Abstract
Resistance to hops is a prerequisite for lactic acid bacteria to spoil beer. In this study we analyzed mechanisms of hop resistance of Lactobacillus brevis at the metabolism, membrane physiology, and cell wall composition levels. The beer-spoiling organism L. brevis TMW 1.465 was adapted to high concentrations of hop compounds and compared to a nonadapted strain. Upon adaptation to hops the metabolism changed to minimize ethanol stress. Fructose was used predominantly as a carbon source by the nonadapted strain but served as an electron acceptor upon adaptation to hops, with concomitant formation of acetate instead of ethanol. Furthermore, hop adaptation resulted in higher levels of lipoteichoic acids (LTA) incorporated into the cell wall and altered composition and fluidity of the cytoplasmic membrane. The putative transport protein HitA and enzymes of the arginine deiminase pathway were overexpressed upon hop adaptation. HorA was not expressed, and the transport of hop compounds from the membrane to the extracellular space did not account for increased resistance to hops upon adaptation. Accordingly, hop resistance is a multifactorial dynamic property, which can develop during adaptation. During hop adaptation, arginine catabolism contributes to energy and generation of the proton motive force until a small fraction of the population has established structural improvements. This acquired hop resistance is energy independent and involves an altered cell wall composition. LTA shields the organism from accompanying stresses and provides a reservoir of divalent cations, which are otherwise scarce as a result of their complexation by hop acids. Some of the mechanisms involved in hop resistance overlap with mechanisms of pH resistance and ethanol tolerance and as a result enable beer spoilage by L. brevis.
Resistance to hops is a prerequisite for the ability of lactic acid bacteria to grow in beer and thus cause beer spoilage. Hop compounds, mainly iso-α-acids, were described as ionophores which dissipate the pH gradient across the cytoplasmic membrane and reduce the proton motive force (PMF). Consequently, the low intracellular pH (pHin) interferes with essential enzyme reactions and PMF-dependent nutrient uptake is hampered, resulting in the death of cells of hop-sensitive strains (34, 37, 49).
Several mechanisms involved in the hop resistance of lactobacilli have recently been characterized (13, 34-36, 38, 24-44, 47, 48). The proteins contributing to hop resistance include multidrug resistance (MDR) transporters that excrete the hop compounds into the outer medium (35, 45) and proton export systems that maintain the intracellular pH. HitA is a putative divalent cation transporter present predominantly in beer-spoiling lactobacilli (13). An alteration of the teichoic acids in the cell wall (50) and a changed lipid composition of the cytoplasmic membranes (34) might additionally contribute to the hop resistance. However, the role of these hop resistance mechanisms in beer-spoiling lactobacilli is not fully understood, especially because as none of them confers high levels of hop resistance in the absence of other mechanisms of hop resistance.
Some potentially hop-resistant strains cannot grow in beer unless they have first been exposed to subinhibitory concentrations of hop compounds, but the mechanisms of adaptation are not understood (39). Apparently, hop resistance of lactobacilli requires multiple resistance mechanisms. This is consistent with the stress conditions acting on bacteria in beer, which mainly consist of acid stress (40) and the antimicrobial effect of the hop compounds in addition to ethanol stress and starvation. For analytical convenience, however, the whole cell is usually separated into cytoplasm, membranes, and cell wall, and the dedicated functions of these parts are explored; in the living cell one part shades into another, and all parts are interdependent in terms of function and formation (33). Consequently, the challenge in hop resistance research is to reassemble the various defense mechanisms at all cellular levels to see the overall functions. In this work we investigated the diversity of metabolism and various aspects of hop resistance in a potential beer-spoiling strain of Lactobacillus brevis and an adapted hop-resistant variant of this strain in order to establish a model for the interaction of the multiple resistance mechanisms and their roles in hop adaptation.
MATERIALS AND METHODS
Microorganisms, media, and culture conditions.
L. brevis TMW 1.465 was selected as the most potent beer-spoiling organism among 31 strains of lactobacilli considered. A variant of this strain adapted to hop stress (see below) was designated L. brevis TMW 1.465A. The properties of L. brevis TMW 1.465A reflect those of L. brevis TMW 1.465 except that the MIC of iso-α-acids was higher, >100 μM (the highest concentration tested was 103.2 μM), at pH 4.0 after 48 h. L. brevis TMW 1.465 was grown in mMRS4 (41). A growth medium containing fructose was chosen because mannitol was detected by high-performance liquid chromatography in spoiled beer, indicating that there was a reduction in the level of fructose. Furthermore, mMRS4 was optimal for growth of L. brevis TMW 1.465 at pH 6.0 (reference conditions) or at pH 4.0 (acid stress conditions). L. brevis TMW 1.465A was grown at pH 4.0 in the presence of isomerized hop extract (Isohop; Nateco2 GmbH u. Co. KG, Mainburg, Hallertau, Germany) added at an iso-α-acid concentration of 86 μM. Cultures were grown at 30°C. Unless indicated otherwise, cells were harvested by centrifugation for each condition at an optical density at 590 nm (OD590) of 0.4 in the early exponential growth phase.
Determination of MDR transport activity.
MDR transport activity of L. brevis TMW 1.465 and TMW 1.465A was assessed with ethidium bromide, Hoechst 33342, and Calcein AM as substrates using previously established protocols (21, 35, 48).
Adaptation conditions and measurement of hop resistance.
Cultures of L. brevis TMW 1.465 were subcultured in media with increasing concentrations of iso-α-acids. The inoculation density in each case was an OD590 of 0.4. The concentrations of iso-α-acids were increased from 17.2 μM to a final concentration of 86 μM within 60 days. Growth curves were determined at days 15, 30, and 60 during the adaptation period. Growth challenges were carried out in microtiter plates with a hop extract dilution series using concentrations of iso-α-acids ranging from 17.2 μM to 103.2 μM in 8.6 μM steps. Media were inoculated to obtain an OD590 of 0.15. A layer of sterile paraffin was used to ensure anaerobic conditions, and the OD590 was measured for 200 h. One-half of the cultures did not survive the adaptation procedure. The hop-resistant variant of L. brevis TMW 1.465 adapted to 86 μM iso-α-acids was designated L. brevis TMW 1.465A.
Acquisition of metabolic data.
Cultures were grown to stationary phase under reference, acid stress, and hop stress conditions, and the culture supernatants were obtained by centrifugation (5,000 × g, 10 min). Metabolite contents were determined by high-performance liquid chromatography. Maltose, glucose, fructose, mannitol, lactic acid, acetic acid, and ethanol were separated on a Polyspher OA KC column (Merck, Darmstadt, Germany), and amino acid contents in the supernatants were determined using an AminoPac PA10 column (Dionex, Idstein, Germany) as previously described (19, 46). Two external amino acid standards containing arginine, ornithine, lysine, citrulline, glutamine, asparagine, alanine, threonine, glycine, valine, serine, proline, isoleucine, leucine, methionine, histidine, phenylalanine, glutamate, aspartate, cystine, cysteine, and tyrosine were used. Norleucine was used as an internal standard. Samples from independent cultures were prepared in duplicate, and the means were calculated.
Growth challenges in the presence of amino acids.
L. brevis TMW 1.465 and TMW 1.465A were incubated in mMRS4 (pH 4.0, 86 μM iso-α-acids) supplemented with different concentrations of arginine, ornithine, alanine, asparagine, leucine, glutamate, methionine, and phenylalanine. The amino acids were added at concentrations ranging from 20 mM to 35 mM in 5 mM steps, and the pH was adjusted to 4.0. Growth challenges were carried out in microtiter plates. The inoculation density used was an OD590 of 0.15. A layer of sterile paraffin was used. The growth was measured photometrically at 590 nm.
Contribution of membrane-associated transport proteins to hop resistance.
The contribution of membrane-associated transport proteins to hop resistance was determined by selective inactivation of membrane transport systems by high-pressure treatment (4, 47). Cells of L. brevis TMW 1.465 and L. brevis TMW 1.465A were subjected to a pressure treatment at 300 MPa and 20°C for 0 to 30 min as previously described (47). The treated cells were subsequently incubated at 30°C in mMRS4 (pH 4.0) containing 0 to 68.8 μM iso-α-acids, and the OD590 of the cultures were determined at 1-h intervals for 28 days. The detection times were obtained by determining the incubation times required to increase the OD590 by 0.2.
Determination of membrane composition.
Cells were grown under reference, acid stress, and hop stress conditions as described above in 50 ml of mMRS4 to the stationary growth phase. Cells from each preparation were lyophilized, packed under an N2 atmosphere, and sent to Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) for analysis. Membrane fatty acids were extracted, transesterified, and analyzed by gas chromatography.
Measurement of membrane fluidity.
The membrane fluidity of L. brevis TMW 1.465 grown under reference and acid stress conditions and the membrane fluidity of L. brevis TMW 1.465A grown under hop stress conditions were determined by Laurdan fluorescence (27). The cells were washed twice with phosphate buffer (50 mM phosphate [pH 6.5], 10 mM glucose) and resuspended to an OD590 of 1.0 in the same buffer. A stock solution of Laurdan (2 mM in ethanol) was added to obtain a Laurdan concentration of 40 μM. Cells were stained at 30°C for 30 min in the dark. After this the cells were washed twice with phosphate buffer (50 mM phosphate [pH 6.5], 10 mM glucose) and resuspended again in phosphate buffer (50 mM phosphate [pH 6.5], 10 mM glucose). The fluorescence spectra (excitation wavelength, 360 nm; emission wavelengths, 380 to 550 nm) were determined with an LS 50B luminescence spectrometer (Perkin-Elmer, Rodgau-Jügesheim, Germany) using 1-nm steps at 5, 10, 15, 20, 25, and 30°C. The Laurdan general polarization (GP) was calculated as follows: GP = (I440 − I490)/I440 + I490), where I440 is relative fluorescence at 440 nm and I490 is relative fluorescence at 490 nm (27). To measure the effects of the hop compounds on the membrane fluidity, the phosphate buffer was replaced with sodium acetate buffer (50 mM, pH 4.0) containing 86 μM iso-α-acids. The measurements were obtained at 30°C.
Preparation and analysis of lipoteichoic acids.
Cells were grown under reference, acid stress, and hop stress conditions as described above to the stationary growth phase. Cells were harvested by centrifugation (5,000 × g, 20 min, 4°C), washed once with cold 0.01 M sodium acetate (pH 4.7) containing 0.9% NaCl, and resuspended at a concentration of 0.4 g cells/ml (15). Cells were broken by an ultrasonic treatment (HD-70/Bandelin; five 30-s cycles; power, 90%; cycle, 30%; intermediate cooling). The dry weight of the broken cell suspension from each preparation was determined and used to normalize analytical data. The lipoteichoic acid (LTA) was extracted and purified on an octylsepharose column essentially as described previously (9, 15). The purification of lipoteichoic acid was controlled online (BioLogic Optics module II OM-11; Bio-Rad, United States) for protein and DNA contamination photometrically. Lipoteichoic acids were concentrated by lyophilization. For characterization, the LTA was chemically deacylated to obtain chemically deacylated LTA (cdLTA) by mild alkali treatment with 0.1 M NaOH for 1 h at 60°C (30), and the cdLTA was analyzed by polyacrylamide gel electrophoresis (PAGE) as described previously (28). The cdLTA was visualized by combined alcian blue and silver staining (24). For determination of the glycerolphosphate content, the cdLTA was hydrolyzed with 2 M HCl for 2 h at 100°C, the buffer was neutralized with NaOH, and the glycerolphosphate concentration was determined enzymatically (26).
Analysis of expression of hop resistance genes at the mRNA level.
For the analysis of expression of hop resistance genes, total RNA was extracted from cells subjected to all three stress conditions (see above) as described by Aiba et al. (1). The RNA was purified, and reverse transcription was performed with random primers and murine leukemia virus reverse transcriptase, using the instructions of the supplier of the reagents (Promega, Mannheim, Germany). The nucleotide sequences of arcA, arcB, arcC and the phosphoketolase gene were determined by the U.S. Department of Energy Joint Genome Institute (http://www.jgi.doe.gov/). The primers for the hop resistance genes were constructed with the Dnasis software (Hitachi Software Engineering Co, Yokohama, Japan) according to the instructions of the manufacturer of the LightCycler (Roche Diagnostics GmbH, Mannheim, Germany). The cDNA was quantified using the QuantiTect SYBR green Mastermix (QIAGEN, Hilden, Germany). The efficiency of each primer pair was determined with a dilution series of chromosomal DNA (22). The expression analysis results (29) were normalized for each experiment using the phosphoketolase gene as a housekeeping gene. Template RNA was included in the LightCycler runs to exclude contamination of the RNA preparation with DNA. The primers and annealing temperatures for the amplification reactions are shown in Table 1.
TABLE 1.
Primers used for analysis of expression of hop resistance genes by LightCycler PCR
| Primer | Sequence (5′ to 3′) | Annealing temp (°C) |
|---|---|---|
| hitA_V | TAGCACGGTCGGGCGATTCGTTG | 59 |
| hitA_R | CCTGACAGCGTCCCGGTAATCGTG | 59 |
| arcA_V | CAAGAGTCATTTTGACAAGGTTATTG | 52 |
| arcA_R | GAATCAAATCTAAATCGTCAAGGTTC | 52 |
| arcB_V | AAATTACTTGTTACCGACGACTTAGC | 52 |
| arcB_R | CCATTTCAGTAATACCGTACTTTTCC | 52 |
| arcC_V | GACCACAAGTTGGTAATTTACTGTTG | 52 |
| arcC_R | CTTAATCTCAGCTTCTGTGTAGAACG | 52 |
| keto_V | TTCTACATTGGTGGTCCTGGTC | 52 |
| keto_R | GTTGCGTGGGAAAGTGAGTAAC | 52 |
Evaluation of resistance mechanisms and their roles in hop resistance.
The roles of the hop resistance mechanisms in the phenotype of L. brevis TMW 1.465A were evaluated by measuring changes in the intracellular pH of cells that were exposed to different concentrations of hop compounds in order to study energy-independent hop barriers, as well as HorA-independent transport processes (45). L. brevis TMW 1.465A was grown under hop stress conditions as described above. The effect of hop compounds on the intracellular pH can be measured only in cells with a “normal” starting pHin. Accordingly, the cells were subcultured once in the same medium without hop compounds to restore their intracellular pH in order to obtain measurements. Cells were harvested by centrifugation, washed twice with phosphate buffer (50 mM, pH 6.5), and resuspended in the same buffer at an OD590 of 1.0. The intracellular pH was measured with the fluorescent dyes cFDASE (pKa 6.5) using a previously described method (23) and with Calcein AM (Invitrogen GmbH, Karlsruhe, Germany). Calcein AM exhibits pKa values (pKa1 2.1, pKa2 2.9, pKa3 4.2, pKa4 5.5, pKa5 10.8, and pKa6 11.7) which make it suitable for measuring pH in the range from pH 3.0 to 6.5. Four micromolar Calcein AM was added to the samples. After 3 h of incubation at 30°C to allow dye internalization and hydrolysis by cellular esterases, the cells were washed twice with sodium acetate buffer (50 mM, pH 4.0), resuspended at an OD590 of 1.0, and cooled to 4°C to achieve low permeability of the bacterial membrane for hop compounds until measurement was started (37). Hop compounds were added to the samples to obtain iso-α-acid concentrations of 0, 2, 20, and 80 μM. For energized samples 10 mM arginine or glucose was added. The ionophores valinomycin (1 μM) and nigericin (1 μM) were used as controls. The fluorescence (excitation wavelength, 485 nm; emission wavelength, 520 nm) was measured using black microtiter plates with a Spectrafluor microtiter plate reader (Tecan, Grödig, Austria) at 30°C for 120 min with shaking before and between measurements. For calibration the pHin and extracellular pH were equilibrated by addition of valinomycin (1 μM) and nigericin (1 μM) (23) (data not shown). We verified that hydrolyzed Calcein AM is not a substrate for MDR transport in L. brevis TMW 1.465A (data not shown). Furthermore, we excluded the possibility that the divalent cations Ca2+, Mg2+, and Mn2+, the latter of which is known to be present at high concentrations in lactic acid bacteria (2), affected hydrolyzed Calcein AM fluorescence when they were added to the buffer at concentrations ranging from 16 μM to 10 mM (data not shown). To take into account the fact that no pH-independent fluorescence could be measured for calibration, we ascertained that a loss of dye was not responsible for the decrease in fluorescence. The rate of recovery of fluorescence for pHin 6.5 equilibrated cells quantified before and after each measurement (as described above) was 97% ± 3%.
RESULTS
Adaptation of L. brevis TMW 1.465 to hop compounds.
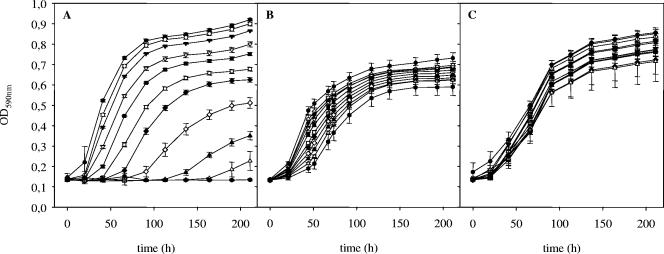
The MIC of iso-α-acids for the unadapted L. brevis TMW 1.465 strain was 17.2 μM at pH 4.0 after 48 h of incubation. This strain has a functional hitA gene (13). It exhibited MDR transport activity when Calcein AM was used as the substrate but not when ethidium bromide and Hoechst 33342, which are accepted as substrates by HorA, were used. To check HorA functionality in this strain, its horA gene (DDBJ accession no. AB167897) was cloned and sequenced to reveal a deletion in base 99, which leads to a stop codon after amino acid 36 and renders the protein nonfunctional. To document the progress of adaptation of L. brevis TMW 1.465 to high concentrations of hop compounds, the hop resistance was measured at three stages of adaptation. The starting iso-α-acid concentration, 17.2 μM, reflected the hop resistance of the unadapted strain L. brevis TMW 1.465. At day 15, the hop resistance had increased to 51.6 μM iso-α-acids, and after 45 days it had increased to the maximum concentration tested, 103.2 μM (Fig. 1). Upon adaptation, the lag phase and the growth rate of L. brevis TMW 1.465A were nearly independent of the iso-α-acid concentration in the growth medium. Adaptation to even higher hop levels was investigated, and L. brevis TMW 1.465A grew with up to 172 μM iso-α-acids; however, the cultures had a extended lag phase and exhibited poor growth compared to the growth of cultures grown under acid stress and optimal conditions.
FIG. 1.
Adaptation of L. brevis TMW 1.465 to increasing concentrations of iso-α-acids. The growth curves were determined at days 15 (A), 45 (B), and 60 (C) of adaptation. The cultures were inoculated onto mMRS4 (pH 4.0) containing 17.2 •, 28.8 ○, 34.4 ▾, 43.0 ▿, 51.6 ▪, 60.2 □, 68.8 ⧫, 77.4 ⋄, 86.0 ▴, 94.6 ▵, or 103.2 • μM iso-α-acids. The values are the means and standard deviations of four independent experiments.
Metabolism of L. brevis TMW 1.465 and L. brevis TMW 1.465A under reference and stress conditions.
The metabolites from maltose, glucose, and fructose of L. brevis TMW 1.465 grown under reference and acid stress conditions and of L. brevis TMW 1.465A grown under hop stress conditions are shown in Table 2. A major change in metabolism was observed when the reference conditions (pH 6.0) were compared to the acid stress conditions (pH 4.0). This trend continued with the higher levels of stress caused by additional hops, although to a lesser extent. Fructose was used predominantly as a carbon source under reference conditions but was used predominantly as an electron acceptor under stress conditions, and there was a concomitant increase in mannitol and acetate production. Lactate production and ethanol production were reduced under stress conditions. One-third of the maltose was not fermented under acid stress and hop stress conditions.
TABLE 2.
Sugar metabolism of L. brevis TMW 1.465 under reference and acid stress conditions and of L. brevis TMW 1.465A under hop stress conditions, as determined by high-performance liquid chromatography analysis
| Compound | Concn (mM) in:
|
|||
|---|---|---|---|---|
| mMRS4 | Reference conditions (TMW 1.465) | Acid stress conditions (TMW 1.465) | Hop stress conditions (TMW 1.465A) | |
| Maltose | 31.5 | 1.1 | 21.4 | 22.1 |
| Glucose | 28.0 | 0.0 | 1.8 | 7.3 |
| Fructose | 28.4 | 0.0 | 1.8 | 1.2 |
| Mannitol | 0.0 | 8.6 | 19.6 | 24.3 |
| Lactate | 0.0 | 74.8 | 42.1 | 24.2 |
| Acetate | 0.0 | 11.1 | 13.2 | 18.8 |
| Ethanol | 0.0 | 80.2 | 31.6 | 10.8 |
The amino acid metabolism under reference, acid stress, and hop stress conditions was also examined. No detectable differences in the amino acid compositions of fermented supernatants and fresh growth medium were observed except for ornithine, which was formed from arginine. Ornithine was not detectable in fresh mMRS4. L. brevis TMW 1.465A accumulated 3.1 mM ornithine under hop stress conditions; after incubation under reference and acid stress conditions, 1.4 and 1.7 mM ornithine were produced, respectively.
To evaluate a possible role of arginine metabolism in hop resistance, L. brevis TMW 1.465 and L. brevis TMW 1.465A were incubated in mMRS4 (pH 4.0) containing 86 μM iso-α-acids and supplemented with various amino acids. L. brevis TMW 1.465 grew only in medium supplemented with arginine, and higehr arginine concentrations resulted in higher growth rates. Hop-containing medium that was supplemented with the other amino acids remained lethal for L. brevis TMW 1.465. The hop-adapted strain L. brevis TMW 1.465A exhibited slightly faster growth with increasing concentrations of any of the amino acids.
Inactivation of membrane-associated transport proteins by high pressure and determination of the resulting decrease in hop resistance.
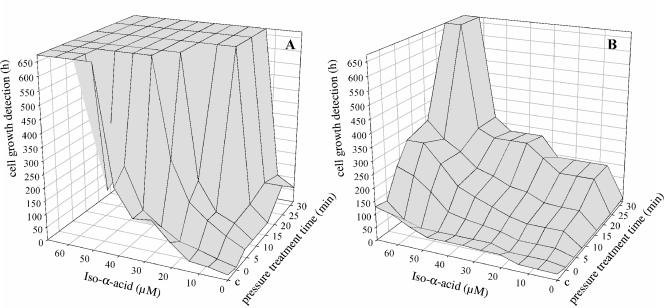
To determine the relevance of membrane-associated transport proteins for hop resistance, the transport proteins were inactivated by treatment of L. brevis with sublethal pressure as described previously (48) and the hop resistance of pressure-treated L. brevis was assessed by determination of detection times in media containing various levels of iso-α-acids. Pressure-treated samples were diluted to obtain the same level of viable cell counts independent of the pressure holding time (data not shown). This ensured that there was a constant inoculation density for all preparations in the growth experiment. Figure 2 shows that addition of hop iso-α-acids to the growth medium delayed the growth of pressure-treated cells. However, for L. brevis TMW 1.465 and TMW 1.465A, 15 min and 24 min (lethal level), respectively, at a pressure of 300 MPa were needed to inhibit the growth. For inactivation of MDR transport activity a 30-s pressure holding time was sufficient (4).
FIG. 2.
Effect of treatment with sublethal high pressure on growth during subsequent storage in hop-containing media. L. brevis TMW 1.465 (A) and L. brevis TMW 1.465A (B) were treated at 300 MPa and 20°C for 0 to 30 min and subsequently incubated in mMRS4 (pH 4.0) containing different concentrations of iso-α-acids. No-pressure controls (c) were included. Cell growth detection times were calculated by determining the time required for the OD590 to increase 0.2 U.
Determination of membrane composition.
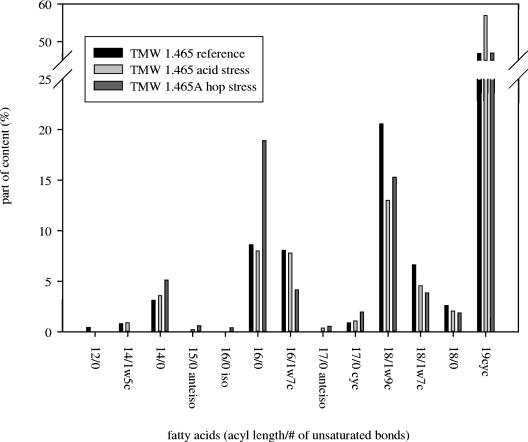
The fatty acid compositions of the membrane of L. brevis TMW 1.465 under reference and acid stress conditions and of L. brevis TMW 1.465A under hop stress conditions were determined (Fig. 3). Acid or hop stress strongly affected the membrane composition. The most prominent shift upon acid stress was an increased level of C19 cyclopropane fatty acids at the expense of 18/1 unsaturated fatty acids. Upon adaptation to hop stress, the content of palmitic acid (19% of the total fatty acids) was strongly increased relative to the content under reference and acid stress conditions (8%). Simultaneously, the 16/1 and 18/1 fatty acid contents were decreased. The ratio of 16/0 fatty acid content to 16/1 fatty acid content, which is considered a “magic number” in proton ionophore resistance (20), increased from 1.0 to 4.75 upon hop adaptation.
FIG. 3.
Fatty acid compositions of L. brevis TMW 1.465 grown under reference and acid stress conditions and of L. brevis TMW 1.465A cytoplasmic membranes.
Membrane fluidity.
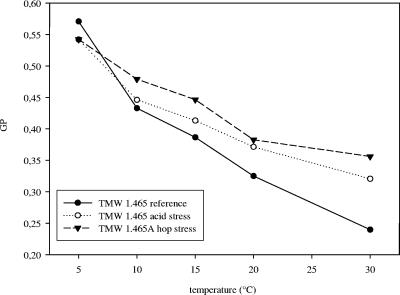
To evaluate the change in fatty acid membrane composition, the fluidity and polarity of the cytoplasmic membrane of L. brevis TMW 1.465 grown under reference and acid stress conditions and the fluidity and polarity of the cytoplasmic membrane of L. brevis TMW 1.465A grown under hop stress conditions were determined by using Laurdan fluorescence. An increase in the GP value correlates with reduced fluidity of the membrane (52). The influence of the hop compounds on the membrane fluidity was determined at 30°C. Addition of hops resulted in an increase in the GP value of approximately 0.06 ± 0.01 U, indicating that there was a decrease in the fluidity of the membrane (data not shown). The effects of adaptation to acid stress and hop stress on membrane fluidity are shown in Fig. 4. The cells grown under acid stress conditions showed a marked decrease in the membrane fluidity at temperatures ranging from 10 to 30°C compared to cells grown under reference conditions. Upon hop adaptation, the membrane fluidity was reduced further. At 5°C, a temperature at which the membrane is in the gel phase, the highest GP was observed in reference cells.
FIG. 4.
GP values for Laurdan incorporated into the cytoplasmic membrane of L. brevis TMW 1.465 and L. brevis TMW 1.465A. Cells were grown to stationary phase under reference and acid stress conditions (TMW 1.465) or in the presence of hops (TMW 1.465A). The data are representative of two independent experiments.
Lipoteichoic acids.
The changes due to the presence of hop compounds in the growth media in the lipoteichoic acid content of the cell walls were measured under reference, acid stress, and hop stress conditions. For qualitative analysis, the lipoteichoic acid was isolated, chemically deacylated, and separated by native PAGE (Fig. 5). As expected based on its heterogeneity, the cdLTA migrated as a smear. Under reference conditions almost no cdLTA was detected on the gel. The cdLTA from acid-adapted cells were visible as a smear in the middle of the gel. The cdLTA from hop-adapted cells formed a thick band in the upper third of the gel and a slight smear comparable to smear observed for cells grown under the acid stress conditions. To obtain quantitative information on the cdLTA levels, the cdLTA was hydrolyzed and the amount of glycerolphosphate, which forms the backbone of LTA, was measured. In agreement with the PAGE analysis, the glycerolphosphate content of the cdLTA increased from 0.6 ± 0.0 mmol g (dry weight)−1 to 2.9 ± 0.2 mmol g (dry weight)−1 and finally to 6.4 ± 0.1 mmol g (dry weight)−1 with increasing stress levels.
FIG. 5.

Analysis of LTA isolated from L. brevis TMW 1.465 and L. brevis TMW 1.465A: electrophoretic separation of cdLTA purified from L. brevis TMW 1.465 grown under reference conditions (lane 1) or acid stress conditions (lane 2) and from hop-adapted L. brevis TMW 1.465A (lane 3). The samples loaded represented equivalent cell dry masses for the different conditions, and the results are representative of two independent LTA isolations.
Analysis of expression of hop resistance genes at the mRNA level.
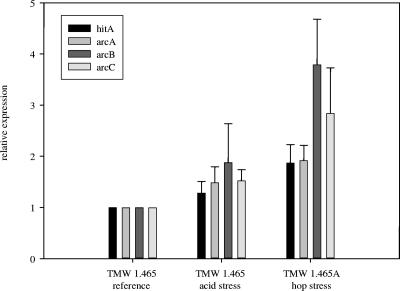
To determine the regulation of mechanisms contributing to hop resistance, the expression of the previously characterized hop resistance gene hitA was measured at the mRNA level. As the metabolite analysis of L. brevis TMW 1.465A indicated that arginine metabolism has a role in hop resistance, the genes of the arginine deiminase (ADI) pathway (arcA, arcB, and arcC) were also quantified. Gene expression was normalized to expression of the phosphoketolase housekeeping gene. The profile of expression of the target genes is shown in Fig. 6. The gene expression under the reference conditions was defined as 1.0. Acid stress resulted in 1.2- to 1.8-fold overexpression of all four genes, whereas arcB and arcC were overexpressed 2.5- to 4-fold upon adaptation to hops.
FIG. 6.
Analysis of the expression of hop resistance genes hitA, arcA, arcB, and arcC of L. brevis TMW 1.465 (reference and acid stress conditions) and L. brevis TMW 1.465A (hop-adapted cells). mRNA levels were quantified by LightCycler PCR using cDNA as the template and pta as the reference. The values are the means and standard deviations of four independent experiments.
Evaluation of resistance mechanisms and their roles in hop resistance.
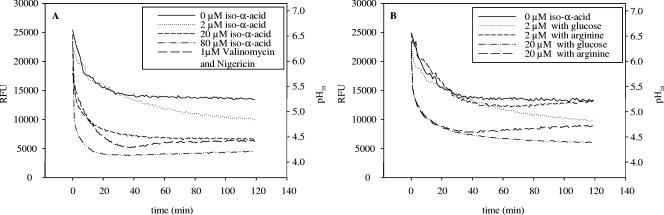
The intracellular pH of L. brevis TMW 1.465A was measured by the cFDASE method and with Calcein AM in order to determine whether barrier functions, transport, or metabolism plays the major role in the high level of resistance of L. brevis TMW 1.465A to hops. Measurements by the cFDASE method and with Calcein AM generally provided consistent results; however, based on the pKa values of the two dyes, Calcein AM was the more appropriate choice. Therefore, only the values obtained from Calcein AM measurements are shown in Fig. 7. As a reference for the experiments, cells of L. brevis TMW 1.465A incubated without an energy source and hop compounds were used. The decrease in fluorescence in these controls was caused mainly by a decrease in the pHin upon the shift from the pH 6.5 phosphate buffer to the pH 4.0 acetate buffer. After 40 min, equilibrium was reached. Figure 7A shows the rapid decrease in the intracellular pH in deenergized cells mediated by hop compounds. Even in hop-adapted cells, addition of 80 μM α-iso-acids strongly decreased the intracellular pH, indicating that the alterations in the cell envelope failed to protect cells against hop compounds in the absence of a source of metabolic energy. Figure 7B shows the intracellular pH of cells that were energized with glucose or arginine concomitant with their exposure to iso-α-acids. No differences in the curves for deenergized and energized cells were observed for the first 40 min of measurement (Fig. 7A and B). In cells energized with arginine, but not in cells energized with glucose, a slow increase in the intracellular pH was detected after 40 min.
FIG. 7.
Measurement of intracellular pH changes in cells of L. brevis TMW 1.465A after addition of different concentrations of hop compounds. (A) Exposure of deenergized cells to 0 to 80 μM iso-α-acids or 1 μM valinomycin and nigericin. (B) Exposure of cells to 2 and 20 μM iso-α-acids with concomitant addition of glucose or arginine as an energy source. The data are representative of two independent experiments. RFU, relative fluorescence units.
DISCUSSION
L. brevis TMW 1.465 can express a variety of hop resistance mechanisms that act at various cellular levels, including metabolism, membrane physiology, and cell wall structure. These mechanisms are not necessarily all used at the same time but can be distinguished to confer low-level or high-level resistance to hops, the latter of which develops only upon prolonged adaptation. To delineate these resistance mechanisms and to study their roles in hop resistance and adaptation, cells were grown under optimal, acid stress, and hop stress conditions. For the investigation of hop stress conditions L. brevis TMW 1.465 had to be adapted to increasing concentrations of iso-α-acids (31, 39). Thus, the responses to three different growth conditions of variants of one strain that strongly differed in hop resistance could be compared. The experimental design allowed differentiation between acid stress and hop stress.
The high level of hop resistance of L. brevis TMW 1.465A could not be attributed to an MDR transport system. It could be demonstrated that there were significant changes in the metabolic products and that the arginine deiminase pathway supported the hop resistance and the adaptation to hops. Furthermore, the altered fatty acid composition of the cytoplasmic membrane and the modified cell wall contributed to the high level of hop resistance of L. brevis TMW 1.465A.
The adaptation of L. brevis TMW 1.465 to 86 μM iso-α-acids was a time-consuming process, which was associated with the loss of several cultures that could not cope with the increasing hop stress conditions. However, after 60 days the hop-resistant variant of L. brevis TMW 1.465 exhibited a lag phase and a growth rate that were nearly independent of the iso-α-acid concentration. L. brevis TMW 1.465A, which grew with 86 μM iso-α-acids, was considered to be comparable to strains that grew under the other conditions and was chosen for the experiments.
Sugar metabolism.
In heterofermentative lactic acid bacteria, like L. brevis, the phosphoketolase pathway generates 1 mol of ATP and 2 mol of NADH + H+ from 1 mol of glucose or 2/3 mol of ATP and 2 mol of NADH + H+ from 1 mol of fructose. The NADH + H+ can be restored by the formation of ethanol from acetyl-P, to maintain the redox balance of the cells. If acetate is formed from acetyl-P instead of ethanol, one additional mol of ATP is created by the acetate kinase. In this case the cellular redox balance has to be maintained by the use of external electron acceptors like fructose, which is converted to mannitol. The sugar metabolism in L. brevis TMW 1.465 and 1.465A changed from the reference conditions at pH 6.0 to the acid stress conditions at pH 4.0. With an increasing stress level (lower pH) the production of lactate and the production of acetate decreased and increased, respectively. This metabolic change was also reflected by elevated mannitol production under acid and hop stress conditions. This way, the formation of ethanol was avoided, allowing the energetically favorable formation of acetate. At a high pH value the fructose acts more as a C source, and only one-third of the fructose is used as an electron acceptor. Thus, L. brevis avoids the formation of ethanol and uses the energetically favorable acetate kinase pathway. The remaining maltose and the decreased lactate production are caused by the fact that a growth-limiting environment, principally a low pH, occurs earlier under acid stress and hop stress conditions.
Amino acid metabolism.
The hop adaptation of L. brevis TMW 1.465A increased the conversion of arginine to ornithine via the ADI pathway (42). The observations concerning the levels of metabolites were substantiated by quantification of the expression of the enzymes of the ADI pathway. Significant increases in arcA and arcC expression and even more significant increases in arcB expression under hop stress conditions were apparent, indicating that the arcB step could be the metabolic rate-limiting step in the arginine deiminase pathway. The conversion of arginine to ornithine, ammonia, and CO2 is coupled to production of ATP and also contributes to the generation of the PMF and an increase in the extracellular pH. Accordingly, arginine metabolism is recognized as an important factor in bacterial acid resistance (5) and contributes to nisin resistance in Lactococcus lactis (18). Glucose transport in L. brevis is PMF dependent (51), and the dissipation of the PMF by hop compounds inhibits sugar uptake (34). An increase in the extracellular pH reduces the activity of hop compounds, which is dependent on the pH (40).
L. brevis TMW 1.465 tolerated hop stress (86 μmol iso-α-acids/liter) only in media supplemented with arginine. In contrast, L. brevis TMW 1.465A grew nearly independent of amino acid supplementation. This indicates that L brevis TMW 1.465A developed additional resistance mechanisms during adaptation, whereas L. brevis TMW 1.465 requires the arginine deiminase pathway for survival. Utilization of this pathway thus appears to be an inducible early step in the adaptation to hop compounds, transiently conferring resistance until full adaptation is achieved.
Role of MDR transport.
Whenever HorA activity was absent, MDR transport activity mediated by other transporters was observed in L. brevis TMW 1.465 and in L. brevis TMW 1.465A. Inactivation of membrane-associated transport proteins via high-pressure treatment and a subsequent growth challenge under hop stress conditions were used to further assess the role of MDR transport proteins in hop resistance (35, 48). Treatment of lactic acid bacteria with sublethal pressure is known to inactivate the F0F1 ATPase, as well as ATP-dependent and PMF-dependent MDR transport enzymes, such as LmrP in L. lactis (23). The glutamate-dependent acid resistance system of Escherichia coli is pressure stable (17). The ability to grow in the presence of hops of L. brevis TMW 1.465A and TMW 1.465 was nearly unchanged after inactivation of the membrane-associated transporters. Measurement of the ionophore effect of iso-α-acids by monitoring the intracellular pH indicated that the efflux rate mediated by possible transport mechanisms other than HorA (e.g., HorC) was far less than the minimum iso-α-acid concentration tested (2 μM). As membrane-bound MDR transporters are easily inactivated by high pressure (10) and hop transport activity was not detectable, the hop resistance of L. brevis TMW 1.465 and its variant TMW 1.465A appears to be based mainly on attributes other than membrane-associated transport proteins.
Role of membrane composition.
Both acid stress and hop adaptation resulted in an altered fatty acid composition of the cytoplasmic membrane. Upon acid stress, the level of cyclopropane fatty acids increased at the expense of the level of long-chain unsaturated fatty acids. Methylation of unsaturated fatty acids to cyclopropane fatty acids is catalyzed by cyclopropane-fatty-acyl-phospholipid synthase (11). In E. coli, cfa expression is up-regulated as part of the stringent response and upon acid and oxidative stress (7, 8). Substitution of monounsaturated fatty acids with cyclopropane fatty acids decreases membrane fluidity (11), as observed in our work with acid-stressed L. brevis TMW 1.465. Adaptation of L. brevis TMW 1.465A to hops had an effect on membrane fluidity similar to that of acid stress but resulted in a different membrane composition. The most important change was the increased content of the 16/0 fatty acid at the expense of 16/1 and 18/1 monounsaturated fatty acids, resulting in a strongly elevated ratio of 16/0 fatty acid to 16/1 fatty acid. The ratio of 16/0 to 16/1 was also strongly increased in proton ionophore-resistant Bacillus subtilis strains (12, 20). It was hypothesized that the increase in the ratio of 16/0 fatty acid to 16/1 fatty acid underlies other membrane-associated changes that are less obviously related to protonophore resistance (20).
Role of lipoteichoic acids and divalent cations.
As the stress level increased, the LTA content in the cell wall of L. brevis TMW 1.465A increased. In cells grown under optimal growth conditions, almost no LTA could be detected. Inserted LTA creates a polyanionic matrix in the cell wall (25). Hughes et al. (14) concluded that in whole cells the ordered array of anionic wall and membrane teichoic acids (LTA) provides a constant reservoir of bound divalent cations that the membrane preferentially interacts with. According to Simpson (37), the ability of hop bitter acids to simultaneously bind to two or more cations may be crucial to the antibacterial action of the acids, and the antibacterial activity of hop bitter acids is strongly reduced by divalent cations. On the other hand, the findings of Archibald and Duong (2) indicate the importance of divalent cations (Mn2+) for the survival of lactobacilli. Thus, the lack of divalent cations mediated by complexation with hop compounds efficiently eliminates growth of lactobacilli. To ensure a sufficient supply of divalent cations, LTA plays an important role in “trafficking ions” (25). LTA may therefore enable hop-resistant cells to establish a larger reservoir of divalent cations in the cell wall than hop-sensitive cells establish. This also could lead to a higher divalent cation content near the membrane (2).
The overexpression of hitA by L. brevis upon hop adaptation further supports the hypothesis that the homeostasis of divalent cations plays an important role in hop resistance. Although the biochemical activity of HitA has not been demonstrated yet, this molecule is a homologue of the NRAMP proteins of Salmonella enterica and E. coli, which are PMF-dependent transport proteins that accumulate manganese in response to oxidative stress (16).
The LTA-mediated acquisition of divalent cations could contribute to the altered decrease in membrane fluidity of L. brevis, which decreased upon acid stress and hop adaptation. Divalent cations interact with the negatively charged head groups of membrane phospholipids and decrease the fluidity of the membrane (3, 32). This effect cannot be assigned to the lipid part of the LTA, which is a very small amount compared to the of membrane phospholipids.
Mechanism of hop adaptation.
Hop adaptation appears to be a multifactorial process, which involves changes in metabolism and in membrane and cell wall composition. The mechanisms involved in hop resistance overlap with the mechanisms involved in pH resistance and as a result enable spoilage of beer. The ADI pathway contributes to hop resistance by generation of energy and additional PMF and appears mainly to ensure the survival of L. brevis TMW 1.465 at the initial stage of hop adaptation. In combination with a higher energy yield from hexose metabolism, the increased ATP yield provides enough survival time to enable adaptation of a small fraction of the population that establishes structural improvements in all the cellular defense mechanisms. Moreover, hop adaptation was attributed to altered membrane composition and the function of LTA, which may act as a divalent cation sequestrant. In contrast to MDR transport and proton extrusion systems, which provide protection against hop compounds at the expense of ATP or the PMF, the structural alterations of the cell envelope can be considered “passive,” i.e., operating largely independent of metabolic energy. However, deenergized cells of L. brevis TMW 1.465A failed to maintain a high intracellular pH when they were exposed to subinhibitory concentrations of iso-α-acids. Consequently, the growth rate of hop-adapted L. brevis TMW 1.465A was virtually independent of the concentration of iso-α-acids, and hop resistance was not eliminated by treatment with sublethal pressure.
As some of the mechanisms involved in hop and pH resistance involve regular metabolic and structural responses that have also been reported for the acid tolerance of Oenococcus oeni (6) and the nisin resistance of L. lactis (18), it is likely that many (e.g., arginine-positive) strains of lactobacilli will develop into beer-spoiling bacteria, although they are considered “harmless” at this time. At the same time, arginine-containing media can also be useful for accelerated detection of present and future beer spoilers. A long-term adaptive response enables growth at hop concentrations greater than those used in any beer and must be avoided by the development of preventive measures.
Acknowledgments
We thank the Wissenschaftsförderung der Deutschen Brauwirtschaft, Nateco2 GmbH u. Co. KG, and Joh. Barth & Sohn GmbH & Co. KG for supporting this work.
We thank Monika Hadek and Holger Teichert (DFG Projekt) for sequencing horA and Franz Reiter for performing high-pressure experiments.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. Crombrugghe. 1981. Evidence for two functional gal promotors in intact Escherichia coli cells. J. Biol. Chem. 256:11905-11910. [PubMed] [Google Scholar]
- 2.Archibald, F. S., and M. N. Duong. 1984. Manganese acquisition by Lactobacillus plantarum. J. Bacteriol. 158:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai, Y., Y. Sano, K. Kikuchi, K. Iwamoto, and S. Watanabe. 2000. The effect of divalent cations on the membrane properties and pharmacokinetics in rat of the lipid A analogue E5531. J. Pharm. Pharmacol. 52:39-45. [DOI] [PubMed] [Google Scholar]
- 4.Behr, J., M. G. Gänzle, and R. F. Vogel. 2004. High pressure inactivation of beer-spoiling lactobacilli, contribution P-73. World Brewing Congr. 2004 Proc., San Diego, 2004, CD ROM 2004.
- 5.Booth, I. R. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49:359-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourdineaud, J.-P. 2006. Both arginine and fructose stimulate pH-independent resistance in the wine bacteria Oenococcus oeni. Int. J. Food Microbiol. 107:274-280. [DOI] [PubMed] [Google Scholar]
- 7.Brown, J. L., T. Ross, R. A. McMeekin, and P. D. Nichols. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37:163-173. [DOI] [PubMed] [Google Scholar]
- 8.Eichel, J., Y. Y. Chang, D. Riesenberg, and J. E. Cronan. 1999. Effect of ppGpp on Escherichia coli cyclopropane fatty acid synthesis is mediated through the RpoS sigma factor (σS). J. Bacteriol. 181:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer, W., H. U. Koch, and R. Haas. 1983. Improved preparation of lipoteichoic acids. Eur. J. Biochem. 133:523-530. [DOI] [PubMed] [Google Scholar]
- 10.Gänzle, M. G., H. M. Ulmer, and R. F. Vogel. 2001. High pressure inactivation of Lactobacillus plantarum in a model beer system. J. Food Sci. 66:1174-1181. [Google Scholar]
- 11.Grogan, D. W., and J. E. Cronan. 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61:429-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guffanti, A. A., S. Clejan, L. H. Falk, D. B. Hicks, and T. A. Krulwich. 1987. Isolation and characterization of uncoupler-resistant mutants of Bacillus subtilis. J. Bacteriol. 169:4469-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, N., M. Ito, S. Horiike, and H. Taguchi. 2001. Molecular cloning of a putative divalent-cation transporter gene as a new genetic marker for the identification of Lactobacillus brevis strains capable of growing in beer. Appl. Microbiol. Biotechnol. 55:596-603. [DOI] [PubMed] [Google Scholar]
- 14.Hughes, A. H., I. C. Hancock, and J. Baddiley. 1973. The function of teichoic acids in cation control in bacterial membranes. Biochem. J. 132:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenni, R., and B. Berger-Bächi. 1998. Teichoic acid content in different lineages of Staphylococcus aureus. Arch. Microbiol. 170:171-178. [DOI] [PubMed] [Google Scholar]
- 16.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 17.Kilimann, K. V., C. Hartmann, R. F. Vogel, and M. G. Gänzle. 2005. Differential inactivation of glucose- and glutamate-dependent acid resistance of Escherichia coli TMW 2.497 by high pressure treatments. Syst. Appl. Microbiol. 28:663-671. [DOI] [PubMed] [Google Scholar]
- 18.Kok, J., B. Buist, A. L. Zomer, S. A. F. T. van Hijum, and O. P. Kuipers. 2005. Comparative and functional genomics of lactococci. FEMS Microbiol. Rev. 29:411-433. [DOI] [PubMed] [Google Scholar]
- 19.Korakli, M., M. Pavlovic, M. G. Gänzle, and R. F. Vogel. 2003. Exopolysaccharide and kestose production by Lactobacillus sanfranciscensis LTH2590. Appl. Environ. Microbiol. 69:2073-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krulwich, T. A., S. Clejan, L. H. Falk, and A. A. Guffanti. 1987. Incorporation of specific exogenous fatty acids into membrane lipids modulates protonophore resistance in Bacillus subtilis. J. Bacteriol. 169:4479-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecureur, V., D. Sun, P. Hargrove, E. G. Schuetz, R. B. Kim, L. B. Lan, and J. D. Schuetz. 2000. Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol. Pharmacol. 57:24-35. [PubMed] [Google Scholar]
- 22.Lewington, J., S. D. Greenaway, and B. J. Spillane. 1987. Rapid small scale preparations of bacterial genomic DNA, suitable for cloning and hybridisation analysis. Lett. Appl. Microbiol. 5:51-53. [Google Scholar]
- 23.Molina-Gutierrez, A., V. Stippl, A. Delgado, M. G. Gänzle, and R. F. Vogel. 2002. In situ determination of the intracellular pH of Lactococcus lactis and Lactobacillus plantarum during pressure treatment. Appl. Environ. Microbiol. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller, H. J., D. Heinegard, and J. H. Poulsen. 1993. Combined alcian blue and silver staining of subnanogram quantities of proteoglycans and glycosaminoglycans in sodium dodecyl sulfate-polyacrylamide gels. Anal. Biochem. 209:169-175. [DOI] [PubMed] [Google Scholar]
- 25.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishihara, M., and Y. Koga. 2000. Enzymatic determination of sn-glycerol-1-phosphate. J. UOEH 22:13-18. [DOI] [PubMed] [Google Scholar]
- 27.Parasassi, T., G. De Stasio, A. d'Ubaldo, and E. Gratton. 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 57:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelkonen, S., J. Hayrinen, and J. Finne. 1988. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J. Bacteriol. 170:2646-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollack, J. H., A. S. Ntamere, and F. C. Neuhaus. 1992. d-Alanyl-lipoteichoic acid in Lactobacillus casei: secretion of vesicles in response to benzylpenicillin. J. Gen. Microbiol. 138:849-859. [DOI] [PubMed] [Google Scholar]
- 31.Richards, M., and R. M. Macrae. 1964. The significance of the use of hops in regard to the biological stability of beer. II. The development of resistance to hop resins by strains of lactobacilli. J. Inst. Brew. 70:484-488. [Google Scholar]
- 32.Riske, K. A., H.-G. Dobereiner, and M. T. Lamy-Freund. 2002. Gel-fluid transition in dilute versus concentrated DMPG aqueous dispersions. J. Phys. Chem. B 106:239-246. [Google Scholar]
- 33.Rogers, H. J. 1988. The bacterial surface—where does it begin and end. American Society for Microbiology, Washington, D.C.
- 34.Sakamoto, K., and W. N. Konings. 2003. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 89:105-124. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto, K., A. Margolles, H. W. van Veen, and W. N. Konings. 2001. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sami, M., K. Suzuki, K. Sakamoto, H. Kadokura, K. Kitamoto, and K. Yoda. 1998. A plasmid pRH45 of Lactobacillus brevis confers hop resistance. J. Gen. Appl. Microbiol. 44:361-363. [DOI] [PubMed] [Google Scholar]
- 37.Simpson, W. J. 1993. Studies on the sensitivity of lactic acid bacteria to hop bitter acids. J. Inst. Brew. 99:405-411. [Google Scholar]
- 38.Simpson, W. J., and J. L. Fernandez. 1994. Mechanism of resistance of lactic acid bacteria to trans-isohumulone. J. Am. Soc. Brew. Chem. 52:9-11. [Google Scholar]
- 39.Simpson, W. J., and J. L. Fernandez. 1992. Selection of beer-spoilage lactic acid bacteria and induction of their ability to grow in beer. Lett. Appl. Microbiol. 14:13-16. [Google Scholar]
- 40.Simpson, W. J., and A. R. Smith. 1992. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 72:327-334. [DOI] [PubMed] [Google Scholar]
- 41.Stolz, P., G. Bocker, R. F. Vogel, and W. P. Hammes. 1993. Utilisation of maltose and glucose by lactobacilli isolated from sourdough. FEMS Microbiol. Lett. 109:237-242. [Google Scholar]
- 42.Suzuki, K., K. Iijima, K. Ozaki, and H. Yamashita. 2005. Study on ATP production of lactic acid bacteria in beer and development of a rapid pre-screening method for beer-spoilage bacteria. J. Inst. Brew. 111:328-335. [Google Scholar]
- 43.Suzuki, K., M. Koyanagi, and H. Yamashita. 2004. Genetic characterization of non-spoilage variant isolated from beer-spoilage Lactobacillus brevis ABBC45. J. Appl. Microbiol. 96:946-953. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, K., K. Ozaki, and H. Yamashita. 2004. Comparative analysis of conserved genetic markers and adjacent DNA regions identified in beer-spoilage lactic acid bacteria. Lett. Appl. Microbiol. 39:240-245. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, K., M. Sami, H. Kadokura, H. Nakajima, and K. Kitamoto. 2002. Biochemical characterization of horA-independent hop resistance mechanism in Lactobacillus brevis. Int. J. Food Microbiol. 76:223-230. [DOI] [PubMed] [Google Scholar]
- 46.Thiele, C., M. G. Gänzle, and R. F. Vogel. 2002. Sample preparation for amino acid determination by integrated pulsed amperometric detection in foods. Anal. Biochem. 310:171-178. [DOI] [PubMed] [Google Scholar]
- 47.Ulmer, H. M., M. G. Gänzle, and R. F. Vogel. 2000. Effects of high pressure on survival and metabolic activity of Lactobacillus plantarum TMW1.460. Appl. Environ. Microbiol. 66:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulmer, H. M., H. Herberhold, S. Fahsel, M. G. Gänzle, R. Winter, and R. F. Vogel. 2002. Effects of pressure-induced membrane phase transitions on inactivation of HorA, an ATP-dependent multidrug resistance transporter, in Lactobacillus plantarum. Appl. Environ. Microbiol. 68:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yansanjav, A., H. Siegumfeldt, L. Jespersen, M. Vancanneyt, J. Swings, I. Hollerova, and J. J. Leisner. 2004. Detection of resistance of lactic acid bacteria to a mixture of the hop analogue compounds tetrahydroiso-alpha-acids by noninvasive measurement of intracellular pH. J Appl. Microbiol. 96:1324-1332. [DOI] [PubMed] [Google Scholar]
- 50.Yasui, T., and K. Yoda. 1997. Purification and partial characterization of an antigen specific to Lactobacillus brevis strains with beer spoilage activity. FEMS Microbiol. Lett. 151:169-176. [DOI] [PubMed] [Google Scholar]
- 51.Ye, J. J., J. W. Neal, X. Cui, J. Reizer, and M. H. Saier, Jr. 1994. Regulation of the glucose:H+ symporter by metabolite-activated ATP-dependent phosphorylation of HPr in Lactobacillus brevis. J. Bacteriol. 176:3484-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, W., P. T. So, T. French, and E. Gratton. 1996. Fluorescence generalized polarization of cell membranes: a two-photon scanning microscopy approach. Biophys. J. 70:626-636. [DOI] [PMC free article] [PubMed] [Google Scholar]