Abstract
Eighty-one tetracycline-resistant Aeromonas sp. strains were isolated from farm-raised catfish. Morphological and biochemical characteristics indicated that 23 of the 81 aeromonads were Aeromonas hydrophila, 7 isolates were Aeromonas trota, 6 isolates were Aeromonas caviae, 42 isolates were Aeromonas veronii, and 3 isolates were Aeromonas jandaei. However, the AluI and MboI restriction fragment length polymorphism (RFLP) patterns of the PCR-amplified 1.4-kb 16S rRNA gene from all 81 tetracycline-resistant aeromonads from catfish were identical to the RFLP banding patterns of A. veronii ATCC 35626, indicating that all 81 isolates were strains of A. veronii. A multiplex PCR assay successfully amplified the 5 tetracycline-resistant genes (tetA to E) from the genomic DNA of all 81 isolates. The assay determined that tetE was the dominant gene occurring in 73/81 (90.0%) of the aeromonads. Plasmids (2.0 to 20 kb) were isolated from 33 of the 81 isolates. Dendrogram analysis of the SpeI pulsed-field gel electrophoresis identified 15 distinct macrorestriction patterns among the isolates. Our results indicate the need for use of 16S rRNA in the identification of Aeromonas spp. and the prevalence of catfish as a reservoir of tet genes.
The United States produces more than $500 million per annum of pond-raised channel catfish (Ictalurus punctatus), primarily in the southeastern part of the country (4). Infectious diseases reduce catfish production by nearly 10% every year. Motile aeromonad septicemia, caused by Aeromonas hydrophila, is one of the common diseases accounting for the reduced production (5). Antimicrobials agents, such as oxytetracycline and Romet 30 (sulfadimethoxine-ormetoprim), are used to prevent the outbreak of infectious diseases (5, 32). However, widespread use of drugs may result in the selection of tetracycline- and sulfonamide-resistant bacteria in the aquaculture environment and may play a role in the dissemination of antibiotic resistance genes to clinical aeromonad strains (20, 22).
Aeromonads have been implicated in the cause of numerous human infections, such as gastroenteritis, cellulitis, meningitis, bacteremia, soft-tissue infections, peritonitis, and bronchopulmonary infections (8, 15, 18-19). Direct contact with contaminated water and soil is the most frequent cause of gastrointestinal and wound infections in humans (15). Broad-spectrum antibiotics, such as tetracycline, are prescribed clinically for the treatment of such infections.
The molecular epidemiology of tetracycline-resistant aeromonads, especially Aeromonas salmonicida, has been well documented (2, 3, 29). These studies indicate that tetracycline resistance is plasmid-encoded and that, among the different classes of tetracycline-resistant genes, tetA is predominant. Schmidt et al. (29) reported the isolation and characterization of oxytetracycline-sulfonamide/trimethoprim-resistant aeromonads from Danish rainbow trout farms. Their PCR data indicate tetE as the predominant determinant, followed by tetA and tetD. Earlier, DePaola et al. (10) had determined that 86% of Aeromonas hydrophila isolated from catfish contained tetA and the rest harbored tetE. However, these investigators identified the pathogen based on biochemical characterization, which is often controversial, erratic, and unreliable (1, 25). Given the severity of aeromonad infections in both fish and humans, correct identification of the infectious agent is essential for the rapid selection of antibiotic therapy. In addition, little information is available on Aeromonas veronii, a food-borne pathogen causing infection in fish, food-producing animals, and humans (5, 14, 16). We, for the first time, report the isolation and identification of 81 tetracycline-resistant A. veronii isolates from farm-raised catfish. These strains were identified as A. veronii based on the restriction fragment length polymorphism (RFLP) pattern of the 16S rRNA gene. We also report the epidemiological distribution of tet genes in A. veronii from catfish. Such information is vital in tracking the spread of tet genes.
MATERIALS AND METHODS
Isolation, characterization, and identification of bacteria.
Bacteria were isolated from the intestines of catfish collected from 16 commercial ponds in Arkansas, Louisiana, and Texas. One gram of the intestinal sample was enriched for 6 h in alkaline phosphate medium (pH 8.0) supplemented with 30 μg/ml of ampicillin (13). Enriched samples were streaked on MacConkey agar plates supplemented with 30 μg/ml of tetracycline and incubated at 37°C overnight. Presumptive positive colonies were further biochemically characterized and identified by the Vitek GNI+ card with VTK-R07-01 software (bioMerieux Vitek, Hazelwood, MO) and by fatty acid methyl ester analysis (MIDI, Newark, DE). All isolates were stored in Luria-Bertani (LB) broth containing 20% glycerol at −70°C. Organisms were grown overnight at 37°C in LB broth or on Trypticase soy agar plates supplemented with 5% sheep's blood. Cultures A. hydrophila ATCC 7966, A. veronii ATCC 35626, Aeromonas caviae ATCC 13136, Aeromonas trota ATCC 49661, and A. salmonicida ATCC 33659 were obtained from American Type Culture Collection, Manassas, Va.
Amplification of 16S rRNA from Aeromonas spp. by PCR.
The following oligonucleotides were used to amplify the 16S rRNA gene (7, 11) in a PCR: oligonucleotide F1 (5′-AGAGTTTGATCATGGTCAG-3′) and oligonucleotide F2 (5′-GGTTACCTTGTTACGACTT-3′). The primers were synthesized by MWG Biotech (High Point, N.C.). The PCR amplification reactions were carried out in a GeneAmp PCR System 9700 (Applied Biosystems, Forrest City, CA). The reaction mixture contained 47 μl of QIAGEN PCR mixture, 2 μl of the PCR primer mix, and 1 μl of the genomic DNA. PCR was performed under the following conditions: denaturation at 93°C for 3 min, followed by 35 cycles at 94°C for 1 min, 56°C for 1 min, and 72°C for 2 min. After the final cycle, an extension at 72°C was allowed for 10 min. The gels were electrophoresed, stained with ethidium bromide, and photographed (23). As a routine negative control, Escherichia coli cells or DNA was used. A reagent blank contained all components of the reaction mixture with the exception of template DNA, for which sterile distilled water was substituted. The thermocycler, tips, and pipettes used for preparing the PCR reagents and template DNA were kept in a different location from where the gels were loaded, stained, and photographed. All reagents used in an experiment were taken from the freezer and discarded at the end of the day.
RFLP of 16S rRNA from aeromonads.
The PCR amplicon was purified with a QIAquick PCR purification kit (QIAGEN, Valencia, CA). Restriction digestions were performed by incubating 10 μl of the amplified PCR product with 5 U of each restriction enzyme (AluI and MboI; New England Biolabs, Beverly, MA) as per the manufacturer's instructions (7, 11). Digestion was performed at 37°C for 4 h. The digested samples were electrophoresed on 4.0% Metaphor agarose gel (FMC Bioproducts, Philadelphia, PA), stained, and photographed (23).
Antibiotic susceptibility testing by disk diffusion.
The antibiotic susceptibility of each aeromonad was determined by the disk diffusion method (6). Aeromonad strains were streaked on Mueller-Hinton agar plates, and the various antibiotic disks were applied on the streaked cultures with a Dispens-O-Disc dispenser (Difco Laboratories, Detroit, MI). Disks of bacitracin (10 μg), kanamycin (30 μg), erythromycin (15 μg), gentamicin (10 μg), trimethoprim-sulfamethoxazole (23:75; 1.25 μg), tetracycline (30 μg), chloramphenicol (30 μg), streptomycin (10 μg), nalidixic acid (30 μg), and ciprofloxacin (5 μg) were used. After 18 h of incubation at 37°C, the zones of inhibition were measured and compared to the manufacturer's instruction and by the criteria of the National Committee for Clinical Laboratory Standards (21). Strains were considered resistant to the above stated antibiotics when no zones of inhibition were observed or when the zone diameters were less than the manufacturer's recommendations.
Plasmid isolation.
Plasmids were isolated from bacterial cultures grown overnight at 37°C in LB broth with a QiaPrep Spin Miniprep kit (QIAGEN, Valencia, CA). The plasmids were electrophoresed on 1.0% agarose gels.
Multiplex PCR of the tetracycline resistance genes.
A multiplex PCR was designed to amplify the tetracycline resistance genes (tetA to E) from the isolates (Table 1). The genomic DNA was isolated by the QIAamp DNA mini kit (QIAGEN). The composition of the mixture was as follows: 10× PCR buffer, 2.5 μl; 10 mM deoxynucleoside triphosphate, 0.5 μl; 10 μM primer mixture, 2.5 μl; Taq DNA polymerase, 0.4 μl; 25 mM MgCl2, 2.0 μl; genomic DNA extract, 2.0 μl; nuclease-free water, 15.1 μl. Samples were overlaid with sterile mineral oil, denatured at 94°C for 2 min, and then cycled at 94°C for 20 s, 53°C for 10 s, and 65°C for 45 s, with 1 cycle at 94°C for 20 s, 53°C for 10 s, and 65°C for 4 min. The samples were maintained afterwards at 4°C. A reagent blank contained all components of the reaction mixture except template DNA, for which sterile distilled water was substituted. This step was included in every PCR procedure. PCR products were electrophoresed on a 0.8% agarose gel in 1× Tris-borate-EDTA (TBE) buffer.
TABLE 1.
Sequences of the oligonucleotide primers used in the study
| Primer | Product size (bp) | Primer sequence (5′-3′) | Position | Length (bp) | Tm (°C)a |
|---|---|---|---|---|---|
| tetAF | 211 | GCTACATCCTGCTTGCCTTC | 869 | 20 | 54.7 |
| tetAR | GCATAGATCGCCGTGAAGAG | 1079 | 20 | 54.4 | |
| ClassB tetAF | 391 | TCATTGCCGATACCACCTCAG | 350 | 21 | 55.7 |
| ClassB tetAR | CCAACCATCATGCTATTCCATCC | 740 | 23 | 55.8 | |
| ClassC tetAF | 897 | CTGCTCGCTTCGCTACTTG | 226 | 19 | 54.6 |
| ClassC tetAR | GCCTACAATCCATGCCAACC | 1122 | 20 | 54.9 | |
| ClassD tetAF | 844 | TGTGCTGTGGATGTTGTATCTC | 276 | 22 | 54.5 |
| ClassD tetAR | CAGTGCCGTGCCAATCAG | 1119 | 18 | 54.3 | |
| ClassE tetAF | 744 | ATGAACCGCACTGTGATGATG | 1 | 21 | 54.9 |
| ClassE tetAR | ACCGACCATTACGCCATCC | 744 | 19 | 55.2 |
Tm, melting temperature.
PFGE.
Genomic DNA samples of the isolates were subjected to pulsed-field gel electrophoresis (PFGE) (23). DNA plugs were digested with 20 U of SpeI (New England Biolabs, Beverly, MA) at 37°C for 5 h. The digested DNA was electrophoresed in 1.0% SeaKem Gold (FMC Corporation, Philadelphia, PA) agarose gel with Chef-Mapper III PFGE (Bio-Rad Laboratories, Hercules, CA) system for 18 h. The gels were stained for 30 min with ethidium bromide, destained with distilled water, and photographed using the Eagle Eye II gel documentation system (Stratagene, La Jolla, CA). The genetic relationship among the tetracycline-resistant aeromonad isolates was analyzed using the Bionumerics software (Applied Maths, Kortrijk, Belgium).
RESULTS
Isolation and identification of Aeromonas spp. from catfish samples.
Approximately 327 bacterial isolates exhibiting the typical aeromonad morphological characteristics, such as buff-colored colonies on Trypticase soy agar plates, gram-negative, oxidase-positive, rod-shaped bacteria resistant to ampicillin, were isolated from 190 catfish samples. Eighty-one of the 327 (ca. 25.0%) isolates were tetracycline resistant. From the biochemical characteristics (GNI+; Vitek), 23 of these isolates were tentatively identified as A. hydrophila, 7 as A. trota, 42 as A. veronii, 6 as A. caviae, and 3 as Aeromonas jandaei. Results from the Vitek database indicated that the percent probabilities of identification of A. veronii were 95 to 99%. However, the percent probabilities of identification of A. hydrophila, A. trota, A. caviae, and A. jandaei were only 65 to 85%.
PCR amplification and RFLP profile of the 16S rRNA gene.
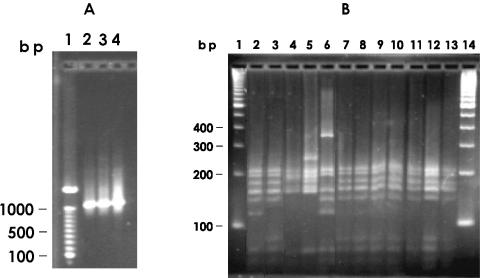
One pair of synthetic 16S rRNA-specific oligonucleotide primers, targeting a 1.4-kb region of the 16S rRNA, was used in the PCR assay. The protocol amplified the 1.4-kb gene from the genomic DNA obtained from all 81 aeromonads (Fig. 1A). The purified PCR amplicon was double digested with AluI and MboI, and the digests were separated on a 4.0% Metaphor agarose gel. Digestion of the 16S rRNA PCR amplicon from A. hydrophila ATCC 7966 yielded 7 restriction fragments measuring 50 to 210 bp (Fig. 1B, lane 2). Digestion of the amplified PCR product from A. veronii ATCC 35626 also yielded 7 restriction fragments measuring 50 to 210 bp (Fig. 1B, lane 3). The RFLP of A. hydrophila had a distinct DNA fragment measuring 125 bp (Fig. 1B, lane 2), whereas A. veronii had a distinct DNA fragment measuring ca. 75 bp (Fig. 1B, lane 3). Restriction digestion of the amplified gene product from A. caviae ATCC 13136 resulted in 5 DNA fragments measuring 50 to 200 kb (Fig. 1B, lane 4). Digestion of the 1.4-kb PCR amplicon from A. trota ATCC 49661 and A. salmonicida ATCC 33659 resulted in 7 and 8 DNA restriction fragments ranging from 50 to 400 bp, respectively (Fig. 1B, lanes 5 and 6). Amplification of the 16S rRNA gene, restriction digestion of the purified PCR product and electrophoresis of the digested DNA fragments of the 23 tetracycline-resistant isolates considered to be A. hydrophila (Fig. 1B, lane 7), 7 tetracycline-resistant isolates considered to be A. trota (Fig. 1B, lane 8), 42 tetracycline-resistant isolates considered to be A. veronii (Fig. 1B, lane 9), 6 tetracycline-resistant isolates considered to be A. caviae (Fig. 1B, lane 10), and 3 tetracycline-resistant isolates considered to be A. jandaei (Fig. 1B, lane 11) yielded 7 identical restriction fragments measuring 50 to 200 bp. The size and number of restriction fragments from these isolates were identical to the size and number of restriction fragments from the genomic DNA of A. veronii ATCC 35626 (Fig. 1B, lane 3). Our results, based on the PCR amplification and RFLP pattern of the 16S rRNA gene from the 81 tetracycline-resistant aeromonads isolated from catfish, indicated that all are strains of A. veronii.
FIG. 1.
(A) PCR amplification of the 1.4-kb 16S rRNA gene from representative strains. (B) AluI plus MboI RFLP profile of the 16S rRNA amplified from representative isolates of Aeromonas spp. from catfish. Lanes: 1 and 14, 100-bp molecular size markers; 2, A. hydrophila ATCC 7966; 3, A. veronii ATCC 35626; 4, A. caviae ATCC 13136; 5, A. trota ATCC 49661; 6, A. salmonicida ATCC 33659; 7, AVT 720; 8, AVT 429; 9, AHT 03; 10, AVT 61; 11, AJT 2; 12, AJT 1; 13, AJT 3.
Antibiotic resistance profiles of Aeromonas isolates.
All 81 strains of A. veronii were resistant to multiple antibiotics (see “Antibiotic susceptibility testing by disk diffusion” above). Besides tetracycline, all isolates were resistant to ampicillin and penicillin. A majority (69/81) of the ampicillin-, penicillin-, and tetracycline-resistant isolates were also resistant to bacitracin. Six of the 81 strains exhibited resistance to streptomycin. Surprisingly, few isolates (6/81) were resistant to trimethoprim-sulfamethoxazole.
PCR amplification of the tetracycline resistance (tet) genes.
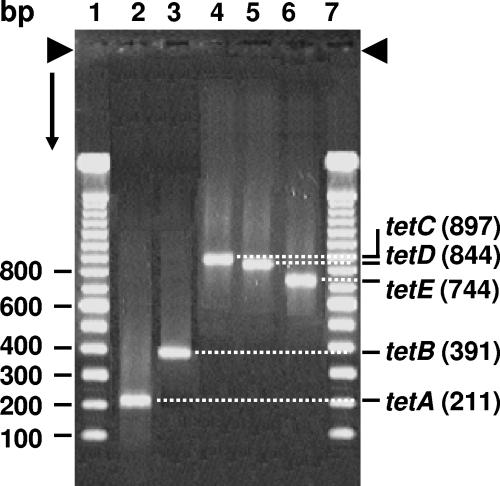
The PCR assay detected tetA in 3/81 isolates by amplifying the PCR amplicon measuring 211 bp (Fig. 2). The protocol detected the presence of tetB in 23/81 strains of tetracycline-resistant aeromonads by amplifying the PCR amplicon measuring 391 bp. Similarly, the multiplex PCR assay detected tetC and tetD in 2/81 aeromonads by amplifying their PCR products measuring 897 bp and 844 bp, respectively, from the DNA of A. veronii. The assay detected the presence of the tetE gene in 73/81 aeromonads by amplifying the PCR product measuring 744 bp from the genomic DNA of these isolates (Fig. 2). Our results indicate that tetE is the predominant tetracycline resistance gene, followed by tetB, in tetracycline-resistant A. veronii isolates from catfish.
FIG. 2.
Detection of tetracycline resistance determinants (tetA to E) by multiplex PCR in aeromonads isolated from catfish. Lanes 1 to 7, 100-bp ladder; lane 2, 211-bp tetA amplicon; lane 3, 391-bp tetB amplicon; lane 4, 897-bp tetC amplicon; lane 5, 844-bp tetD-amplified DNA; lane 6, 744-bp tetE-amplified DNA.
Isolation and characterization of plasmids.
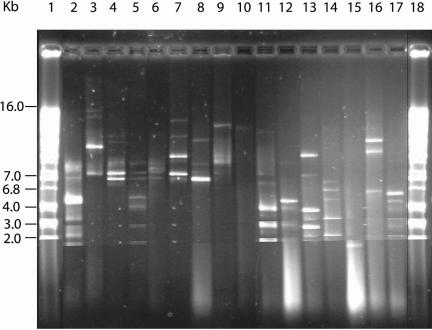
Attempts were made to isolate plasmids from the 81 tetracycline-resistant Aeromonas veronii strains. Only 33 of the 81 strains contained plasmids. These plasmids varied in sizes ranging from 2.5 kb to 16.0 kb (Fig. 3, lanes 2 to 17). Strains Aht22 (lane 10) and AvtB6 (lane 15) were distinct from other plasmid-containing strains by containing just one plasmid. Strain Aht22 had a plasmid measuring 14.0 kb, and strain AvtB6 contained a plasmid measuring less than 2.0 kb. Strains Avt1031 (lane 14) and strain Avt901 (lane 17) contained multiple plasmids measuring less than 7.0 kb. However, strains Aht920 (lane 3), Avt429 (lane7), and Avt620 (lane 8) contained plasmids measuring more than 7.0 kb.
FIG. 3.
Plasmid profiles of tetracycline-resistant A. veronii isolates from catfish. Lanes 1 and 18 are plasmids of known sizes. Plasmids were from strains AE 11 (lane 2), Aht620 (lane 3), AE 2 (lane 4), Avt603 (lane 5), Aht1 (lane 6), Avt429 (lane 7), Avt620 (lane 8), Avt004 (lane 9), Aht22 (lane 10), Avt 720 (lane 11), Avt431 (lane 12), Aet603 (lane 13), Avt1031 (lane 14), AvtB6 (lane 15), Avt013 (lane 16), and Avt901 (lane 17).
Pulsed-field gel electrophoresis.
Sixteen of the 81 tetracycline-resistant aeromonads were untypeable by the PFGE methodology used in this study. SpeI restriction digestion yielded 15 to 25 DNA fragments measuring between 23.1 and 437 kb in size. SpeI PFGE identified 15 distinct macro restriction patterns among the 65 isolates that were typeable (Fig. 4). Dendrogram analysis indicated that the SpeI PFGE profiles of Aht918 (Fig. 4, lane 6), Aht620 (Fig. 4, lane 7), and Aht03 (Fig. 4, lane 8) were identical. Similarly, the analysis also indicated that the SpeI PFGE profiles of Avt48 (Fig. 4, lane 9) and Avt410 (Fig. 4, lane 10) were identical. The dendrogram analysis indicated that the DNA banding patterns of the other 13 different pulsotypes had a similarity index of less than 70%.
FIG. 4.
SpeI PFGE of the genomic DNA of selected aeromonads isolated from catfish and the dendrogram analysis of the macrorestriction patterns by the bionumeric software.
DISCUSSION
The Vitek GNI+ card with Vitek version VTK-R07-01 software, a fully automated method, easily characterized and identified the 81 tetracycline-resistant Aeromonas spp. as 5 different species within 12 h of incubation. Among the 5 species, A. veronii had the highest percent probability of correct identification (95 to 99.0%). The Vitek diagnostic system could only identify 42/81 (52.0%) of the isolates as A. veronii. The other species had a lower percent probability (less than 85.0%), and in some cases, the system indicated that the probability of A. hydrophila or A. caviae was similar to that of A. trota or members of the Vibrionaceae. The characterization and identification of the members of the genus Aeromonas by conventional methods is difficult because many of the 16 species have overlapping characteristics (17, 25). Furthermore, it is not unusual for the diagnostic system to misidentify aeromonads as members of the Vibrionaceae (1, 9). Since biochemical properties do not accurately reflect the genomic complexity of a given species and the diagnostic results may be influenced by physical parameters, such as pH, temperature, and growth substrate concentrations, unambiguous identification of the different members of the genus by biochemical reactions is impossible. Thus, molecular methods, such as PCR amplification and restriction digestion of the16S rRNA, are invaluable for the identification of these isolates (17, 25, 28).
The PCR protocol for amplification of 16S rRNA successfully amplified the 1.4-kb genes from the genomic DNA of all 81 tetracycline-resistant Aeromonas spp., indicating the specificity and accuracy of the oligonucleotide probes and confirming that all 81 isolates are indeed members of the genus Aeromonas. The RFLP patterns of the 16S rRNA gene from 81 isolates matched the patterns of A. veronii ATCC 35626, indicating that all 81 tetracycline-resistant Aeromonas isolated from catfish were A. veronii. Restriction digestion of the amplified gene resulted in 7 different DNA fragments measuring a total of 1.1 kb. Our inability to account for all of the DNA fragments to measure a total of 1.4 kb may be due either to overlap in some of these fragments or to loss of some of the smaller fragments from the gel.
Numerous investigations have been conducted to isolate and characterize Aeromonas hydrophila from aquaculture systems because of its status as a human pathogen (10, 29, 30, 32). Significant differences in the antibiograms of these isolates compared to the 81 tetracycline-resistant A. veronii isolates were observed. Son et al. (30), on characterizing A. hydrophila in tilapia (Oreochromis mossambica), indicated that 48% of these isolates were resistant to tetracycline, 57% were resistant to streptomycin, and 43% were resistant to erythromycin. Rhodes et al. (26) indicated that 52% of tetracycline-resistant aeromonads isolated from fish hatcheries were also resistant to nalidixic acid and 40% of the total aeromonads were resistant to streptomycin (26). Schmidt et al. (29) isolated tetracycline-resistant aeromonads from rainbow trout and indicated that a majority of the isolates were resistant to sulfadiazine/trimethoprim. The prevalence of large numbers of aeromonads from aquaculture resistant to streptomycin, erythromycin, and nalidixic acid may indicate the usage of these antibiotics in these ecosystems (12). Since streptomycin and erythromycin are not approved for use in the U.S. aquaculture industry, we were unable to isolate large numbers of Aeromonas spp. resistant to these drugs.
The genetics of tetracycline resistance in aeromonads has been investigated previously (2, 3, 27, 29, 33). Five classes of genetically distinguishable tetracycline resistance determinants, designated A through E, have been described among aerobic enteric gram-negative bacteria. Most of these determinants are tetracycline inducible and provide resistance to other tetracycline analogs, such as oxytetracycline (29). However, the distribution of these determinants is not uniform. In aquatic aeromonads, more than three different tetracycline resistance genes are known among oxytetracycline-resistant isolates (2).
DePaola et al. (10) reported that a majority of the tetracycline-resistant A. hydrophila strains from catfish contained either tetA or tetE. However, Schmidt et al. (29) reported that only 30% of the tetracycline-resistant aeromonads from Danish rainbow trout contained either tetA or tetE and that tetA was plasmid borne. Adams et al. (2) reported that 19 of 29 A. salmonicida strains isolated from salmon hatcheries contained tetA localized on a 5.4-kb plasmid. In contrast, our study indicates that tetE was the dominant gene in A. veronii from catfish because the PCR protocol amplified the 744-bp tetE gene from the cell extracts of 73/81 (90.0%) A. veronii isolates. The other dominant tetracycline-resistant gene (tetA) was found in less than 3.0% (2/81) of the catfish strains of A. veronii. These two isolates also harbored the 897-bp tetC and 844-bp tetD genes. Interestingly, we also detected the occurrence of tetB, a plasmid-borne gene on 23/81 (28.0%) A. veronii strains. Although tetB has been reported in other gram-negative bacteria (26, 33), this is the first time this gene has been reported in aquaculture aeromonad isolates.
Characterization of pathogenic bacteria responsible for the outbreak of infectious diseases by phenotypic and genotypic methods can provide useful epidemiological data (23, 24, 30, 31). These methods may provide valuable insight into antibiotic resistance profiles, rates of transmission, reservoirs of infection, mechanisms of infection, and nomenclature and evolution of bacteria. The aeromonads described here were divided into 6 different groups based on their antibiograms, 16 different groups based on plasmid profiles, and 5 different groups based on the presence of tetracycline resistance determinants (tetA to E). However, based on the results of SpeI PFGE, the 81 tetracycline-resistant A. veronii isolates could be further put into 15 different groups.
REFERENCES
- 1.Abbott, S. L., L. S. Seli, M. Carino, Jr., M. A. Hartley, and J. M. Janda. 1998. Misidentification of unusual Aeromonas species as members of the genus Vibrio: a continuing problem. J. Clin. Microbiol. 36:1103-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, C. A., B. Austin, P. G. Meaden, and D. McIntosh. 1998. Molecular characterization of plasmid-mediated oxytetracycline resistance in Aeromonas salmonicida. Appl. Environ. Microbiol. 64:4194-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, S. R., and R. A. Sandaa. 1994. Distribution of tetracycline resistance determinants among gram-negative bacteria isolated from polluted and unpolluted sediments. Appl. Environ. Microbiol. 60:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aquaculture Outlook. 2001. Livestock, dairy and poultry situation and outlook supplement. U.S. Department of Agriculture, Washington, D.C.
- 5.Austin, B., and D. A. Austin. 1993. Bacterial fish pathogens: disease in farmed and wild fish, 2nd ed. Ellis Horwood, Chichester, United Kingdom.
- 6.Bauer, A. W., W. M. M. Kirby, J. C. Sherris, and M. Truck. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 7.Borrell, N., S. G. Acinas, M. J. Figueras, and A. J. Martinez-Murcia. 1997. Identification of Aeromonas clinical isolates by restriction fragment length polymorphism of PCR-amplified 16S rRNA genes. J. Clin. Microbiol. 35:1671-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouqui, P., and D. Raoult. 2001. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 14:177-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colwell, R. R., M. T. MacDonell, and J. De Ley. 1986. Proposal to recognize the family Aeromonadaceae fam. nov. Int. J. Syst. Bacteriol. 36:473-477. [Google Scholar]
- 10.DePaola, A., P. A. Flynn, R. M. McPhearson, and S. B. Levy. 1988. Phenotypic and genotypic characterization of tetracycline and oxytetracycline resistant Aeromonas hydrophila from cultured channel catfish (Ictalurus punctatus) and their environments. Appl. Environ. Microbiol. 54:1861-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueras, M. J., L. Soler, M. R. Chacon, J. Guarro, and A. J. Martinez-Murcia. 2000. Extended method for discrimination of Aeromonas spp. by 16S rRNA RFLP analysis. Int. J. Syst. Evol. Microbiol. 50:2069-2073. [DOI] [PubMed] [Google Scholar]
- 12.Grave, K., M. Engelstad, N. E. Soli, and T. Hastein. 1990. Utilization of antibacterial drugs in salmonid farming in Norway during 1980-1988. Aquaculture 86:347-358. [Google Scholar]
- 13.Havelaar, A. H., M. During, and J. E. Versteegh. 1987. Ampicillin-dextrim agar medium for the enumeration of Aeromonas species in water by membrane filtration. J. Appl. Bacteriol. 62:279-287. [DOI] [PubMed] [Google Scholar]
- 14.Isonhood, J. H., and M. Drake. 2002. Aeromonas species in foods. J. Food Prot. 65:575-582. [DOI] [PubMed] [Google Scholar]
- 15.Janda, J. M., L. S. Guthertz, R. P. Kokka, and T. Shimada. 1994. Aeromonas species in septicemia: laboratory characteristics and clinical observations. Clin. Infect. Dis. 19:77-83. [DOI] [PubMed] [Google Scholar]
- 16.Janda, J. M., and S. L. Abbott. 1996. Human pathogens, p. 151-173. In B. Austin, M. Attwegg, P. J. Gosling, and S. Joseph. (ed.), The genus Aeromonas, John Wiley and Sons, New York, N.Y.
- 17.Janda, J. M., and S. L. Abbott. 2002. Bacterial identification for publication: when is enough? J. Clin. Microbiol. 40:1887-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko, W. C., H. C. Lee, Y. C. Chuang, C. C. Liu, and J. J. Wu. 2000. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J. Infect. 40:267-273. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn, I., J. M. Albert, M. Ansaruzzaman, N. A. Bhuiyan, S. A. Alabi, M. S. Islam, P. K. B. Neogi, G. P. Huys, J. K. Kersters, and R. Mollby. 1997. Characterization of Aeromonas spp. isolated from humans with diarrhea, from healthy controls and from surface water in Bangladesh. J. Clin. Microbiol. 35:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Midtved, T., and E. Lingaas. 1992. Putative public health risks of antibiotic resistance development in aquatic bacteria, p. 302-314. In C. Michel and D. Alderman (ed.) Chemotherapy in aquaculture: from theory to reality. Office International des Epizooties, Paris, France.
- 21.National Committee for Clinical Laboratory Standards. 1994. Performance standards for antimicrobial disk susceptibility tests. Fifth information supplement. M100-S5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.Nawaz, M. S., B. D. Erickson, A. A. Khan, S. A. Khan, J. V. Pothuluri, F. Rafii, J. B. Sutherland, R. D. Wagner, and C. E. Cerniglia. 2001. Human health impact and regulatory issues involving antimicrobial resistance in the food animal production environment. Regul. Res. Perspect. 1:1-8. [Google Scholar]
- 23.Nawaz, M. S., S. A. Khan, A. A. Khan, R. Nayak, R. Steele, D. Paine, and R. Jones. 2003. Molecular characterization of fluoroquinolone-resistant Campylobacter spp. isolated from poultry. Poultry Sci. 82:251-258. [DOI] [PubMed] [Google Scholar]
- 24.On, S. W. L. 1998. In vitro genotypic variation of Campylobacter coli documented by pulsed field gel electrophoresis DNA profiling: implications for epidemiological studies. FEMS Microbiol. Lett. 165:341-346. [DOI] [PubMed] [Google Scholar]
- 25.Park, T. S., S. H. Oh, E. Y. Lee, T. K. Lee, K. H. Park, M. J. Figueras, and C. L. Chang. 2003. Misidentification of Aeromonas veronii biovar sobria as Vibrio alginolyticus by Vitek system. Lett. Appl. Microbiol. 37:349-353. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes, G., G. Huys, J. Swings, P. McGann, M. Hiney, P. Smith, and R. W. Pickup. 2000. Distribution of oxytetracycline resistance plasmids between aeromonads in hospitals and aquaculture environments: implication of Tn1721 in dissemination of the tetracycline resistance determinant Tet A. Appl. Environ. Microbiol. 66:3883-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, M. C., M. O. Chung, and D. E. Roe. 1996. Characterization of tetracycline and erythromycin resistance determinants in Treponema denticola. Antimicrob. Agents Chemother. 40:1690-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosselio-Mora, R., and R. Amann. 2001. The species concept for prokaryotes. FEMS Microbiol. Lett. 25:39-67. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, A. S., M. S. Bruun, I. Dalsgaard, and J. L. Larsen. 2001. Incidence, distribution and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl. Environ. Microbiol. 67:5675-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son, R., G. Rusul, A. M. Sahilah, A. Zainuri, A. R. Raha, and I. Salmah. 1997. Antibiotic resistance and plasmid profile of Aeromonas hydrophila isolates from cultured fish, Telapia (Telapia mossambica). Lett. Appl. Microbiol. 24:479-482. [DOI] [PubMed] [Google Scholar]
- 31.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelson, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai, G. J., and T. H. Chen. 1996. Incidence and toxigenicity of Aeromonas hydrophila in seafood. Int. J. Food Microbiol. 31:121-131. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi, A. 1997. Bacterial resistance mechanisms for tetracyclines. Nippon Rinsho 55:1245-1251. [PubMed] [Google Scholar]