Abstract
We investigated the effects of O2 on Bifidobacterium species using liquid shaking cultures under various O2 concentrations. Although most of the Bifidobacterium species we selected showed O2 sensitivity, two species, B. boum and B. thermophilum, demonstrated microaerophilic profiles. The growth of B. bifidum and B. longum was inhibited under high-O2 conditions accompanied by the accumulation of H2O2 in the medium, and growth was restored by adding catalase to the medium. B. boum and B. thermophilum grew well even under 20% O2 conditions without H2O2 accumulation, and growth was stimulated compared to anoxic growth. H2O-forming NADH oxidase activities were detected dominantly in cell extracts of B. boum and B. thermophilum under acidic reaction conditions (pH 5.0 to 6.0).
Although anaerobes are defined as being unable to grow in the presence of O2, their degree of O2 sensitivity exhibits wide variation (2, 3, 10, 11, 14, 15, 16, 17, 18, 20, 21, 25, 28). The genus Bifidobacterium is a well-investigated anaerobe known to be beneficial to human health. Its sensitivity to O2 causes a loss of viability during manufacture and storage as well as after incorporation into the human body (36). The O2 sensitivity differs among strains and species (13). de Vries and Stouthamer (7) classified Bifidobacterium species into three categories according to their sensitivities to O2. They proposed that some O2-sensitive species produce H2O2 through NADH oxidase activity. Since then, there have been several approaches taken to investigate bifidobacterial oxidative growth inhibition (1, 6, 9, 32, 33, 37); however, the mechanism of growth inhibition under oxic conditions remains unclear.
In this study, microaerophilic Bifidobacterium species were found. The main objectives of the present study were to (i) determine growth characteristics with respect to O2 using several Bifidobacterium species, (ii) determine the factor responsible for aerobic growth inhibition using O2-sensitive species, (iii) investigate the metabolic properties of O2-sensitive and microaerophilic Bifidobacterium species under oxic growth conditions, and (iv) investigate the properties of O2 reduction systems that should differ between O2-sensitive and microaerophilic Bifidobacterium species.
Effect of O2 on the growth of Bifidobacterium species.
Bifidobacterium species are classified as typical anaerobic bacteria; however, their differing degrees of O2 sensitivity in liquid shaking culture are not well characterized. We selected several species by referring to reports concerning the physiological effects of O2. B. bifidum, B. longum, B. breve, and B. infantis were selected from among strains often used in milk products and intestinal probiotics. B. asteroides is reported to possess catalase (13, 30). B. indicum has characteristics similar to those of B. asteroides but shows catalase activity only when hemin is added to the medium (13, 30). B. boum, B. globosum, and B. thermophilum are reported to form colonies under atmospheric conditions of 90% air-10% CO2 without the cells becoming catalase or pseudo-catalase positive (29, 31). Strains were grown at 37°C in modified MRS medium (without 0.5% sodium acetate) containing 1% (wt/vol) glucose, 1% proteose peptone, 0.2% beef extract, 0.5% yeast extract, 0.2% ammonium citrate, 0.02% MgCl2, 0.2% K2HPO4, and 0.005% MnSO4. The culture medium was sparged with O2-free N2 gas, and 30 ml of anoxic medium was transferred into 150-ml serum bottles with rubber tube stoppers, which were then sealed and autoclaved; the pH was approximately 6.7. To avoid the effect of CO2 on oxic growth, CO2-free gas was used in the anaerobic atmospheres. Sterile 100% O2 was added to the headspace of the culture bottles to the concentrations indicated. Headspace gas conditions were checked with a Shimadzu GC-14 gas chromatograph equipped with a thermal conductivity detector and a Shincarbon-ST column (Shimadzu, Tokyo, Japan). Anoxically grown strains were inoculated into the sealed serum bottles, and the bottles were placed horizontally and cultured with vigorous shaking (150 rpm) at 37°C. After inoculation, the starting medium pH decreased to 6.2 or 6.3. Most of the species, except B. boum and B. thermophilum, showed significant growth inhibition under 20% O2-80% N2 conditions (Table 1).
TABLE 1.
Growth of Bifidobacterium species in liquid shaking culture under 20% O2-80% N2 conditions
| Straina | % O2 | Maximum growthb |
|---|---|---|
| B. asteroides JCM8230T | 0 | 1.45 ± 0.03 |
| 20 | 0.30 ± 0.15 | |
| B. bifidum JCM1255T | 0 | 1.59 ± 0.18 |
| 20 | 0.30 ± 0.04 | |
| B. boum JCM1211T | 0 | 2.47 ± 0.09 |
| 20 | 3.90 ± 0.30 | |
| B. breve JCM1192T | 0 | 0.98 ± 0.10 |
| 20 | 0.20 ± 0.02 | |
| B. globosum JCM7089 | 0 | 1.13 ± 0.34 |
| 20 | 0.24 ± 0.07 | |
| B. indicum JCM1302T | 0 | 0.90 ± 0.04 |
| 20 | 0.28 ± 0.09 | |
| B. infantis JCM1222T | 0 | 1.18 ± 0.10 |
| 20 | 0.27 ± 0.02 | |
| B. longum JCM1217T | 0 | 1.71 ± 0.11 |
| 20 | 0.22 ± 0.01 | |
| B. thermophilum JCM1207T | 0 | 1.35 ± 0.07 |
| 20 | 2.11 ± 0.49 |
Strains were provided by the Japan Culture Collection Center.
Maximum growth during 28 h of culture (measured at an optical density of 660 nm [OD660]). When the OD660 rose above 1.0, the cell density was measured by diluting the culture medium with uninoculated medium. None of the growth inhibition was reversed after 28 h. Data are the means ± standard deviations of two independent experiments.
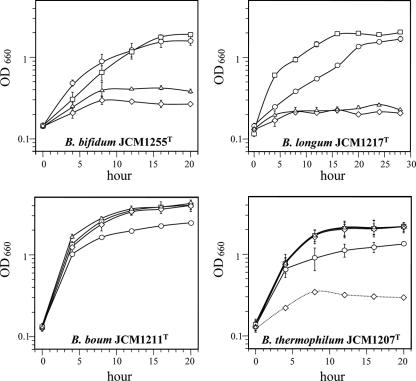
The growth profiles of the selected species are shown in Fig. 1. B. bifidum, a type species of the genus Bifidobacterium, stopped growing under 10% O2. B. longum stopped growing under 10% O2, but, interestingly, growth under 5% O2 was stimulated compared to anoxic growth. B. boum and B. thermophilum grew well in the presence of O2, and their growth was stimulated compared to that under anoxic conditions. The growth of B. boum and B. thermophilum reached a maximum at 16 h after inoculation, when approximately 2% to 3% of the starting O2 in the headspace (5%, 10%, and 20% O2) was consumed. The growth of B. thermophilum under 20% O2 was sometimes completely halted at the initial phase of growth, accompanied by the production of H2O2 (approximately 20% of cultures showed arrested growth; otherwise, the cultures grew well) (Fig. 1; Table 2).
FIG. 1.
Growth of Bifidobacterium species under various oxygen conditions in liquid shaking culture. Growth was measured as the increase in optical density at 660 nm (OD660) by taking 0.2 ml of the 30-ml culture into a gas-tight syringe. Circles, 100% N2 cultures; squares, 5% O2-95% N2 cultures; triangles, 10% O2-90% N2 cultures; and diamonds, 20% O2-80% N2 cultures. The growth of B. thermophilum was sometimes arrested in cultures grown under 20% O2-80% N2 (dashed line).
TABLE 2.
Growth and H2O2 accumulation of Bifidobacterium species under 0% to 20% O2 conditionsa
| Species and % O2 | Maximum growthb | H2O2 concn (mM)d |
|---|---|---|
| B. bifidum JCM1255T | ||
| 0 | 1.59 ± 0.18 | ND |
| 5 | 1.89 ± 0.05 | ND |
| 10 | 0.42 ± 0.03 | 0.18 ± 0.02 |
| 20 | 0.30 ± 0.04 | 0.32 ± 0.04 |
| 20 (+ catalase)c | 1.10 ± 0.16 | ND |
| B. longum JCM1217T | ||
| 0 | 1.71 ± 0.11 | ND |
| 5 | 2.05 ± 0.09 | ND |
| 10 | 0.27 ± 0.01 | 0.26 ± 0.02 |
| 20 | 0.23 ± 0.01 | 0.33 ± 0.04 |
| 20 (+ catalase)c | 1.15 ± 0.19 | ND |
H2O2 accumulation in the culture medium of B. boum JCM1211T and B. thermophilum JCM1207T under 0 to 20% O2 conditions was not detected (less than 0.01 mM). B. thermophilum sometimes stopped growing at the initial phase of growth under 20% O2 conditions, and H2O2 accumulation was detected (maximum optical density at 660 nm [OD660], 0.37 ± 0.03; H2O2 concentration, 0.38 ± 0.02 mM). Data are the means ± standard deviations of two independent experiments.
Maximum OD660 for 28 h.
Filter-sterilized catalase was added before inoculation (25 U catalase/ml medium).
ND, not detected (less than 0.01 mM).
Determination of H2O2 production and growth recovery by catalase.
The accumulation of H2O2 in the culture medium was measured. The culture supernatants were diluted 6:4 with a solution containing 0.01% horseradish peroxidase, 0.2% Triton X-100, and 0.63 mM o-dianisidine dihydrichloride in 50 mM acetate buffer, pH 5.0. The production of H2O2 was monitored spectrophotometrically at 460 nm by detecting the oxidation of o-dianisidine dihydrochloride (4). In all samples in which growth inhibition was observed, H2O2 accumulation was detected (Table 2). No H2O2 accumulation was seen in the culture medium of B. boum or B. thermophilum under 0% to 20% O2 conditions.
The growth inhibition of B. bifidum and B. longum under 20% O2 conditions was partially reversed when catalase (Roche, Japan) was added to the medium (Table 2). The inability of exogenously added catalase to decompose intracellular H2O2 might be a reason for the failure to obtain complete growth recoveries. These results indicate that the primary factor in aerobic inhibition is the production of H2O2 derived from O2 reduction.
Fermentation under various O2 concentrations.
Glucose consumption and fermentation products in the late exponential phase of growth under various O2 concentrations were analyzed. Glucose consumption as well as acetate and lactate production were determined with a Waters LC module 1-plus high-performance liquid chromatograph equipped with a Shodex RI detector and a Shodex SH1011 column (300 mm by 8 mm; Shodex, Tokyo, Japan); the column temperature was 60°C, and the mobile phase was 0.01 N H2SO4 at a flow rate of 1.0 ml/min. Data are the means of two independent experiments. The mean standard deviations were less than 5%. In the absence of O2, B. bifidum, B. boum, and B. thermophilum showed similar lactate/acetate molar ratio profiles (glucose consumed:acetate produced:lactate produced, 1:1.34:0.82 [for B. bifidum], 1:1.32:1.14 [B. boum], and 1:1.29:1.03 [B. thermophilum]). B. longum showed the highest production of lactate among the tested species (1:1.17:1.49). In the presence of 5% O2, no dramatic change in the lactate/acetate production ratio was observed for B. bifidum and B. thermophilum. In the case of B. longum, a slight increase in acetate production and slight decrease in lactate production were observed (1:1.27:1.21). For B. boum, the ratio of acetate production was slightly decreased under 10% and 20% O2 conditions (1:1.02 to 1.18:0.91 to 0.93). No other fermentation products (CO2, acids, and alcohols from C1 to C6 other than acetate and lactate) were detected under any set of culture conditions (detected by gas chromatograph; data not shown). We expected an aerobic metabolic shift from lactate to acetate for B. boum and B. thermophilum as well because of the drainage of reducing power to O2, which has been speculated upon in some reports on lactic acid bacteria and Bifidobacterium species (5, 34, 35), but these metabolic shifts were not observed.
Determination and characterization of NADH oxidase activities.
O2-sensitive Bifidobacterium species accumulated H2O2 under high-O2 conditions. Several reports have mentioned a correlation between the production of H2O2 and NADH oxidation activity (6, 7, 32, 33, 37). NADH oxidases have been divided into several groups based on their final reaction products: H2O, H2O2, O2−, or mixtures thereof (8, 12, 22, 23, 26, 38, 39). To our knowledge, none of the NADH oxidases in Bifidobacterium species have been characterized in terms of their function. To characterize the O2 reduction system in Bifidobacterium species, several substrate-dependent oxidase activities in cell extracts were tested using glucose, lactate, pyruvate, alanine, and NADH as electron donors. These oxidase activities were detected as the reduction of O2 monitored by an O2 electrode, as described previously (15, 16). The reactions were carried out under different pH conditions in sodium phosphate buffer (pH 5.0 to 7.0). None of the substrates other than NADH reduced O2. NADH-dependent H2O2 reductase activity, assayed anaerobically as described previously (15, 16, 18), was also detected in cell extracts of every tested species, but the activities were very low (0.5 to 1.8 mU/mg protein at a pH range of 5.0 to 7.0 in every tested species) compared to the NADH oxidase activities, and no significant inductions were detected by aeration. These activities are also very low compared to the activities detected in O2-tolerant species as reported by Shimamura et al. (approximately 100 mU/mg protein was detected in cell extracts) (33).
To investigate the difference in H2O2 accumulation profiles between O2-sensitive and microaerophilic species, the enzymatic properties of NADH-dependent oxidase activities were characterized. NADH-dependent oxidase activities from four tested species were potassium cyanide (2 mM) and NaN3 (2 mM) insensitive (no inhibitions were detected; data not shown), suggesting that those activities are not related to the cytochrome-type multiple-terminal oxidase activity (19). B. bifidum showed an optimum pH in the acidic region (28.8 mU/mg protein at pH 5.0, 21.4 mU/mg protein at pH 6.0, and 12.4 mU/mg protein at pH 7.0). B. longum showed the strongest activity among the tested species, with a pH optimum of 6.0 (64.6 mU/mg protein at pH 5.0, 77.8 mU/mg protein at pH 6.0, and 44.7 mU/mg protein at pH 7.0). B. boum showed rather low activity compared to B. bifidum and B. longum under all pH conditions (13.8 mU/mg protein at pH 5.0, 11.4 mU/mg protein at pH 6.0, and 13.8 mU/mg protein at pH 7.0). B. thermophilum also showed low activity (10.7 mU/mg protein at pH 5.0, 9.3 mU/mg protein at pH 6.0, and 10.4 mU/mg protein at pH 7.0). Aeration slightly increased the activity (about 1.2 to 1.5 times) of every tested species. Although total activity and the O2-induced properties of NADH oxidase activity did not differ significantly among species at any tested pH, the final reaction product differed significantly. H2O2 production from NADH oxidation was detected by monitoring O2 production with an O2 electrode after the addition of catalase to the NADH oxidation reaction (15, 16, 26, 27, 38). The ratio of H2O2-forming types of NADH oxidase activity in cell extracts was estimated by calculating the stoichiometric production of H2O2 during the NADH-dependent oxidase reaction, which is detectable by adding catalase to the reaction vessels (the H2O2-forming type of NADH oxidase produces 50% O2 after the addition of catalase to the total amount of O2 consumed by the NADH oxidase reaction) (15, 16, 26, 27, 38). The absence of O2− production by the NADH oxidase reactions was confirmed by monitoring the reduction of ferricytochrome c by O2− as described elsewhere (16, 23, 24, 38). As shown in Table 3, H2O2-forming NADH oxidase activity was predominant at all pH conditions in B. bifidum and B. longum. In B. boum, the NADH oxidase activity in the cell extract obtained from microoxically grown cells produced no H2O2 at all over the reaction pH range of 5.0 to 6.0. In the case of B. thermophilum, 10% to 20% of the NADH oxidase activity was of the H2O2-forming variety at pH 5.0 in CFE from anoxically grown cells, and this decreased to 0% in the CFE from microoxically grown cells.
TABLE 3.
Percentage of H2O2-forming NADH oxidase activity in the total NADH oxidase activity under different pH conditions
| Species | Growth condition | % H2O2-forming NADH oxidase activity at pHc:
|
||||
|---|---|---|---|---|---|---|
| 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | ||
| B. bifidum JCM1255T | ANa | 100 | 100 | 100 | 100 | 100 |
| AEb | 85 ± 3 | 88 ± 1 | 85 ± 3 | 90 ± 3 | 94 ± 5 | |
| B. boum JCM1211T | AN | 0 | 22 ± 10 | 67 ± 13 | 63 ± 5 | 100 |
| AE | 0 | 0 | 0 | 52 ± 18 | 100 | |
| B. longum JCM1217T | AN | 74 ± 4 | 60 ± 5 | 46 ± 3 | 48 ± 5 | 63 ± 7 |
| AE | 100 | 75 ± 8 | 66 ± 5 | 69 ± 2 | 78 ± 2 | |
| B. thermophilum JCM1207T | AN | 16 ± 6 | 38 ± 16 | 91 ± 9 | 97 ± 3 | 100 |
| AE | 0 | 0 | 8 ± 8 | 53 ± 15 | 100 | |
AN, cell extracts were obtained from cells grown under anoxic conditions.
AE, cell extracts were obtained from cells grown under microoxic conditions (grown in aerobic static culture by stirring the medium with a magnetic stirrer).
Data are the means ± standard deviations of two independent experiments.
Conclusions.
B. boum and B. thermophilum show growth stimulation in the presence of O2. These two species do not form colonies under air conditions, so they are reasonably classified as anaerobes. The accumulation of H2O2 in O2-sensitive species must be the end product of O2 reduction. NADH-dependent oxidase activities were detected as part of an O2 reduction system. Although the total activity of NADH-dependent oxidase in the CFE was similar among species, the activity profiles differed between O2-sensitive and microaerophilic species. Further investigation of NADH-dependent oxidase activity will clarify the difference in the H2O2 accumulation profiles of O2-sensitive and microaerophilic species.
Acknowledgments
We thank Tohru Kodama and Junichi Nakagawa for valuable discussions. We also thank Masahiro Nagasaku, Jun Anzai, Nobuko Sato, Shingo Tamaru, Mitsunori Todoroki, Tomoko Kohno, and Kurara Emi for helpful technical assistance at the Tokyo University of Agriculture.
Footnotes
Published ahead of print on 1 September 2006.
REFERENCES
- 1.Ahn, J. B., H. J. Hwang, and J. H. Park. 2001. Physiological responses of oxygen-tolerant anaerobic Bifidobacterium longum under oxygen. J. Microbiol. Biotechnol. 11:443-451. [Google Scholar]
- 2.Baughn, A. D., and M. H. Malamy. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441-444. [DOI] [PubMed] [Google Scholar]
- 3.Boga, H. I., and A. Brune. 2003. Hydrogen-dependent oxygen reduction by homoacetogenic bacteria isolated from termite guts. Appl. Environ. Microbiol. 69:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brent, E., and H. U. Bergmeyer. 1962. Anorganische peroxyde, p. 633-635. In H. U. Bergmeyer (ed.), Methoden der enzymatischen analyse. Verlag Chemie, Weinheim, Germany.
- 5.Condon, S. 1987. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 46:269-280. [Google Scholar]
- 6.Cox, R. P., and N. Marling. 1992. High-affinity oxygen uptake by Bifidobacterium bifidum. Antonie Leeuwenhoek 62:291-297. [DOI] [PubMed] [Google Scholar]
- 7.de Vries, W., and A. H. Stouthamer. 1969. Factors determining the degree of anaerobiosis of Bifidobacterium strains. Arch. Mikrobiol. 65:275-287. [DOI] [PubMed] [Google Scholar]
- 8.Dolin, M. I. 1955. The DPNH-oxidizing enzymes of Streptococcus faecalis. II. The enzymes utilizing oxygen, cytochrome c, peroxide and 2,6-dichlorophenol-indophenol or ferricyanide as oxidants. Arch. Biochem. Biophys. 55:415-435. [Google Scholar]
- 9.Gonzalez, R., A. Blancas, R. Santillana, A. Azaola, and C. Wacher. 2004. Growth and final product formation by Bifidobacterium infantis in aerated fermentations. Appl. Microbiol. Biotechnol. 65:606-610. [DOI] [PubMed] [Google Scholar]
- 10.Gossner, A. S., K. Kusel, D. Schulz, S. Trenz, G. Acker, C. R. Lovell, and H. L. Drake. 2006. Trophic interaction of the aerotolerant anaerobe Clostridium intestinale and the acetogen Sporomusa rhizae sp. nov. isolated from roots of the black needlerush Juncus roemerianus. Microbiology 152:1209-1219. [DOI] [PubMed] [Google Scholar]
- 11.Holland, K. T., J. S. Knapp, and J. G. Shoesmith. 1987. Anaerobes and oxygen, p. 4-12. In K. T. Holland, J. S. Knapp, and J. G. Shoesmith (ed.), Anaerobic bacteria. Chapman and Hall, New York, N.Y.
- 12.Hoskins, D. D., H. R. Whiteley, and B. Mackler. 1962. The reduced diphosphopyridine nucleotide oxidase of Streptococcus faecalis: purification and properties. J. Biol. Chem. 237:2647-2651. [PubMed] [Google Scholar]
- 13.Jones, D., and M. D. Collins. 1986. Irregular, nonsporing gram-positive rods, p. 1261-1434. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. Williams and Wilkins Co., Baltimore, Md.
- 14.Karnholz, A., K. Kuse, A. Gossner, A. Schramm, and H. L. Drake. 2002. Tolerance and metabolic response of acetogenic bacteria toward oxygen. Appl. Environ. Microbiol. 68:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasaki, S., T. Nakagawa, Y. Nishiyama, Y. Benno, T. Uchimura, K. Komagata, K. Kozaki, and Y. Niimura. 1998. Effect of oxygen on the growth of Clostridium butyricum (type species of the genus Clostridium), and the distribution of enzymes for oxygen and for active oxygen species in clostridia. J. Ferment. Bioeng. 86:368-372. [Google Scholar]
- 16.Kawasaki, S., J. Ishikura, D. Chiba, T. Nishino, and Y. Niimura. 2004. Purification and characterization of an H2O-forming NADH oxidase from Clostridium aminovalericum: existence of an oxygen-detoxifying enzyme in an obligate anaerobic bacteria. Arch. Microbiol. 181:324-330. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki, S., J. Ishikura, Y. Watamura, and Y. Niimura. 2004. Identification of O2-induced peptides in an obligatory anaerobe, Clostridium acetobutylicum. FEBS Lett. 571:21-25. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki, S., Y. Watamura, M. Ono, T. Watanabe, K. Takeda, and Y. Niimura. 2005. Adaptive responses to oxygen stress in obligatory anaerobes Clostridium acetobutylicum and Clostridium aminovalericum. Appl. Environ. Microbiol. 71:8442-8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kita, K., K. Konishi, and Y. Anraku. 1984. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J. Biol. Chem. 259:3375-3381. [PubMed] [Google Scholar]
- 20.Kusel, K., A. Karnholz, T. Trinkwalter, R. Devereux, G. Acker, and H. L. Drake. 2001. Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl. Environ. Microbiol. 67:4734-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loesche, W. J. 1969. Oxygen sensitivity of various anaerobic bacteria. Appl. Microbiol. 18:723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan, C., and S. G. Mayhew. 2000. Cloning, overexpression, and characterization of peroxiredoxin and NADH peroxiredoxin reductase from Thermus aquaticus. J. Biol. Chem. 275:30019-30028. [DOI] [PubMed] [Google Scholar]
- 23.Maeda, K., K. Truscott, X. L. Liu, and K. Scopes. 1992. A thermostable NADH oxidase from anaerobic extreme thermophiles. Biochem. J. 284:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCord, J. M., and I. Fridovich. 1969. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244:6049-6055. [PubMed] [Google Scholar]
- 25.Morris, J. G. 1976. Oxygen and the obligate anaerobe. J. Appl. Bacteriol. 40:229-244. [DOI] [PubMed] [Google Scholar]
- 26.Niimura, Y., K. Ohnishi, Y. Yarita, M. Hidaka, H. Masaki, T. Uchimura, H. Suzuki, M. Kozaki, and T. Uozumi. 1993. A flavoprotein functional as NADH oxidase from Amphibacillus xylanus Ep01: purification and characterization of the enzyme and structural analysis of its gene. J. Bacteriol. 175:7945-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niimura, Y., L. B. Poole, and V. Massey. 1995. Amphibacillus xylanus NADH oxidase and Salmonella typhimurium alkyl-hydroperoxide reductase flavoprotein components show extremely high scavenging activity for both alkyl hydroperoxide and hydrogen peroxide in the presence of S. typhimurium alkyl-hydroperoxide reductase 22-kDa protein component. J. Biol. Chem. 270:25645-25650. [DOI] [PubMed] [Google Scholar]
- 28.Rolfe, R. D., D. J. Hentges, B. J. Campbell, and J. T. Barrett. 1978. Factors related to the oxygen tolerance of anaerobic bacteria. Appl. Environ. Microbiol. 36:306-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scardovi, V., L. D. Trovatelli, F. Crociani, and B. Sgorbati. 1969. Bifid bacteria in bovine rumen. New species of the genus Bifidobacterium: B. globosum n. sp. and B. ruminale n. sp. Arch. Mikrobiol. 68:278-294. [PubMed] [Google Scholar]
- 30.Scardovi, V., and L. D. Trovatelli. 1969. New species of bifid bacteria from Apis mellifica L. and Apis indica F. A contribution to the taxonomy and biochemistry of the genus Bifidobacterium. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Zweite Abt. 123:64-88. [PubMed] [Google Scholar]
- 31.Scardovi, V., L. D. Trovatelli, B. Biavati, and G. Zani. 1979. Bifidobacteriumcuniculi, Bifidobacterium choerinum, Bifidobacterium boum, and Bifidobacterium pseudocatenulatum: four new species and their deoxyribonucleic acid homology relationships. Int. J. Syst. Bacteriol. 29:291-311. [Google Scholar]
- 32.Shimamura, S., F. Abe, N. Ishibashi, H. Miyakawa, T. Yaeshima, and M. Tomita. 1990. Endogenous oxygen uptake and polysaccharide accumulation in Bifidobacterium. Agric. Biol. Chem. 54:2869-2874. [Google Scholar]
- 33.Shimamura, S., F. Abe, N. Ishibashi, H. Miyakawa, T. Yaeshima, T. Araya, and M. Tomita. 1992. Relationship between oxygen sensitivity and oxygen metabolism of Bifidobacterium species. J. Dairy. Sci. 75:3296-3306. [DOI] [PubMed] [Google Scholar]
- 34.Smart, J. B., and D. T. Thomas. 1987. Effect of oxygen on lactose metabolism in lactic streptococci. Appl. Environ. Microbiol. 53:533-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talwalkar, A., and K. Kailasapathy. 2003. Metabolic and biochemical responses of probiotic bacteria to oxygen. J. Dairy Sci. 86:2537-2546. [DOI] [PubMed] [Google Scholar]
- 36.Talwalkar, A., and K. Kailasapathy. 2004. The role of oxygen in the viability of probiotic bacteria with reference to L. acidophilus and Bifidobacterium spp. Curr. Issues Intest. Microbiol. 5:1-8. [PubMed] [Google Scholar]
- 37.Uesugi, I., and M. Yajima. 1978. Oxygen and ‘strictly anaerobic’ intestinal bacteria. II. Oxygen metabolism in strictly anaerobic bacteria. Z. Allg. Mikrobiol. 18:593-601. [DOI] [PubMed] [Google Scholar]
- 38.van Niel, E. W., K. Hofvendahl, and B. Hahn-Hägerdal. 2002. Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions. Appl. Environ. Microbiol. 68:4350-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, D. E., C. J. Donnelly, M. E. Mullendore, J. van der Oost, W. M. de Vos, and E. J. Crane. 2001. The NADH oxidase from Pyrococcus furiosus. Implications for the protection of anaerobic hyperthermophiles against oxidative stress. Eur. J. Biochem. 268:5816-5823. [DOI] [PubMed] [Google Scholar]