, A., and K. Miura. 2005. Detection of ‘Candidatus Cardinium’ bacteria from the haploid host Brevipalpus californicus (Acari: Tenuipalpidae) and effect on the host. Exp. Appl. Acarol. 37:107-116. [DOI] [PubMed] [Google Scholar]
- 7.Dieckmann, U., J. A. J. Metz, M. W. Sabelis, and K. Sigmund. 2002. Adaptive dynamics of infectious diseases. Cambridge University Press, Cambridge, United Kingdom.
- 8.Dobson, S., E. Marsland, and W. Rattanadechakul. 2002. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics 160:1087-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas, A. E. 1989. Mycetocyte symbiosis in insects. Biol. Rev. 64:409-434. [DOI] [PubMed] [Google Scholar]
- 10.Ebbert, M. A. 1995. Variable effects of crowding on Drosophila hosts of male-lethal and non-male-lethal spiroplasmas in laboratory populations. Heredity 74:227-240. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 2005. PHYLIP (Phylogeny Inference Package) version 3.6. Department of Genome Sciences, University of Washington, Seattle. [Online.] http://evolution.gs.washington.edu/phylip.html.
- 12.Fleury, F., F. Vavre, N. Ris, P. Fouillet, and M. Bouletreau. 2000. Physiological cost induced by the maternally-transmitted endosymbiont Wolbachia in the Drosophila parasitoid Leptopilina heterotoma. Parasitology 5:493-500. [DOI] [PubMed] [Google Scholar]
- 13.Fry, A., and D. Rand. 2002. Wolbachia interactions that determine Drosophila melanogaster survival. Evolution 56:1976-1981. [DOI] [PubMed] [Google Scholar]
- 14.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter, M. S., S. J. Perlman, and S. E. Kelly. 2003. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. Lond. B 270:2185-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst, G. D. D., and M. E. N. Majerus. 1993. Why do maternally inherited microorganisms kill males? Heredity 71:81-95. [Google Scholar]
- 17.Hurst, G. D., J. H. Graf von der Schulenburg, T. M. Majerus, D. Bertrand, I. A. Zakharov, J. Baungaard, W. Volkl, R. Stouthamer, and M. E. Majerus. 1999. Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol. Biol. 8:133-139. [DOI] [PubMed] [Google Scholar]
- 18.Hurst, G. D. D., F. M. Jiggins, and M. E. N. Majerus. 2003. Inherited microorganisms that selectively kill male hosts: the hidden players of insect evolution?, p. 177-197. In K. Bourtzis and T. A. Miller (ed.), Insect symbiosis. CRC Press, New York, N.Y.
- 19.Ikeda, T., H. Ishikawa, and T. Sasaki. 2003. Infection density of Wolbachia and level of cytoplasmic incompatibility in the Mediterranean flour moth, Ephestia kuehniella. J. Invertebr. Pathol. 84:1-5. [DOI] [PubMed] [Google Scholar]
- 20.Jacob, C., F. Nouzieres, S. Duret, J. M. Bove, and J. Renaudin. 1997. Isolation, characterization, and complementation of a motility mutant of Spiroplasma citri. J. Bacteriol. 179:4802-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiggins, F. M., G. D. Hurst, C. D. Jiggins, J. H. Graf von der Schulenburg, and M. E. Majerus. 2000. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 120:439-446. [DOI] [PubMed] [Google Scholar]
- 22.Koga, R., T. Tsuchida, and T. Fukatsu. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. Lond. B 270:2543-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo, N., M. Shimada, and T. Fukatsu. 2005. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol. Lett. 1:488-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majerus, T. M., J. H. Graf von der Schulenburg, M. E. Majerus, and G. D. Hurst. 1999. Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect. Mol. Biol. 8:551-555. [DOI] [PubMed] [Google Scholar]
- 25.McCullagh, P., and J. A. Nelder. 1989. Generalized linear models, 2nd ed. Chapman and Hall, London, United Kingdom.
- 26.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montenegro, H., W. N. Souza, D. L. Leite, and L. B. Klaczko. 2000. Male killing selfish cytoplasmic element causes sex-ratio distortion in Drosophila melanogaster. Heredity 85:465-470. [DOI] [PubMed] [Google Scholar]
- 28.Montenegro, H., V. N. Solferini, L. B. Klaczko, and G. D. D. Hurst. 2005. Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol. Biol. 14:281-287. [DOI] [PubMed] [Google Scholar]
- 29.Montllor, C. B., A. Maxmen, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189-195. [Google Scholar]
- 30.Oliver, K. M., J. A. Russell, N. A. Moran, and M. S. Hunter. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 100:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ota, T., M. Kawabe, K. Oishi, and D. F. Poulson. 1979. Non-male-killing spiroplasmas in Drosophila hydei. J. Heredity 70:211-213. [Google Scholar]
- 32.Poinsot, D., K. Bourtzis, G. Markakis, C. Savakis, and H. Mercot. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150:227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Development Core Team. 2005. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 34.Riegler, M., S. Charlat, C. Stauffer, and H. Mercot. 2004. Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: investigating the outcomes of host-symbiont coevolution. Appl. Environ. Microbiol. 70:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell, J. A., and N. A. Moran. 2005. Horizontal transfer of bacterial symbionts: heritability and fitness effects in a novel aphid host. Appl. Environ. Microbiol. 71:7987-7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto, H., Y. Ishikawa, T. Sasaki, S. Kikuyama, S. Tatsuki, and S. Hoshizaki. 2005. Transinfection reveals the crucial importance of Wolbachia genotypes in determining the type of reproductive alteration in the host. Genet. Res. 85:205-210. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai, M., R. Koga, T. Tsuchida, X.-Y. Meng, and T. Fukatsu. 2005. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl. Environ. Microbiol. 71:4069-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarborough, C. L., J. Ferrari, and H. C. Godfray. 2005. Aphid protected from pathogen by endosymbiont. Science 310:1781. [DOI] [PubMed] [Google Scholar]
- 39.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuchida, T., R. Koga, and T. Fukatsu. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchida, T., R. Koga, M. Sakurai, and T. Fukatsu. 2006. Facultative bacterial endosymbionts of three aphid species, Aphis craccivora, Megoura crassicauda and Acyrthosiphon pisum, sympatrically found on the same host plants. Appl. Entomol. Zool. 41:129-137. [Google Scholar]
- 42.Veneti, Z., M. E. Clark, S. Zabalou, T. L. Karr, C. Savakis, and K. Bourtzis. 2003. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics 164:545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veneti, Z., M. E. Clark, T. L. Karr, C. Savakis, and K. Bourtzis. 2004. Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl. Environ. Microbiol. 70:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitcomb, R. F., and J. G. Tully. 1979. The mycoplasmas, vol. 3. Plant and insect mycoplasmas. Academic Press, New York, N.Y.
- 45.Williamson, D. L., and D. F. Poulson. 1979. Sex ratio organisms (spiroplasmas) of Drosophila, p. 175-208. In R. F. Whitcomb and J. G. Tully (ed.), The mycoplasmas, vol. 3. Academic Press, New York, N.Y. [Google Scholar]
- 46.Yamada, M.-A., S. Nawa, and T. K. Watanabe. 1982. A mutant of SR organism (SRO) in Drosophila that does not kill the host males. Jpn. J. Genet. 57:301-305. [Google Scholar]
- 47.Ye, F., U. Melcher, and J. Fletcher. 1997. Molecular characterization of a gene encoding a membrane protein of Spiroplasma citri. Gene 189:95-100. [DOI] [PubMed] [Google Scholar]
- 48.Zchori-Fein, E., and S. J. Perlman. 2004. Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 13:2009-2016. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, W., F. Rousset, and S. O'Neill. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]