Abstract
Noble-rotted grapes are colonized by complex microbial populations. I isolated pigment-producing Metschnikowia strains from noble-rotted grapes that had antagonistic activity against filamentous fungi, yeasts, and bacteria. A red-maroon pigment was formed from a diffusible colorless precursor released by the cells into the medium. The conversion of the precursor required iron and could occur both in the cells (red colonies) and in the medium (red halos around colonies). The intensity of pigmentation was correlated with the intensity of the antimicrobial activity. Mutants that did not form pigment also lacked antifungal activity. Within the pigmented halos, conidia of the sensitive fungi did not germinate, and their hyphae did not grow and frequently lysed at the tips. Supplementation of the medium with iron reduced the size of the halos and the inhibition zones, while it increased the pigment accumulation by the colonies. The iron-binding agent tropolone had a similar effect, so I hypothesize that pigmented Metschnikowia isolates inhibit the growth of the sensitive microorganisms by pigment formation, which depletes the free iron in the medium. As the pigment is a large nondiffusible complex produced in the presence of both low and high concentrations of ferric ions, the proposed mechanism is different from the mechanisms operating in microbes that release siderophores into the environment for iron acquisition.
The important problems for postharvest protection of fruit include the declining effectiveness of registered fungicides, public pressure to reduce fungicide use, and public demand for produce free of synthetic pesticides. One solution is to use microorganisms with antifungal effects as biocontrol agents to reduce or inhibit the rate of propagation of destructive fungi during storage. Antagonistic yeasts have received particular attention, as their activity usually does not depend on the production of antibiotics or other toxic secondary metabolites. For example, strains of Candida, Cryptococcus, Debaromyces, Metschnikowia, Pichia, Rhodotorula, Sporobolomyces, and Trichosporon all have been reported to inhibit postharvest decay of fruit due to their antifungal effects (6, 18, 36, 38, 40, 44, 50, 52). The modes of action proposed for the inhibition process include competition for space and nutrients, parasitism, direct interaction with the pathogen, production of cell wall lytic enzymes, and induced resistance in the host tissue (for a review, see reference 47).
Fruit-borne strains of Metschnikowia pulcherrima can be effective in protecting apples, peaches, and grapes against postharvest rot caused by Botrytis cinerea and other postharvest pathogens (14, 36, 48). The related species Metschnikowia fructicola is an effective biocontrol agent for postharvest diseases of grapes (25). M. pulcherrima, but not M. fructicola, produces a red pigment, pulcherrimin, that accumulates in the cells and in the medium near a colony (26, 27). Pulcherrimin is a large complex formed nonenzymatically from a dibasic acid, pulcherriminic acid, and ferric ions (11, 29). The mechanism of the antifungal antagonism and its relationship to the production of pigment have not been studied yet.
M. pulcherrima is common on wine grapes at the time of harvest (for a review, see reference 20) and in grape must during the early stages of wine fermentation (9, 10, 19, 31). M. pulcherrima occurs more frequently on damaged berries (37), on berries used to produce ice wine (7), and in botrytized (noble-rotted) wines (1). Ice wine is a late-harvest wine produced from grapes left on the vine until the first frost hits. These grapes are overripe and frequently rupture and partially desiccate before harvest. Noble rot of grapes occurs when the berries are infected by B. cinerea. Water evaporates through the Botrytis-generated skin lesions, and the grapes desiccate, resulting in high levels of sugar. The ability of M. pulcherrima to survive under these low-water-availability conditions (41) may be a reason for its prevalence on noble-rotted and ice wine grapes.
The objectives of this study were (i) to isolate pigment-producing strains of Metschnikowia from noble-rotted grapes, (ii) to test these isolates to determine their ability to antagonize the growth of filamentous fungi, yeasts, and bacteria, (iii) to determine the role of pigment production in the antagonism observed, and (iv) to identify the cytological target(s) of the inhibitory agent. Since pulcherrimin is a nondiffusible complex (11, 26, 29), I propose that Metschnikowia inhibits the growth of other microbes by immobilizing iron in the medium. This possibility has not been considered in previous reports on the antifungal antagonism of Metschnikowia strains. The proposed mechanism of iron depletion is different from the mechanisms operating in microbes that release siderophores (low-molecular-weight, ferric ion-specific chelators) into the environment for the purpose of absorbing iron (for a review, see reference 24). As iron is essential for the growth of many microorganisms and microbial pathogenesis, iron sequestration by nonpathogenic microbes can be exploited in novel and more environmentally benign systems for postharvest protection against destructive fungi.
MATERIALS AND METHODS
Organisms, media, and chemicals.
All fungal strains (Table 1) were maintained on potato dextrose agar (PDA) (49). For production of conidia, strains were grown on PDA at 15 to 20°C for 1 week with daylight (on a laboratory bench in an air-conditioned room). Conidia were harvested by washing the mycelium with 10 ml of sterile distilled water. Yeast strains were grown on yeast extract peptone agar (YPD) (49) or in liquid YPDL medium (YPD without agar) at 30°C. Oenococcus oeni was cultured on MRS medium (16) at 30°C. Escherichia coli was maintained on LB agar (42) at 37°C. Synthetic minimal agar (SMA), SML (SMA without agar), yeast extract agar (YEA), and YEL (YEA without agar) (46) were used in pigment production tests. The sensitivity of strains to iron depletion was tested with tropolone (2-hydroxycyclohepta-2,4,6-trienone; T7387; Sigma-Aldrich Co., St. Louis, Mo.), a chelating agent with a strong affinity for ferric ions (12, 17).
TABLE 1.
List of strainsa
| Strain | Source or reference |
|---|---|
| Aspergillus clavatus IMI 15949 | UKNCC |
| Aspergillus niger IMI 17454 | UKNCC |
| Aureobasidium pullulans 1.3.11 | This study |
| Botrytis cinerea 980 | 22 |
| Botrytis cinerea 3318 | This study |
| 10-432 Candida stellata CBS 157T | CBS |
| 10-372 Candida zemplinina CBS 9494T | 45 |
| Escherichia coli DH5 | Invitrogen |
| Gilbertella persicaria IMI 101638 | UKNCC |
| 10-511 Hanseniaspora uvarum CBS 314 | CBS |
| Metschnikowia pulcherrima isolates 02.4.3.38 (= NCAIM Y0177071), 02.11.1.21 (= NCAIM Y0177072), 11.2.38 (= NCAIM Y0177071), 4a.3.11, 7.3.10, 7.3.37, 9.4.60, 17.1.IV, 17.3.1 | This study |
| Metschnikowia pulcherrima mutants A-1 (isolated from 02.11.1.21), A-2 (isolated from 02.11.1.21), B-1 (isolated from 02.11.1.21), B-2 (isolated from 02.11.1.21), C (isolated from 02.11.1.21), D (isolated from 02.11.1.21), E (isolated from 02.11.1.21), F (isolated from 02.11.1.21), G-21 (isolated from 02.11.1.21), G-38 (isolated from 02.4.3.38), H-1 (isolated from 02.4.3.38), H-2 (isolated from 02.4.3.38) | This study |
| Mucor piriformis NRRL 3636 | NRRL |
| Mucor circinelloides ATCC 1216 | ATCC |
| Oenococcus oeni B16 | Faculté d'Oenologie |
| Rhizopus stolonifer var. stolonifer CBS 109.76 | CBS |
| 10-157 Saccharomyces cerevisiae s288c | YGS |
| 10-408 Saccharomyces uvarum | 2 |
| 7-1 Schizosaccharomyces japonicus var. japonicus CCY-44-5-1 | CCY |
| 0-1 Schizosaccharomyces pombe var. pombe L972 | Bern Collection |
| 0-39 Schizosaccharomyces pombe var. pombe leu1 | Bern Collection |
Abbreviations: ATCC, American Type Culture Collection, Manassas, Va.; Bern Collection, Institut für Allgemeine Mikrobiologie, Universität Bern, Bern, Switzerland; CBS, Centraalbureau voor Schimmelcultures, Delft, The Netherlands; CCY, Czechoslovak Collection of Yeasts, Institute of Chemistry, Bratislava, Slovakia; Faculté d'Oenologie, Faculté d'Oenologie de Bordeaux UMR1219, Talence, France; NCAIM, National Collection of Agricultural and Industrial Microorganisms, Budapest, Hungary; NRRL, National Center for Agricultural Utilization Research, Peoria, Ill.; UKNCC, United Kingdom National Culture Collection; YGS, Yeast Genetic Stock, Berkeley, Calif. Numbers 0-1, 0-39, 7-1, 10-157, 10-372, 10-408, 10-432, and 10-511 are codes used for the strain collection of the Department of Genetics, University of Debrecen (Hungary).
Strain isolation and taxonomic identification.
Grape berries were collected aseptically from noble-rotted bunches. Each berry was homogenized separately in 2 ml of sterile water, and samples of the resulting must were spread on YPD plates. After incubation at room temperature (15 to 20°C) for 5 days, red or reddish colonies fringed by reddish halos in the medium were isolated. Each isolate was spread once or twice on YPD plates to obtain clones originating from single cells. Morphological examination and tests for carbon and nitrogen source utilization for species identification were conducted as previously described (4, 49). Ten colonies of Aureobasidium pullulans and 10 colonies with Botrytis morphology also were recovered. The sequences of the D1/D2 domains at the 5′ end of the large-subunit 26S rRNA genes of the yeast isolates and the Botrytis isolates were amplified with primers NL-1 and NL-4 (35) by using PCR conditions described previously (45). Both strands of the fragments were sequenced with an ABI PRISM 3700 sequencer (AME Bioscience Ltd., Sharnbrook, United Kingdom) by using the PCR primers. Sequence similarity searches were performed with the BLAST network service of the NCBI database (http://www.ncbi.nlm.nih.gov/BLAST).
Tests for pigment production.
To compare pigment production in colonies growing on agar plates, cells of the isolates either were streaked on agar plates or were suspended in sterile water (∼107 cells per ml), and 15-μl samples of the suspensions were dropped onto the surfaces of agar plates. The widths of the reddish halos around the yeast colonies were measured after 5 to 10 days of incubation at 25°C. Pigment production also was tested in shake cultures at 25°C (50 ml medium in a 200-ml Erlenmeyer flask; incubation at 100 rpm in a gyratory shaking incubator for 14 h; inoculum, 104 cells/ml).
Tests for antagonism.
Inhibition of conidial germination was examined on PDA plates flooded with suspensions of conidia. One yeast isolate was streaked or dropped on each plate and incubated at 25°C. To examine the inhibition of hyphal growth, spores were streaked on PDA plates and incubated for 2 days at 25°C to allow them to develop mycelia. The yeast isolates tested were inoculated as colonies from 15-μl spots placed 5 mm ahead of the growing edges of the mycelia. The plates were checked for inhibition twice a day for 7 days because the growth rates of the fungal strains differed.
The effects of Metschnikowia isolates 02.11.1.21 and 02.4.3.38 on yeasts and bacteria were tested on plates flooded with cells of the test organisms. The media used were PDA for yeasts, MRS agar for O. oeni, and LB agar for E. coli. One yeast isolate was streaked or dropped on each plate. The incubation temperatures were 25°C for yeasts, 30°C for O. oeni, and 37°C for E. coli.
Effect of iron and tropolone on pigment production and antagonism.
The effect of ferric ions on pigment production was studied on PDA plates supplemented with FeCl3. The plates were flooded with conidia. One yeast isolate was then inoculated into the center of each plate. The sensitivity of conidial germination to tropolone was tested by placing 50-μl samples of aqueous solutions of the compound into wells (diameter, 5 mm) cut into PDA plates previously flooded with suspensions of Botrytis conidia. The widths of the pigmented halos and inhibition zones were measured after 3 to 10 days of incubation at 25°C.
Mutagenesis and mutant characterization.
Yeast cells in overnight YPDL cultures (50 ml medium in a 200-ml Erlenmeyer flask; incubation at 100 rpm in a gyratory shaking incubator for 14 h at 25°C; inoculum, 104 cells/ml) were collected by centrifugation (1,500 × g, 5 min, 10°C), resuspended in fresh YPDL (107 cells/ml) supplemented with 300 μg/ml 1-methyl-3-nitro-1-nitrosoguanidine (catalog no. 12,994-1; Sigma-Aldrich), and incubated in a shaking incubator (100 rpm) at 25°C with daylight (on a laboratory bench in an air-conditioned room). After 30 min, samples were diluted and spread on PDA plates supplemented with 10 μg/ml FeCl3. After 5 days of incubation at 25°C in continuous darkness, colonies that differed from the wild type in colony color or in the size of the pigmented halo were isolated. Colonies with these morphological properties occurred with a frequency of 8 × 10−4. The survival rate was 23%. The 26S rRNA gene from these colonies was sequenced, and strains that had sequences that were different from that of the mutagenized wild type were discarded. Pigmentation, halo formation, and antifungal activity of the mutants were tested as described above. Because of the lack of hybridization methods for Metschnikowia, the mutants were not tested for allelism or to determine the numbers of mutations in their genomes.
Nucleotide sequence accession numbers.
Partial 26S rRNA gene sequences of the following strains were deposited in the GenBank database: Metschnikowia strains 02.4.3.38 (accession no. DQ666681) and 02.11.1.21(DQ666682) and B. cinerea strain 3318 (DQ666677).
RESULTS
Isolation and taxonomic identification of M. pulcherrima-like strains.
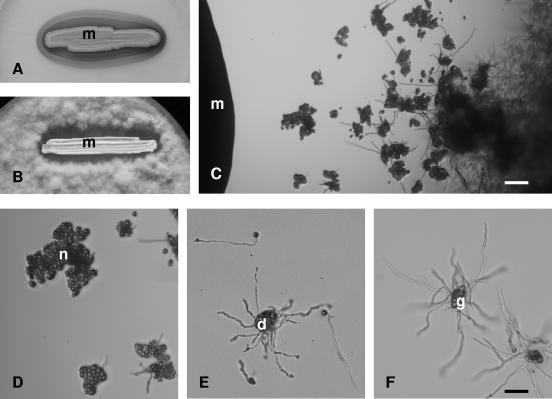
Grape berries were collected from botrytized bunches in two vineyards (Tarcal and Mad) in the Hungarian portion of the Tokaj wine-growing region in 2002 and 2003. Yeasts producing reddish or pink colonies surrounded by reddish halos (Fig. 1A) were recovered from 48/60 berries examined and were more abundant in berries in more advanced stages of noble rotting.
FIG. 1.
Halo formation and inhibition of germination of B. cinerea conidia by Metschnikowia isolate 02.11.1.21. (A) Pigmented halo on YPD supplemented with 0.005 mg/ml FeCl3. (B) Inhibition zone on YPD. (C to F) Microscopic images of conidia on PDA. m, Metschnikowia colony; n, group of nongerminating conidia (within a colored halo); d, conidia with dying germination tubes (at the edge of a colored halo); g, group of germinating conidia developing mycelium (outside a colored halo). (C) Bar = 120 μm. (F) Bar = 50 μm.
One isolate from each berry was identified to the species level as previously described (49). The carbon and nitrogen source utilization patterns were consistent with identification of these strains as M. pulcherrima (see reference 30 for Metschnikowia taxonomy). The sequences of the D1/D2 domains of the 26S rRNA gene regions of 10 of these isolates were 96 to 98% identical to those of the type strain of M. pulcherrima (accession no. U45736).
Pigment production in cultures of the isolates.
Pulcherrimin production is a characteristic of M. pulcherrima, but the intensity of pigmentation varies and depends on medium composition (21, 26). The strains isolated in this study varied in color intensity and in the size of the reddish halos that surrounded the colonies (Table 2). The most pigment was produced on YPD, while colonies on PDA and SMA plates either were colorless (white) or turned slightly pink with a barely visible pink halo. Supplementation of the media with FeCl3 enabled pigment production on these media, and the color intensity increased with the iron concentration (Table 2). This finding is consistent with the proposed role of iron in the chemical structure of pulcherrimin (26).
TABLE 2.
Formation of pigmented halo and inhibition of the growth of B. cinerea around the colonies of selected Metschnikowia isolates on PDA platesa
| Strain | No FeCl3
|
0.005 mg/ml FeCl3
|
0.01 mg/ml FeCl3
|
0.015 mg/ml FeCl3
|
0.02 mg/ml FeCl3
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colony color | Width of colored halo (mm)b | Width of inhibition zone (mm) | Colony color | Width of colored halo (mm) | Width of inhibition zone (mm) | Colony color | Width of colored halo (mm) | Width of inhibition zone (mm) | Colony color | Width of colored halo (mm) | Width of inhibition zone (mm) | Colony color | Width of colored halo (mm) | Width of inhibition zone (mm) | |
| 02.4.3.38c | White | 6 | 7 | Pink | 2.5 | 3.5 | Red | 1.5 | 1.5 | Red | 1.5 | 1.5 | Dark red | 0.5 | 0.5 |
| 02.11.1.21 | White | 7 | 8 | Pink | 3 | 3 | Red | 1.5 | 1.5 | Red | 0.5 | 0.5 | Dark red | 0 | 0 |
| 7.3.37 | White | 7 | 8 | Pink | 2 | 3 | Red | 1 | 1 | Red | 0.5 | 0.5 | Dark red | 0 | 0 |
| 17.1.IV | Pink | 5.5 | 6 | Pink | 2 | 3.5 | Red | 2 | 2 | Red | 0.5 | 0.5 | Dark red | 0 | 0 |
| 17.3.1. | White | 7 | 8 | Pink | 3 | 4 | Red | 2 | 2 | Red | 1.0 | 1.0 | Dark red | 0.5 | 0.5 |
Results were determined on the 10th day of incubation. Data for four experiments are shown. The widths of the halos and inhibition zones were measured manually. Due to the identical conditions and because of the resolution limit of manual measurement, no detectable deviations were observed.
Faintly visible.
Strains 4a.3.11, 7.3.10, 9.4.60, and 11.3.38 give similar but somewhat weaker reactions.
Increasing the concentration of FeCl3 also increased the amount of pigment produced in the liquid media. Without supplementation, YPDL and YEL cultures were pink and SML cultures were colorless. If these cultures were supplemented with 0.1 mg/ml FeCl3 in early stationary phase, they turned red almost instantly, which suggests that the cells produce a colorless precursor that is converted to pigment as soon as iron is available. If these red cultures were centrifuged, then both the sediment, consisting of yeast cells, and the cell-free supernatant were red. If 0.1-ml samples of the supernatants were placed into wells cut into PDA and SMA plates, no pigment halos appeared around the wells. Presumably, the pigment could not diffuse into the medium, perhaps because it was suspended rather than dissolved in the sample. With time, the pigment slowly precipitated to form a maroon sediment if the supernatant was not agitated. The precipitated material did not redissolve in water.
If the pigment is insoluble in water and cannot diffuse into the medium, then it must be formed in situ from its soluble precursors. Pulcherrimin is formed from leucine through the intermediates cyclo-l-leucyl-l-leucyl and pulcherriminic acid (29). Since all of these compounds are water soluble, any of them could be the secreted precursor from which the pigmented halo is formed around the colonies. To test the possibility that leucine is the secreted precursor, the most active Metschnikowia isolates were streaked on SMA plates seeded with cells of the leucine-dependent strain Schizosaccharomyces pombe leu1. No cross-feeding of the Schizosaccharomyces mutant was observed around the Metschnikowia colonies, indicating that the Metschnikowia cells did not excrete leucine and that the secreted precursor of pulcherrimin is not leucine.
Inhibition of germination of Botrytis conidia.
Fifteen colonies with Botrytis morphology were isolated, and their D1/D2 rRNA gene domains were sequenced, which confirmed their identification as B. cinerea (100% identity with the accession no. AF250919 sequence, the sequence of a B. cinerea [Botryotinia fuckeliana] strain isolated from Californian grapes). One of these isolates, strain 3318, was used as a tester (GenBank accession no. DQ666677). The other tester was strain 980, a strain isolated from grapes in France (22).
All Metschnikowia isolates inhibited germination of the conidia of both B. cinerea testers (Fig. 1C and D) if the spores were within 0 to 7 mm (depending on the strain and the amount of FeCl3 added to the medium) of the Metschnikowia colony. At 0 to 6.5 mm from the Metschnikowia colony (depending on the strain and the amount of FeCl3 added to the medium), the conidia germinated but died after forming short germination tubes (Fig. 1E), suggesting that the presence of the Metschnikowia isolates could inhibit hyphal growth. The distance across which the Metschnikowia isolates caused this antagonistic effect varied by strain. Strains that produced larger halos usually also had larger inhibition zones (Table 2). Outside the inhibition zones, the conidia germinated uniformly and formed dense mycelium (Fig. 1B and F). After 5 to 6 days of incubation, the mycelium began to grow slowly into the inhibition zone and gradually reduced the size of the zone. No differences were detected between the two Botrytis tester strains.
Inhibition of growth of Botrytis hyphae.
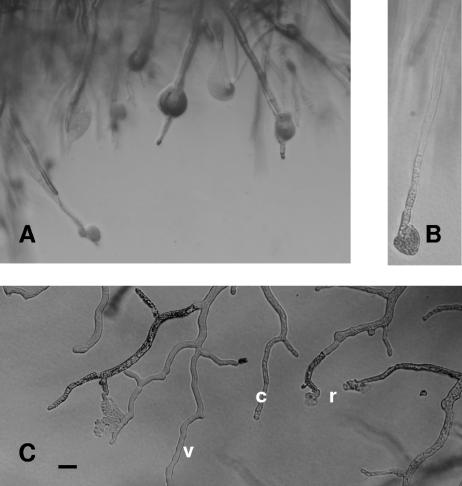
The Metschnikowia isolates also were inoculated ahead of the growing front of the Botrytis mycelium. After a few hours of incubation, mycelia near the yeast colony stopped growing and the hyphal morphology changed (Fig. 2A). Many hyphae lysed at the tip, and in others extensive protoplasmic coagulation occurred. Forty-eight to 72 h later some hyphae resumed growing and grew slowly toward the yeast colony.
FIG. 2.
Degeneration of hyphae at the edge of the inhibition zone around a colony of Metschnikowia isolate 02.11.1.21. (A) B. cinerea 3318. (B) A. pullulans 27/2.36. (C) M. piriformis. v, viable hypha; r, hypha ruptured near its tip; c, hypha with coagulated cytoplasm. Bar = 20 μm.
Iron and antifungal antagonism.
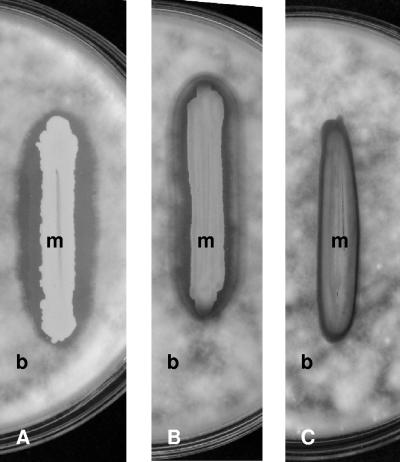
Five Metschnikowia isolates were inoculated onto PDA plates supplemented with various amounts of FeCl3 and then flooded with Botrytis conidia. The greater the amount of iron added, the narrower the halos and inhibition zones were (Table 2). At exogenous FeCl3 concentrations greater than 20 μg/ml no red halos appeared, and the growth of Botrytis was not inhibited (Fig. 3). Increasing the iron concentration reduced the size of the halos but increased the pigmentation of the yeast colonies. At FeCl3 concentrations greater than 20 μg/ml the yeast colonies were dark red, suggesting that all of the pigment was retained in the cells. The simultaneous reductions in the sizes of the pigment halos and inhibition zones by iron supplementation suggests that the Metschnikowia isolates inhibited B. cinerea by depleting the iron in the medium.
FIG. 3.
Effect of FeCl3 on halo formation and inhibition of the growth of B. cinerea on PDA. (A) Without FeCl3. (B) With 0.005 mg/ml FeCl3. (C) With 0.02 mg/ml FeCl3. m, Metschnikowia colony; b, Botrytis mycelium.
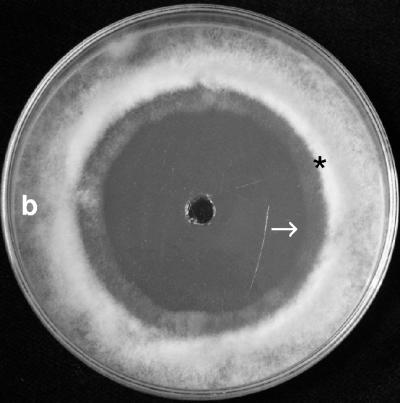
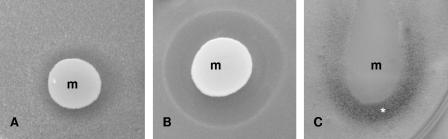
Tropolone is a chelating agent with a strong affinity for ferric ions (12, 17). Tropolone was placed into holes (0.1, 0.2, 0.5, 1.0, 1.5, and 2.0 mg/hole) in PDA plates seeded with Botrytis conidia, which resulted in growth inhibition similar to that observed with the Metschnikowia isolates (Fig. 4). This effect could be reversed by supplementing the medium with FeCl3, demonstrating that iron depletion inhibits conidial germination. The Botrytis testers each formed a ring of thick mycelium around the inhibition zone (Fig. 4), which could be attributed to nutrient diffusion from the inhibition zone. As observed with the Metschnikowia-generated inhibition, hyphae began growing slowly toward the wells after 4 to 5 days of incubation. Tropolone also inhibited the formation of the red or maroon pigment. When 1.0 mg tropolone was placed into a hole cut into the medium (15 μg/ml FeCl3) 10 mm from a Metschnikowia colony, the colony did not turn red and did not form a halo in the agar.
FIG. 4.
Inhibition of B. cinerea by tropolone. Fifty microliters of a solution containing 0.5 mg tropolone was placed into a hole in the middle of the plate. The asterisk indicates the ring of stimulated mycelial growth. The arrow indicates hyphae growing into the inhibition zone. b, Botrytis mycelium.
Correlation between pigment production and antifungal activity in mutants.
Cells of isolates 02.11.1.21 and 02.4.3.38 were mutagenized with nitrosoguanidine, and mutant colonies that differed from the wild-type colonies in pigmentation were recovered. Three white (colorless) mutants, eight pink mutants (with various degrees of pigmentation), and one mutant whose colonies formed halos that were broader than the halos of the wild-type colonies were selected for further examination. The mutants were tested for pigment production and the ability to inhibit germination of B. cinerea conidia on PDA supplemented with various amounts of FeCl3. The colonies formed by colorless mutants A-1, A-2, and G-21 were white at all concentrations of iron and did not affect the growth of Botrytis. Mutant G-38 was more pigmented and had a wider halo and stronger inhibitory effect on Botrytis than the wild-type parent. The other mutants had lower, but not uniform, amounts of pigmentation and antifungal antagonism (Table 3). The white and low-pigment mutants grew as well as the wild-type strains at all concentrations of iron tested (0 to 0.5 mg/ml) at pH 5.0 and 6.5. These conditions did not inhibit the growth of Botrytis.
TABLE 3.
Production of pigmented halos and the inhibition of B. cinerea growth around colonies of wild-type Metschnikowia isolates and mutants on PDA platesa
| Strain | No FeCl3
|
0.01 mg/ml FeCl3
|
0.015 mg/ml FeCl3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Colony colorb | Width of colored halo (mm)c | Width of inhibition zone (mm) | Colony color | Width of colored halo (mm) | Width of inhibition zone (mm) | Colony color | Width of colored halo (mm) | Width of inhibition zone (mm) | |
| Wild-type strains | |||||||||
| 02.4.3.38 | FP | 4.5 | 4 | R | 1.0 | 1 | DR | 0.5 | 0 |
| 02.11.1.21 | FP | 4 | 4 | R | 1.5 | 1 | DR | 0.5 | 0 |
| Mutant strains isolated from 02.4.3.38 | |||||||||
| G-38 | FP | 6 | 4 | P | 4 | 2 | R | 3.5 | 2 |
| H-1 | FP | 4 | 2.5 | R | 0 | 0 | R | 0 | 0 |
| H-2 | FP | 3.5 | 2.5 | R | 0.5 | 0 | R | 0.5 | 0 |
| Mutant strains isolated from 02.11.1.21 | |||||||||
| A-1 | W | 0 | 0 | W | 0 | 0 | W | 0 | 0 |
| A-2 | W | 0 | 0 | W | 0 | 0 | W | 0 | 0 |
| B-1 | W | 0 | 0 | FP | 0 | 0 | FP | 0 | 0 |
| B-2 | W | 0 | 0 | FP | 0 | 0 | FP | 0 | 0 |
| C | W | 0 | 0 | P | 0.5 | 0 | R | 0 | 0 |
| D | FP | 4 | 2 | P | 0.5 | 0 | R | 1 | 0.5 |
| E | W | 0 | 0.5 | P | 0.5 | 0 | R | 0 | 0 |
| F | FP | 4 | 3 | P | 2.5 | 1.5 | R | 1 | 0.5 |
| G-21 | W | 0 | 0 | W | 0 | 0 | W | 0 | 0 |
Results were determined after 6 days of incubation. Data for four experiments are shown. The widths of the halos and inhibition zones were measured manually. Due to the identical conditions and because of the resolution limit of manual measurement, no detectable deviations were observed.
W, white (colorless); FP, faint pink; P, pink; R, red; DR, dark red.
Faintly visible.
Activity against other microorganisms.
The Metschnikowia strains also were tested for activity against other fungal species (Table 1). Most of these fungi were as sensitive to the Metschnikowia strains as B. cinerea was (Table 4). Aspergillus niger conidial germination was not affected, but mycelial growth was retarded. Massive lysis of hyphae occurred in A. pullulans and Mucor piriformis (Fig. 2B and C).
TABLE 4.
Antagonistic effects of Metschnikowia against various microorganisms
| Strain | Width of inhibition zone (mm) around colonies of Metschnikowia straina:
|
Remarks | |
|---|---|---|---|
| 02.4.3.38 | 02.11.1.21 | ||
| Aspergillus clavatus IMI 15949 | 2 | 2.5 | |
| Aspergillus niger IMI 17454 | 0 | 0 | Growth reduction |
| Aureobasidium pullulans 27/2.36 | 1 | 0 | Growth reduction with no sharp edge; dark band around the Metschnikowia colonyb |
| Botrytis cinerea 980 | 3.5 | 4 | Growth stimulation zone, 1 mm |
| Botrytis cinerea 3318 | 4 | 4 | Growth stimulation zone, 1 mm |
| 10-432 Candida stellata | 0 | 0 | |
| 10-372 Candida zemplinina | 3 | 5 | Growth stimulation zones, 3 mm for 02.4.3.38 and 0.5 mm for 02.11.1.21c |
| Escherichia coli DH5 | 2 | 2.5 | |
| Gilbertella persicaria IMI 101638 | 3.5 | 4.5 | |
| 10-511 Hanseniaspora uvarum | 0 | 0 | |
| Mucor piriformis NRRL 3636 | 2 | 2 | |
| Mucor circinelloides ATCC 1216 | 2 | 2 | |
| Oenococcus oeni B16 | 0 | 0.5 | Growth stimulation zone, 3 mm |
| Rhizopus stolonifer var. stolonifer CBS 109.76 | 4.5 | 5.5 | |
| Saccharomyces cerevisiae S288c | 0 | 0 | Growth reduction with no sharp edged |
| 10-408 Saccharomyces uvarum | 0 | 0 | Growth reduction with no sharp edge |
| 7-1 Schizosaccharomyces japonicus var. japonicus CCY-44-5-1 | 0 | 0 | |
| 0-1 Schizosaccharomyces pombe var. pombe L972 | 0 | 0 | |
Results were determined after 6 days of incubation. Data for four experiments are shown. The widths of the halos and inhibition zones were measured manually. Due to the identical conditions and because of the resolution limit of manual measurement, no detectable deviations were observed.
See Fig. 5C.
See Fig. 5B.
See Fig. 5A.
The environment from which the Metschnikowia strains were isolated is rich in yeasts and bacteria (1, 3, 45). Therefore, I also tested the Metschnikowia isolates for activity against yeasts and bacteria. The growth of Candida stellata, Hanseniaspora uvarum, S. pombe, and Schizosaccharomyces japonicus was not altered by the presence of Metschnikowia. The growth of Saccharomyces cerevisiae and Saccharomyces uvarum was somewhat retarded around the Metschnikowia colonies, but no inhibition zones with sharp edges were formed (Fig. 5A). A similar reaction was described by Nguyen and Panon (34). Candida zemplinina also was sensitive; its growth was severely inhibited near the Metschnikowia colonies, but thin rings of facilitated growth fringed the inhibition zones (Fig. 5B).
FIG. 5.
Effect of Metschnikowia isolate 02.11.1.21 on yeasts. (A) S. cerevisiae. (B) C. zemplinina. (C) A. pullulans (yeast phase). m, Metschnikowia colony. There is a zone (marked with an asterisk) in which there is increased melanin production in the A. pullulans lawn.
O. oeni did not grow close to a Metschnikowia colony but did form a broad halo of vigorous growth around the very thin inhibition zone. E. coli was more sensitive to Metschnikowia; it was inhibited more severely and did not grow beyond the zone of inhibition.
DISCUSSION
The red or maroon pigment (pulcherrimin) of M. pulcherrima has been the subject of numerous previous studies. This pigment forms reddish halos around colonies of M. pulcherrima. These pigmented halos contain a water-insoluble complex of ferric ions and pulcherriminic acid (11, 26). The reddish brown colonies of the Metschnikowia isolates analyzed in this study also were surrounded by colored halos, and their pigment also was insoluble in water.
The reduction in halo size observed when the medium was supplemented with FeCl3 suggests that the cells do not secrete the pigment but instead secrete a soluble, diffusible precursor that forms the pigment in the medium when it encounters iron. At low iron concentrations the precursor diffuses further from the yeast colony before it is immobilized by iron, resulting in a wider but paler halo. At higher iron concentrations the halos are smaller because the precursor molecules do not diffuse as far before they bind sufficient iron for pigment production. Since the pigment in these cases is concentrated in a smaller area, the color of the resulting halos is more intense. At even higher concentrations of iron, no halo is produced, suggesting that the entire precursor pool is converted to pigment within the cells. At these concentrations the yeast colonies are dark red (maroon).
The pigment, pulcherrimin, is synthesized from l-leucine through cyclo-l-leucyl-l-leucyl and pulcherriminic acid (29). Since the Metschnikowia colonies do not cross-feed yeast leucine auxotrophs, leucine can be ruled out as the secreted precursor. Chemical analysis of the medium is needed to determine which of the other two intermediates is released.
As indicated by Kluyver et al. (26), Beijerinck hypothesized that pigment production is a defense reaction of the M. pulcherrima cells against the presence of excess iron. However, both Beijerinck and Kluyver et al. (26) described colorless colonies in their M. (Torula, Candida) pulcherrima cultures, which argues against such a role. If the organism does need protection against iron, then the inability to form pigment should be selected against. The pigmentless mutants isolated in this study grew like the wild type even at iron concentrations that turned the wild-type colonies dark red. Thus, pigment production is not essential for growth, even under high-iron conditions, so a protection function is unlikely and pigment production must benefit the organism in some other manner.
The antimicrobial activity of the isolates suggests that a biological function for pigment production might be to inhibit the growth of other microbes that are potential competitors for nutrients. The pulcherrimic acid-ferric ion complex formed in the halos depletes the iron in the substrate and creates an environment unsuitable for growth of microbes that require more iron for growth. This hypothesis is based on the observed correlation between the size of the pigmented halos around the Metschnikowia colonies and the size of the zones within which the growth of Botrytis was inhibited. Metschnikowia isolates that produced wider halos also had a stronger antagonistic effect on B. cinerea. The mutants that lacked pigment did not affect Botrytis, and strains producing low levels of pigment also had lower levels of antifungal activity. Any reduction in halo size due to supplementation of the medium with iron also reduced the size of the inhibition zone.
An alternative hypothesis is that either the pigment or its precursors have antimicrobial activity and that iron depletion is an incidental and harmless side effect. I cannot exclude this possibility as I have not tested purified pigment or its precursors for this activity; however, the iron-binding agent tropolone (17) also inhibits the germination of conidia and the growth of the test organisms. Iron also may be involved in plant antifungal defense mechanisms since infection by B. cinerea increased the Fe(III) levels in tissues of Capsicum annuum and Arabidopsis thaliana (15, 32).
Sequestration of iron is a widespread mechanism of antimicrobial antagonism. Siderophores (low-molecular-weight, ferric ion-specific chelating agents) that deplete the iron in the environment by transporting it into the cells of the siderophore-excreting microorganisms can inhibit the growth of other microbes that do not have similar mechanisms (6, 23, 24, 33). The ferrisiderophore complexes are water soluble, which makes them accessible to the specific membrane receptor and transport systems that mediate their transport into the cell (for a review, see reference 51). The pigment produced by the Metschnikowia cultures also might act as a siderophore, but the results of this study make this possibility unlikely. The Metschnikowia pigment is not soluble in water and cannot diffuse in the agar medium, so rather than solubilizing the iron in the environment, it immobilizes it. Nevertheless, low-molecular-weight forms of the pigment complex might diffuse to the cell and deliver ferric ions.
The accumulation of the pigment within a Metschnikowia colony at higher iron concentrations also argues against a siderophore function. Microscopic observations made by Kluyver et al. (26) indicate that in highly pigmented colonies the pigment is encrusted on the cell wall and partially covers the outside of the cell. Siderophore-iron complexes do not accumulate in the cell but instead dissociate to free the iron for cellular metabolism (13). Another difference between the Metschnikowia pigment and siderophores is in the regulation of their production. Siderophores usually are produced under iron-limiting conditions (for a review, see reference 24), whereas the Metschnikowia pigment is formed constitutively, at both low and high ferric ion concentrations.
Based on microscopic observations of the test organisms, the iron-limited environment created by a Metschnikowia colony is lethal to germinating conidia. This finding is consistent with the results of Charlang et al. (8), who found that the conidia of Aspergillus nidulans and Penicillium chrysogenum required intake of a large amount of iron for germination. More recently, rhodotorulic acid, a siderophore produced by Rhodotorula glutinis, was shown to reduce conidial germination of Penicillium expansum (6) and B. cinerea (43). However, there have been no studies of the cytological effects of iron limitation on mycelium. The observation that hyphae crack when they enter the pigmented zones that form around the Metschnikowia colonies is a new finding and demonstrates that iron starvation elicits complex physiological changes in the fungal cells.
Interestingly, the inhibition of hyphal growth is transitory, and after a lag period of a few days the mycelium recovers from the shock and begins to grow slowly into the inhibition zone. The pigment may degrade with time (and release iron), or iron may be supplied to the growing hyphal tips by cytoplasmic transport from pigment-free areas of the medium. Since recovery also was observed at the edges of the tropolone-generated inhibition zones, the latter possibility is more likely. Numerous fungi can transport nutrients, including iron, between various parts of the mycelium (5, 17).
Some test organisms (O. oeni, B. cinerea, and C. zemplinina) formed rings of thicker growth around the inhibition zones. The mechanism of this stimulation is not known, but the stimulation may be attributable to the diffusion of nutrients from the inhibition zones. For example, for O. oeni, a bacterium that is common on grapes and in wines (28), the ring of facilitated growth was much wider than the inhibition zone. The polymorphic organism A. pullulans also responded to the presence of Metschnikowia in two ways. In addition to growth inhibition, the presence of Metschnikowia also elicited melanin production by the yeast-like cells, a process characteristic of chlamydospores (39).
In summary, Metschnikowia yeasts growing on noble-rotted grapes can strongly antagonize the growth of various filamentous fungi, yeasts, and bacteria by producing an insoluble pigment that depletes the iron in the environment. The immobilization of iron rather than incorporation of iron into the antagonizing strain is a novel method of extracting iron from the environment for biological control. Such a mechanism has not been demonstrated previously and may provide a new strategy for use in the biological control of various plant pathogens.
Acknowledgments
I thank Iren Pasztor and Ilona Lakatos for technical assistance.
This research was supported by grant GND RET provided by the Hungarian National Office for Research and Technology.
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Antunovics, Z., H. Csoma, and M. Sipiczki. 2003. Molecular and genetic analysis of the yeast flora of botrytized Tokaj wines. Bull. O. I. V. (Off. Int. Vigne Vin) 76:380-397. [Google Scholar]
- 2.Antunovics, Z., H.-V. Nguyen, C. Gaillardin, and M. Sipiczki. 2005. Gradual genome stabilisation by progressive reduction of the Saccharomyces uvarum genome in an interspecific hybrid with Saccharomyces cerevisiae. FEMS Yeast Res. 5:1141-1150. [DOI] [PubMed] [Google Scholar]
- 3.Barbe, J.-C., G. de Revel, A. Joyeux, A. Bertrand, and A. Lonvaud-Funel. 2001. Role of botrytized grape micro-organism in SO2 binding phenomena. J. Appl. Microbiol. 90:34-42. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, J. A., R. W. Payne, and D. Yarrow. 1990. Yeasts: characterization and identification. Cambridge University Press, Cambridge, United Kingdom.
- 5.Boddy, L. 1999. Saprophytic cord-forming fungi: meeting the challenge of heterogeneous environments. Mycologia 91:13-32. [Google Scholar]
- 6.Calvente, V., D. Benuzzi, and M. I. S. de Tosetti. 1999. Antagonistic action of siderophores from Rhodotorula glutinis upon the postharvest pathogen Penicillium expansum. Int. Biodeterior. Biodegrad. 43:167-172. [Google Scholar]
- 7.Chamberlain, G., J. Husnik, and R. E. Subden. 1997. Freeze-desiccation survival in wild yeasts in the bloom of icewine grapes. Food Res. Int. 30:435-439. [Google Scholar]
- 8.Charlang, G., B. Ng, N. H. Horowitz, and R. M. Horowitz. 1981. Cellular and extracellular siderophores of Aspergillus nidulans and Penicillium chrysogenum. Mol. Cell. Biol. 1:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemente-Jimenez, J. M., L. Mingorance-Cazorla, S. Martinez-Rodroguez, F. J. Las Heras-Vazquez, and F. Rodriguez-Vico. 2004. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 21:149-155. [Google Scholar]
- 10.Combina, M., A. Elia, L. Mercado, C. Catania, A. Ganga, and C. Martinez. 2005. Dynamics of indigenous yeast populations during spontaneous fermentation of wine from Mendoza, Argentina. Int. J. Food Microbiol. 99:237-243. [DOI] [PubMed] [Google Scholar]
- 11.Cook, A. H., and C. A. Slater. 1956. The structure of pulcherrimin. J. Chem. Soc. 1956:4133-4135. [Google Scholar]
- 12.Coulanges, V. P., P. Andre, and D. Vidon. 1998. Effect of siderophores, catecholamines and catechol compounds on Listeria spp. growth in iron-complexed medium. Biochem. Biophys. Res. Commun. 249:526-530. [DOI] [PubMed] [Google Scholar]
- 13.Crosa, J. H. 1997. Signal transduction and transcriptional and posttranslational control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev. 61:319-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Curtis, E., S. Torriani, E. Rossi, and V. De Cicco. 1996. Selection and use of Metschnikowia pulcherrima as a biological control agent for postharvest rots of peaches and table grapes. Ann. Microbiol. Enzymol. 46:45-55. [Google Scholar]
- 15.Deighton, N., I. Muckenschnabel, B. A. Goodman, and B. Williamson. 1999. Lipid peroxidation and the oxidative burst associated with infection of Capsicum annuum by Botrytis cinerea. Plant J. 20:485-492. [DOI] [PubMed] [Google Scholar]
- 16.De Mann, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 17.Diouf, P. N., N. Delbarre, D. Perrin, P. Gerardin, C. Rapin, J. P. Jacquot, and E. Gelhaye. 2002. Influence of tropolone on Poria placenta wood degradation. Appl. Environ. Microbiol. 68:4377-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan, Q., and S. Tian. 2001. Postharvest biological control of grey mold and blue mold on apple by Cryptococcus albidus (Saito) Skinner. Postharvest Biol. Technol. 21:341-350. [Google Scholar]
- 19.Fleet, G. H., and G. M. Heard. 1993. Yeasts: growth during fermentation, p. 27-54. In G. H. Fleet (ed.), Wine microbiology and biotechnology. Harwood Academic Publishers, Philadelphia, Pa.
- 20.Fleet, G. H., C. Prakitchaiwattana, A. I. Beh, and G. Heard. 2002. The yeast ecology of wine grapes, p. 1-17. In M. Ciani (ed.), Biodiversity and biotechnology of wine yeasts. Research Signpost, Kerala, India.
- 21.Gimenez-Jurado, G., M. J. Valderrama, I. SaNogueira, and I. Spencer-Martins. 1995. Assessment of phenotypic and genetic diversity in the yeast genus Metschnikowia. Antonie Leeuwenhoek 68:101-110. [DOI] [PubMed] [Google Scholar]
- 22.Giraud, T., D. Fortini, C. Levis, and Y. Brygoo. 1998. The minisatellite MSB1, in the fungus Botrytis cinerea, probably mutates by slippage. Mol. Biol. Evol. 15:1524-1531. [DOI] [PubMed] [Google Scholar]
- 23.Gram, L., L. Ravn, M. Rasch, J. B. Bruhn, A. B. Christensen, and M. Givskov. 2002. Food spoilage—interactions between food spoilage bacteria. Int. J. Food Microbiol. 78:79-97. [DOI] [PubMed] [Google Scholar]
- 24.Howard, D. H. 1999. Acquisition, transport, and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 12:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karabulut, O., J. Smilanick, F. Gabler, M. Mansour, and S. Droby. 2003. Near-harvest applications of Metschnikowia fructicola, ethanol, and sodium bicarbonate to control postharvest diseases of grape in central California. Plant Pathol. 87:1384-1389. [DOI] [PubMed] [Google Scholar]
- 26.Kluyver, A. J., J. P. van der Walt, and A. J. van Triet. 1953. Pulcherrimin, the pigment of Candida pulcherrima. Proc. Natl. Acad. Sci. USA 39:583-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurzman, C. P., and S. Droby. 2001. Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 24:395-399. [DOI] [PubMed] [Google Scholar]
- 28.Lonvaud-Funel, A. 1999. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Leeuwenhoek 76:317-331. [PubMed] [Google Scholar]
- 29.MacDonald, J. C. 1965. Biosynthesis of pulcherriminic acid. Biochem. J. 96:533-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, M., and H. J. Phaff. 1998. Metschnikowia Kamienski, p. 256-267. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts, a taxonomic study. Elsevier Science B.V., Amsterdam, The Netherlands.
- 31.Mills, D. A., E. A. Johannsen, and L. Cocolin. 2002. Yeast diversity and persistence in Botrytis-affected wine fermentation. Appl. Environ. Microbiol. 68:4884-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muckenschnabel, I., B. A. Goodman, B. Williamson, G. D. Lyon, and N. Deighton. 2002. Infection of leaves of Arabidopsis thaliana by Botrytis cinerea: changes in ascorbic acid, free radicals and lipid peroxidation products. J. Exp. Bot. 53:207-214. [DOI] [PubMed] [Google Scholar]
- 33.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 45:26723-26726. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen, H.-V., and G. Panon. 1998. The yeast Metschnikowia pulcherrima has an inhibitory effect against various yeast species. Sci. Aliments 18:515-526. [Google Scholar]
- 35.O'Donell, K. 1993. Fusarium and its near relatives, p. 225-233. In D. R. Reynolds and J. W. Taylor (ed.), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, United Kingdom.
- 36.Piano, S., V. Neyrotti, Q. Migheli, and M. L. Gullino. 1997. Biocontrol capability of Metschnikowia pulcherrima against Botrytis postharvest rot of apple. Postharvest Biol. Technol. 11:131-140. [Google Scholar]
- 37.Prakitchaiwattana, C. J., G. H., Fleet, and G. M. Heard. 2004. Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res. 4:865-877. [DOI] [PubMed] [Google Scholar]
- 38.Qin, G., S. Tian, and Y. Xu. 2004. Biocontrol of postharvest diseases on sweet cherries by four antagonistic yeasts in different storage conditions. Postharvest Biol. Technol. 31:51-58. [Google Scholar]
- 39.Ramos, S., and I. G. Garcia-Acha. 1975. A vegetative cycle of Pullularia pullulans. Trans. Br. Mycol. Soc. 64:129-135. [Google Scholar]
- 40.Reyes, M. E. Q., K. G. Rohrbach, and R. E. Paull. 2004. Microbial antagonists control postharvest black rot of pineapple fruit. Postharvest Biol. Technol. 33:193-203. [Google Scholar]
- 41.Rousseau, S., and B. Doneche. 2001. Effect of water activity (aw) on the growth of some epiphytic microorganisms isolated from grape berry. Vitis 40:75-78. [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Sansone, G., I. Rezza, V. Calvente, D. Benuzzi, and M. I. S. de Tosetti. 2005. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Technol. 35:245-251. [Google Scholar]
- 44.Scherm, B., G. Ortu, A. Muzzu, M. Budroni, G. Arras, and Q. Migheli. 2003. Biocontrol activity of antagonistic yeasts against Penicillium expansum on apple. J. Plant Pathol. 85:205-213. [Google Scholar]
- 45.Sipiczki, M. 2003. Candida zemplinina sp. nov., an osmotolerant and psychrotolerant yeast that ferments sweet botrytized wines. Int. J. Syst. Evol. Microbiol. 53:2079-2083. [DOI] [PubMed] [Google Scholar]
- 46.Sipiczki, M., and L. Ferenczy. 1978. Enzymic methods for enrichment of fungal mutants. I. Enrichment of Schizosaccharomyces pombe mutants. Mutat. Res. 50:153-173.349371 [Google Scholar]
- 47.Spadaro, D., and M. L. Gullino. 2004. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 91:185-194. [DOI] [PubMed] [Google Scholar]
- 48.Spadaro, D., R. Vola, S. Piano, and M. L. Gullino. 2002. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 24:123-134. [Google Scholar]
- 49.van der Walt, J. P., and D. Yarrow. 1984. Methods for the isolation, maintenance, classification and identification of yeasts, p. 45-104. In N. J. W. Kreger-van Rij (ed.), The yeasts: a taxonomic study. Elsevier, Amsterdam, The Netherlands.
- 50.Wilson, C. L., and E. Chalutz. 1989. Postharvest biological control of Penicillium rots of citrus with antagonistic yeasts and bacteria. Sci. Hortic. 40:105-112. [Google Scholar]
- 51.Winkelmann, G. 2002. Microbial siderophore-mediated transport. Biochem. Soc. Trans. 30:691-696. [DOI] [PubMed] [Google Scholar]
- 52.Yao, H., S. Tian, and Y. Wang. 2004. Sodium bicarbonate enhances biocontrol efficacy of yeasts on fungal spoilage of pears. Int. J. Food Microbiol. 93:297-304. [DOI] [PubMed] [Google Scholar]