Abstract
We have developed a rapid endospore viability assay (EVA) in which endospore germination serves as an indicator for viability and applied it to (i) monitor UV inactivation of endospores as a function of dose and (ii) determine the proportion of viable endospores in arctic ice cores (Greenland Ice Sheet Project 2 [GISP2] cores; 94 m). EVA is based on the detection of dipicolinic acid (DPA), which is released from endospores during germination. DPA concentrations were determined using the terbium ion (Tb3+)-DPA luminescence assay, and germination was induced by l-alanine addition. The concentrations of germinable endospores were determined by comparison to a standard curve. Parallel EVA and phase-contrast microscopy experiments to determine the percentage of germinable spores yielded comparable results (54.3% ± 3.8% and 48.9% ± 4.5%, respectively), while only 27.8% ± 7.6% of spores produced CFU. EVA was applied to monitor the inactivation of spore suspensions as a function of UV dose, yielding reproducible correlations between EVA and CFU inactivation data. The 90% inactivation doses were 2,773 J/m2, 3,947 J/m2, and 1,322 J/m2 for EVA, phase-contrast microscopy, and CFU reduction, respectively. Finally, EVA was applied to quantify germinable and total endospore concentrations in two GISP2 ice cores. The first ice core contained 295 ± 19 germinable spores/ml and 369 ± 36 total spores/ml (i.e., the percentage of germinable endospores was 79.9% ± 9.3%), and the second core contained 131 ± 4 germinable spores/ml and 162 ± 17 total spores/ml (i.e., the percentage of germinable endospores was 80.9% ± 8.8%), whereas only 2 CFU/ml were detected by culturing.
Bacterial spores (i.e., endospores) are dormant structures that exhibit remarkable longevity, with reports ranging from thousands of years (6, 20, 31, 35, 46) to one-quarter of a billion years (3, 47). They are also highly resistant to chemical, physical, and radiation sterilization processes (31). In fact, Bacillus subtilis spores have survived for 6 years in space while exposed to high vacuum, temperature extremes, and intense solar and galactic radiation (14), which has led to the suggestion that endospores are the most likely to survive an interplanetary lithopanspermic journey (13, 20, 31, 32).
Bacterial spores are the last organisms to remain viable during sterilization processes and environmental extremes that readily kill vegetative bacteria. For that reason, they are employed as biological indicators for monitoring the effectiveness of sterilization processes, such as vaporized hydrogen peroxide treatments (17, 19), autoclaving, and UV irradiation (25, 30).
Two spore-forming genera of medical importance, Bacillus and Clostridium, contain the causative species for anthrax, tetanus, botulism, and gas gangrene (28). Since spores are resistant to sterilization treatments, food that has been incorrectly or minimally processed can give rise to food-borne diseases. For example, Bacillus cereus is a common aerobic food-borne pathogen (24), and Clostridium botulinum and Clostridium perfringens are anaerobic food-borne pathogens commonly associated with food poisoning from canned foods (28). This health issue is exacerbated by the fact that minimally treated foods are becoming extremely popular (50).
Similarly, spore-forming bacteria can survive disinfectant treatments for medical equipment used in hospitals (28). The survival of pathogenic spore-forming bacteria is one of the main causes of infections acquired in hospitals. For example, Clostridium difficile is a major cause of infective hospital-acquired diarrhea (26, 27). In addition, a number of Bacillus species are deliberately released because they produce toxins for agricultural pest control, including Bacillus thuringiensis, which accounts for approximately 90% of the bioinsecticide market (4). Unfortunately, the most ominous use of spore formers is the release of Bacillus anthracis as a bioweapon (16, 42, 48).
Currently, the standard method for quantifying endospores as CFU after heat shock treatment requires several days of incubation. In contrast, the process of endospore germination can be initiated on a timescale of minutes by the addition of trigger molecules, such as l-alanine, l-asparagine, or glucose (7, 9-11, 44). During the first stage of germination, most of the dipicolinic acid (DPA) is released from the core of the spore into solution (7, 41, 49).
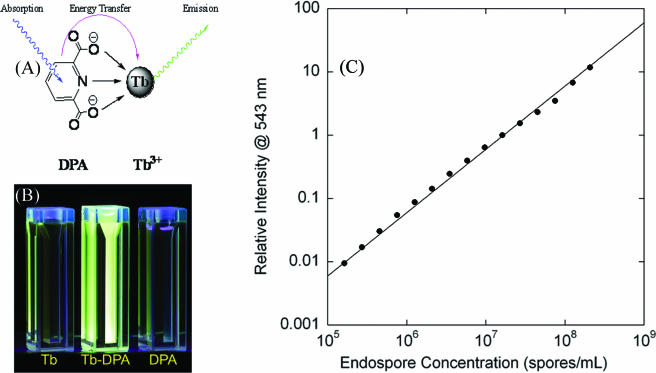
DPA was first identified as a major spore component in 1953 (36). DPA-free mutants were shown to be less resistant to heat inactivation (1); however, further studies with DPA-free, thermoresistant spores have indicated that DPA might help maintain heat resistance as well as facilitate germination (8). Chronologically, the methods developed for DPA detection include UV absorbance measurement (29, 34), iron(II)-DPA colorimetry (15), and most recently, Tb3+-DPA luminescence determination (Fig. 1) (12, 18, 21-23, 33, 39, 41).
FIG. 1.
(A) Absorption-energy transfer-emission mechanism for the Tb3+-DPA luminescence assay. (B) Cuvettes containing solutions of 1 mM Tb3+, 1 mM Tb3+ plus 1 μM DPA, and 1 mM DPA under UV (254 nm) light. (C) Tb-DPA luminescence intensity calibration curve. Error bars represent 1 standard deviation.
Given an average DPA content of 1.7 × 108 DPA molecules per Bacillus atrophaeus spore, the concentration of endospores that germinate can be determined. To assess the efficiencies of sterilization processes, it is sufficient to simply compare the numbers of germinable spores/ml before and after an inactivation process is applied. The endospore viability assay (EVA) protocol reported here, however, also enables the determination of the total spore population via DPA release by autoclaving. Consequently, the proportion of DPA released during germination with respect to the DPA content of the whole spore population provides a measure of the percentage of spores capable of germination.
While the definition of viability of microorganisms is neither simple nor straightforward (40), we employ endospore germination as an indicator of viability, and hence we chose the name endospore viability assay. In this report, we validate and test the EVA against parallel experiments, using phase-contrast microscopy and traditional heterotrophic plate counts. In addition, we report the first application of EVA to monitor the efficiency of a sterilization process. Specifically, we focused on UV inactivation (254 nm) of Bacillus atrophaeus endospores by monitoring the reduction of germinable spore concentrations as a function of UV dose. Finally, we applied EVA to determine the germinable and total spore concentrations in Greenland Ice Sheet Project 2 (GISP2) arctic ice cores, from which we calculated the percentage of endospores capable of germination within the GISP2 ice cores.
MATERIALS AND METHODS
Materials.
Deionized water (18.2 MΩ/cm) was obtained from an ultrafilter system (Water Pro PS; LabConco, Kansas City, MO). Terbium(III) chloride hexahydrate (99.999%), dipicolinic acid (99%; 2,6-pyridinedicarboxylic acid), and l-alanine were obtained from Aldrich (Milwaukee, WI) and were used without further purification. Bacillus atrophaeus (ATCC 9372; formerly called B. subtilis var. Niger) spore water suspensions were purchased from Raven Biological Laboratory (Omaha, NE) and stored at 4°C. The purity of the stock suspension was verified by phase-contrast microscopy to have <0.1% vegetative cell material. The spore stock suspension was determined to contain 1.3 × 1010 CFU/ml and 4.6 × 1010 spores/ml, as determined by titration on tryptic soy agar (TSA; Difco, Sparks, MD), with incubation at 37°C for 48 h, and by direct microscopic enumeration, respectively. Ion chromatography (DX-500; Dionex, Sunnyvale, CA) separation coupled with UV absorbance monitoring at 270 nm (AD-25 absorbance detector; Dionex) was used to determine the number of DPA molecules per spore.
Endospore quantitation using Tb-DPA luminescence.
Tb-DPA luminescence excitation (λex = 250 to 360 nm; λem = 544 nm) and emission (λex = 270 nm; λem = 515 to 580 nm) spectra were recorded at room temperature with a fluorimeter (Jobin Yvon, Edison, N.J.) consisting of a 450-W Xe lamp for excitation, two double-monochromators set with a 4-nm-band-pass filter, and a Pelletier-cooled photomultiplier tube (Products for Research, Inc.). A 500-nm-cutoff filter (Omega Filters, Brattleboro, VT) was placed at the entrance of the emission monochromator. Emission intensities collected on different days were calibrated with respect to an external Tb-DPA luminescence standard solution. To correlate luminescence intensities to spore concentrations, a calibration curve of autoclaved spores ranging from 105 to 108 spores/ml was obtained.
EVA.
All endospore suspensions for EVA experiments were prepared in a final volume of 1.5 ml and contained 10 mM l-alanine and 100 μM unbuffered TbCl3 at pH 6.2.
Determination of germinable spore concentrations.
To induce germination, 150 μl of 0.1 M l-alanine was added to the spore suspension, yielding a final concentration of 10 mM. The solution was then incubated at 37°C for 16 h to allow the germination process to go to completion. Following germination, the luminescence emission and excitation spectra were recorded. The luminescence intensity was converted to the number of germinable spores/ml by comparison to the calibration curve.
Determination of total spore concentrations.
Following germination, the samples were sealed and autoclaved at 136°C for 60 min to release DPA from spores that did not germinate. After cooling to room temperature, the samples were transferred to a 1.4-ml quartz cuvette (Starna, Atascadero, CA), and the same procedure as that described above was followed to obtain the total spores/ml.
Determination of total spore concentrations and germinable spore concentrations by direct microscopic enumeration.
Five-microliter aliquots of a spore suspension were placed in a Petroff-Hausser counting chamber (model 3900; Hausser Scientific, Horsham, PA), and spores were observed at a magnification of ×400 using a phase-contrast microscope (Nikon Eclipse 80i; AG Heinze Co., Lake Forest, CA) mounted with a digital camera (Nikon Digital Sight DS-5 M). The smallest squares in the counting chamber are 0.05 mm by 0.05 mm, of which 36 were analyzed to obtain the average spore count per square. To obtain statistically significant counts, the spore concentration was held above 108 spores/ml. Spore suspensions in the range of 105 to 108 spores/ml were prepared by applying a known dilution factor to the stock suspension. By phase-contrast microscopy, nongerminated, intact spores appear as phase-bright bodies, while germinated spores appear as phase-dark bodies. Vegetative cells appear as larger phase-dark bodies and were not counted. Concentrations of total spores were determined by counting phase-bright bodies. Concentrations of germinable spores were determined by counting the proportion of phase-dark bodies after germination.
Determination of CFU concentration (CFU/ml).
All materials were sterilized in an autoclave at 121°C for 15 min (Tuttnauer-Brinkmann, Westbury, NY). Growth plates of TSA (Difco, Sparks, MD) were prepared in a biological hood (Sterilgard III Advance; The Baker Company, Sanford, ME). Spores were taken from the stock spore suspension and diluted, using 60 s of vortexing to ensure thorough mixing. Aliquots of 100 μl with expected concentrations of 1,000 spores/ml, 500 spores/ml, and 100 spores/ml were plated in triplicate and incubated at 37°C (VWR, West Chester, PA). Colonies were counted manually after intervals of 24 h and 48 h.
UV inactivation experiments.
Endospore suspensions (2 ml at ∼108 spores/ml; A254, ∼0.1) were exposed to 254-nm (4.5 mW/cm2) light from a mercury lamp (UVP, Upland, CA) through methylacrylate cuvettes (A254, ∼1) with caps (Perfector Scientific, Atascadero, CA) as a function of time. Dosages were calculated from this by multiplying the power of the lamp and the time irradiated, accounting for the absorption of the cuvette. The samples were stirred during the entire exposure time, after which they were refrigerated (4°C) until all samples were irradiated. The concentration of UV-irradiated spore suspensions was then determined in terms of germinable spores/ml, as measured by EVA and phase-contrast microscopy, and CFU/ml. The conversion function that correlates the numbers of germinable spores/ml and CFU/ml as a function of dosage was determined by fitting both data sets to exponential functions and calculating the proportionality function between the two. Each data point represents the average of five trials; the standard deviation is too small to be plotted.
Analysis of GISP2 ice cores. (i) Preparation of GISP2 ice cores for analysis.
Initially stored at −78°C, the GISP2 ice cores (ice core 1 no., G2-271 [volume, ∼1 liter]; ice core 2 no., G2-276 [volume, ∼500 ml]) were warmed to −25°C for 8 h and then brought to 0°C within an ice machine prior to decontamination. Decontamination was performed per the modified decontamination protocol of Rogers (38). Briefly, under aseptic conditions within a biological hood (Sterilgard III Advance; The Baker Company, Sanford, ME), the ice core was soaked for 10 seconds in a solution of cold 0.26% sodium hypochlorite (Chlorox bleach) and then rinsed three times with 200 ml of cold, sterile water each time. The rinsed ice core was then melted within a sterile beaker. The melted ice core (434 ml) was vacuum filtered through a 0.2-μm-pore-size polycarbonate filter (Whatman, Florham Park, NJ) and resuspended in 3 ml of cold, sterile water, giving rise to a concentration factor of 145. One milliliter was used to determine the number of germinable spores/ml and, subsequently, total spores/ml, while the rest was used to determine CFU/ml (1/100 R2 agar; Difco, Becton Dickinson, Sparks, Md.).
(ii) Determination of germinable and total spores/ml in GISP2 ice cores.
All endospore suspensions were prepared in a final volume of 3 ml and contained 100 mM l-alanine and 1 μM TbCl3 (unbuffered, pH 6.2). To determine the number of germinable spores/ml, 50 μl of 1 M l-alanine was added to 450 μl of the concentrated ice core meltwater, which was then allowed to germinate to completion in a 37°C water bath for 16 h. Following germination, 150 μl of germinated solution, 300 μl of 10 μM Tb3+, and 2.55 ml water were mixed in a cuvette. Luminescence emission and excitation spectra were recorded as described above. Using a calibration curve for 1 μM Tb3+ ranging over 103 to 107 spores/ml, the integrated intensities were converted to numbers of germinable spores/ml. The same protocol was followed to determine total spores/ml, except that the concentrated ice core meltwater aliquot was autoclaved at 124°C for 20 min. The reported concentrations of spores within the dilute ice core were corrected for the dilution factor by dividing the obtained concentrations of spores by 7.25. The fraction of germinable endospores was found by taking the ratio of germinable spores to total spores.
RESULTS
The fundamental objectives of this investigation were to validate the EVA with respect to traditional phase-contrast microscopy and heterotrophic plate counts and to apply EVA to measure both UV inactivation of B. atrophaeus spores and environmental spores contained within GISP2 ice cores. To this end, we first demonstrated that Tb-DPA luminescence intensity can be reliably converted to spore concentration. We then applied the Tb-DPA luminescence method as part of the EVA to monitor spore germination induced by l-alanine addition. EVA results were compared directly to those of phase-contrast enumeration, which was used to observe spore germination under identical conditions. Germination results were then correlated with traditional heterotrophic plate count results to establish the quantitative relationship between the methods observing germination and those observing full outgrowth to colony formation. We subsequently monitored UV inactivation of spore suspensions, using EVA, phase-contrast, and CFU enumeration. Finally, we used the EVA to determine germinable and total endospore concentrations within arctic ice cores.
Correlation of Tb-DPA luminescence intensity with spore concentration.
Using ion chromatography with UV absorbance detection (λobs = 270 nm), the average spore DPA content was determined to be 1.7 × 108 molecules per spore, which is comparable to the value of 2.2 × 108 molecules per spore previously reported (12). Given an average DPA content per spore, the calibration curve of Tb-DPA luminescence intensity versus direct microscopic enumeration (Fig. 1C) exhibited a linear correlation over 3 orders of magnitude for the concentration, ranging from 105 to 108 spores/ml. From the slope of the best linear fit (R = 0.989), Tb-DPA luminescence intensities can be correlated to endospore concentrations. For subsequent EVA experiments, results were reported in terms of germinable spores and total spores, where DPA was released via germination and autoclaving, respectively.
Endospore viability assay validation.
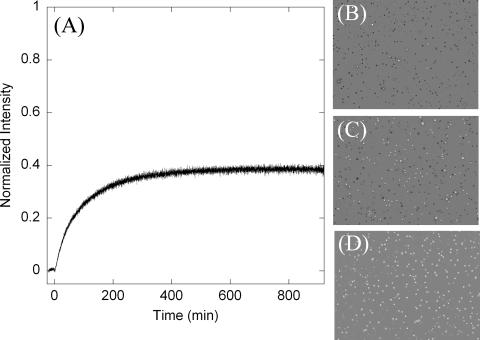
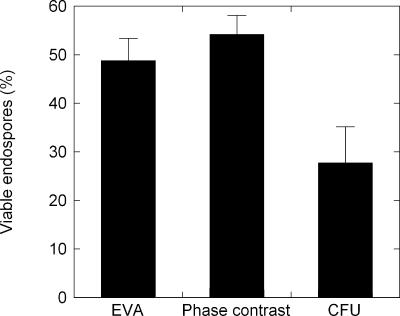
The assumption inherent with the EVA is that there is an average DPA content per spore, which can vary by an order of magnitude across species. Given an average known DPA content within a spore population, the DPA concentration can be correlated to the spore concentration. The addition of l-alanine to a spore suspension triggered the germination process after an induction period of a few minutes. Figure 2A shows the Tb-DPA luminescence intensity increase upon l-alanine-triggered germination, during which DPA is released from the core of the spore into the bulk solution. After the germination process was complete (i.e., the plateau region), the resultant Tb-DPA luminescence intensity was converted to the number of germinable spores/ml, using the conversion factor established from the calibration curve shown in Fig. 1C. Similarly, the conversion factor was applied to convert the intensity after heat inactivation by autoclaving to total spores/ml. The phase-contrast images in Fig. 2 correspond to the spore suspension before germination (D), after germination (C), and after autoclaving (B). Phase-bright bodies indicate nongerminated, intact spores. The percentage of spores that transitioned from phase-bright to phase-dark after l-alanine addition was measured as 48.9% ± 4.5%, which is comparable to the percentage of spores that released DPA in the Tb-DPA luminescence assay (54.3% ± 3.8%), whereas 27.8% ± 7.6% formed colonies on TSA (Fig. 3).
FIG. 2.
(A) Germination time course of B. atrophaeus spores, which shows Tb-DPA luminescence intensity as a function of time. l-Alanine addition at time zero triggers the germination process, during which DPA is released. The luminescence intensity after autoclaving was normalized. (B to D) Phase-contrast images of spores, which appear as phase-bright bodies when intact, after autoclaving (B), after germination is complete (C), and prior to l-alanine addition (D). The image contrast has been enhanced for clarity.
FIG. 3.
Percentage of spores in the total population that can be determined by EVA, phase-contrast microscopy, and CFU enumeration. Error bars represent 1 standard deviation.
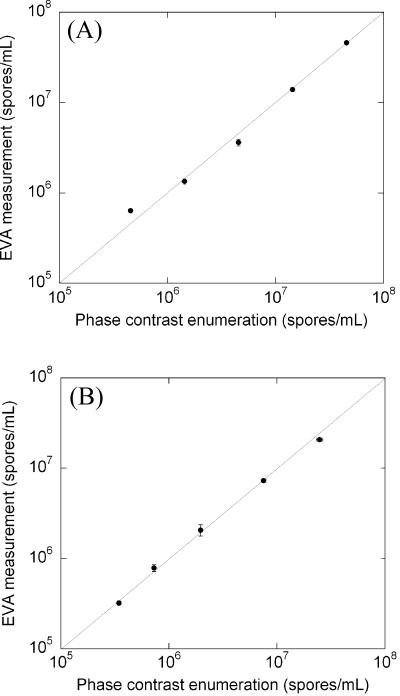
To further validate the EVA, we performed comparison experiments between EVA and phase-contrast enumeration over the concentration range of 105 to 108 spores/ml. For each spore concentration, the Tb-DPA intensities after germination and autoclaving were converted to numbers of germinable spores/ml and total spores/ml, respectively, using the calibration curve (Fig. 1). Figure 4A shows a good fit to a line with a slope of unity that indicates a one-to-one correlation between phase-contrast and EVA measurements for total spores/ml. Similarly, Fig. 4B shows a good one-to-one linear correlation between numbers of germinable spores/ml determined by EVA and phase-contrast microscopy. These one-to-one relationships validate the EVA methodology with respect to the established phase-contrast microscopy methodology to enumerate total spore and germinable populations over 2 orders of magnitude in spore concentration.
FIG. 4.
(A) Correlation between phase-contrast enumeration and Tb3+-DPA luminescence assay for quantification of total spore concentration over 2 orders of magnitude. (B) Correlation between phase-contrast enumeration and Tb3+-DPA luminescence assay for quantification of germinable spores over 2 orders of magnitude. The lines shown indicate a 1:1 correspondence. Error bars represent 1 standard deviation.
Application of EVA to monitor UV inactivation of spores.
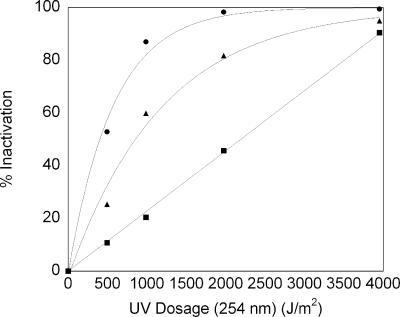
Figure 5 shows spore inactivation as a function of UV-irradiation dosage, as measured by EVA, phase-contrast microscopy, and CFU reduction. Percent inactivation was calculated by using the following equation: % inactivation = (1 − ND/N0) × 100, where ND represents the population measured at a UV dose (D) of >0 and N0 represents the population when D = 0. Populations (N) were evaluated in terms of germinable spores/ml and CFU/ml.
FIG. 5.
Percentage of spore inactivation as a function of UV dosage. •, CFU inactivated by exposure; ▴, inactivation measured by EVA; ▪, inactivation measured by phase-contrast microscopy. Error bars represent 1 standard deviation but are hidden because they are smaller than the data point symbols.
Spore inactivation measured by CFU and EVA followed exponential trends, while spore inactivation measured by phase-contrast microscopy followed a linear trend. From the exponential fits to the CFU and EVA data, we obtained exponential parameters (A), where ACFU = 0.00175 and AEVA = 0.000839. The slope for the linear fit to the phase-contrast data was 0.0228. From the respective fits to the inactivation data, 90% inactivation dose (ID90) values were determined to be 2,773 J/m2, 3,947 J/m2, and 1,322 J/m2 for EVA, phase-contrast microscopy, and CFU enumeration, respectively.
The UV inactivation data reaffirm that germination can serve as an indicator of viability, as illustrated by the reproducible correlation of spore germination (via EVA) to colony formation (i.e., CFU), from which we determined a corresponding conversion function. This conversion function varies with the UV dose (J/m2), as demonstrated in Fig. 5. Thus, the conversion of the germinable spore concentration to the CFU concentration at a given UV dose can be determined with the following equation: CFU/ml = 1.3 × exp(−0.00089D) × GS/ml, where D is the UV dose in J/m2 and GS are germinable spores.
Thus, the magnitude of germinable spore inactivation obtained with the EVA method can be obtained experimentally on the timescale of hours and subsequently correlated to the expected amount of CFU inactivation.
Application of EVA to quantify germinable and total spore concentrations in GISP2 ice cores.
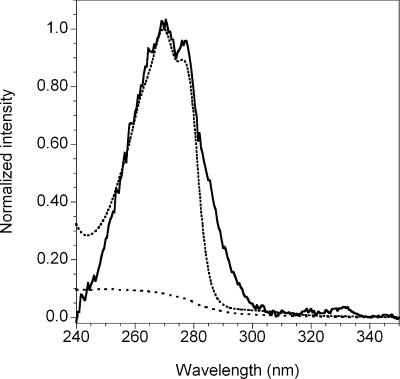
Because of the low expected endospore concentrations in ice cores, we established a new calibration curve using 1 μM Tb3+, which yielded an improved detection limit (1,000 spores/ml) and a linear response from 103 spores/ml to 107 spores/ml (see Fig. S1 in the supplemental material). After the germination process for ice core sample G2-271 was complete, concentrations of endospores within the native ice core were determined to be 295 ± 19 germinable spores/ml and 369 ± 36 total spores/ml, corresponding to 79.9% ± 9.3% germinable spores. A second ice core from a comparable depth, G2-276, yielded slightly different results, with 131 ± 4 germinable spores/ml and 162 ± 17 total spores/ml, corresponding to 80.9% ± 8.8% germinable spores. The excitation spectra showed maximum emission intensities at excitation wavelengths of 270 nm and 278 nm (Fig. 6), which are characteristic of Tb-DPA complexes. Negative controls, in which membrane-filtered water or ice core filtrate was processed in the same manner as the ice cores, did not contain the characteristic DPA excitation spectrum. These data demonstrate that the EVA methodology can be applied to environmental ice core samples.
FIG. 6.
Excitation spectrum of ice core concentrate with 1 μM Tb3+, monitoring emission intensity at 543 nm (solid line). The dotted line shows the excitation spectrum for 1 μM DPA with 10 μM Tb3+. The dashed line shows the negative control for an ice core with 1 μM Tb3+.
DISCUSSION
Comparison of EVA with culture-dependent assays.
As a spore proceeds through the germination process towards cell division, there are various stages, including spore activation; stage 1 germination, during which DPA is released and water partially rehydrates the core; stage 2 germination, during which cortex hydrolysis occurs and metabolism begins; and finally, outgrowth, during which cell division occurs (43). In our experiments, (i) EVA measurements probed DPA release during stage 1 germination via the Tb-DPA luminescence assay; (ii) phase-contrast measurements probed water uptake during stage 1 and stage 2 germination, as indicated by the loss of spore refractility under a phase-contrast microscope; and (iii) CFU measurements probed the population capable of outgrowth under the given set of growth conditions.
The underlying principles of the EVA approach are that (i) the potential to germinate is an indicator of endospore viability, (ii) germination occurs much earlier than outgrowth, and (iii) endospore germination can be observed with simple analytical methods for DPA quantitation (e.g., the Tb3+-DPA luminescence assay) on the timescale of hours. Thus, rather than requiring full outgrowth before enumeration, we probe for viability much earlier, during stage 1 germination, when DPA is released and water begins to enter the core.
Finally, germination and colony formation assays are complementary in that they set upper and lower bounds for the actual viable endospore population. For a given population of spores, a subset will germinate, and a subset of the population capable of germination will form colonies. The actual viable spore population will fall between the populations capable of outgrowth and germination, since culture-based assays underestimate viable endospore bioburden due to viable but nonculturable populations (5), while EVA most likely overestimates the viable endospore bioburden, since not every spore that germinates will be capable of outgrowth. It is possible, however, that the observed number of germinable spores will underestimate the total population capable of germination if significant superdormant populations are present. Superdormant spores will not germinate under the conditions provided or in the timescale of the experiment. In this case, EVA would provide a closer approximation to the viable spore population than do culture-based assays.
UV inactivation and mechanistic considerations for germination.
With respect to the reported series of events during germination, where first DPA is released and then water enters the spore (44), we were intrigued that the UV inactivation data shown in Fig. 5 formed a linear UV dose-response curve when germination was observed by phase-contrast microscopy and showed an exponential response when germination was observed with the Tb-DPA luminescence assay. The linear UV dose-response curve for phase-contrast microscopy leads to a smaller apparent inactivation at any given UV dose than that measured by EVA, such that ID90(phase contrast) = 3,950 J/m2 and ID90(EVA) = 2,770 J/m2. Put another way, at a UV dosage of 1,000 J/m2, we observed 60% EVA inactivation, in contrast to only 20% inactivation by phase-contrast microscopy. Interestingly, the mechanism responsible for the phase-bright-to-phase-dark transition in spores (i.e., water uptake) is less susceptible to 254-nm UV irradiation than that of DPA release. This observation strongly suggests that during stage 1 germination, the mechanisms for DPA release and water uptake consist of separate components that operate independently (i.e., not in series). A mechanism in which one component initiates first DPA release and then water uptake would necessitate an ID90(phase contrast) of less than the ID90(EVA); we observed the opposite.
The ID90(CFU) of 1,300 J/m2 is consistent with reported literature values (2, 37, 45). Not surprisingly, the ability to form colonies exhibited the most sensitivity to UV since outgrowth requires many more mechanisms to remain intact than those required to reach phase 1 germination.
Potential applications of EVA.
Beyond applications for measuring the efficiency of sterilization (where germinable spores are measured before versus after sterilization), EVA can be used to determine the percentage of germinable spores as an indicator of the viable fraction in environmental samples. We envision that EVA will find applications in the determination of viable endospore fractions in environmental samples, including soils, permafrost, and polar ices, from the most extreme environments on earth. To this end, we have demonstrated EVA experiments with GISP2 ice cores. Our initial success in determining numbers of germinable spores/ml and total spores/ml from GISP2 ice cores supports the prospect that EVA will find applications in determining the survival of endospores, the most durable form of life, in extreme environments.
In addition, EVA should find application as part of a suite of assays that may be applied to observe various inactivation points. Using this approach, outgrowth mechanisms may be probed as a function of environmental stress factors. Finally, EVA may be employed to optimize germination conditions in an effort to investigate superdormancy.
Summary.
We have validated a novel EVA that can be used as a rapid alternative to culture-based assays for quantifying (i) the efficiencies of sterilization, bioburden reduction, and decontamination operations (i.e., germinable spores before versus after a sterilization regimen is applied) and (ii) the percentages of germinable endospores embedded in arctic ice cores.
Supplementary Material
Acknowledgments
We thank Wayne Nicholson, Don Obenhuber, Stephanie Connon, Elizabeth Lester, and Michael Kempf for helpful discussions and Pun To Yung for help with contrast enhancement of phase-contrast micrographs. We also thank the National Science Foundation and the National Ice Core Laboratory for providing Greenland ice cores.
The research described in this paper was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Balassa, G., P. Milhaud, E. Raulet, M. T. Silva, and J. C. Sousa. 1979. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J. Gen. Microbiol. 110:365-379. [DOI] [PubMed] [Google Scholar]
- 2.Blatchley, E. R., A. Meeusen, A. I. Aronson, and L. Brewster. 2005. Inactivation of Bacillus spores by ultraviolet or gamma radiation. J. Environ. Eng. 131:1245-1252. [Google Scholar]
- 3.Cano, R. J., and M. K. Borucki. 1995. Revival and identification of bacterial spores in 25-million-year-old to 40-million-year-old Dominican amber. Science 268:1060-1064. [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay, A., N. B. Bhatnagar, and R. Bhatnagar. 2004. Bacterial insecticidal toxins. Crit. Rev. Microbiol. 30:33-54. [DOI] [PubMed] [Google Scholar]
- 5.Colwell, R., and D. Grimes. 2000. Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 6.Gest, H., and J. Mandelstam. 1987. Longevity of microorganisms in natural environments. Microbiol. Sci. 4:69-71. [PubMed] [Google Scholar]
- 7.Gould, G. W., and A. Hurst. 1969. The bacterial spore. Academic Press, New York, N.Y.
- 8.Hanson, R. S., H. O. Halvorson, M. V. Curry, and J. V. Garner. 1972. Mutants of Bacillus cereus strain T that produce thermoresistant spores lacking dipicolinate and have low levels of calcium. Can. J. Microbiol. 18:1139-1143. [DOI] [PubMed] [Google Scholar]
- 9.Hills, G. M. 1950. Chemical factors in the germination of spore-bearing aerobes—observations on the influence of species, strain and conditions of growth. J. Gen. Microbiol. 4:38-47. [DOI] [PubMed] [Google Scholar]
- 10.Hills, G. M. 1949. Chemical factors in the germination of spore-bearing aerobes—the effect of yeast extract on the germination of Bacillus anthracis and its replacement by adenosine. Biochem. J. 45:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hills, G. M. 1949. Chemical factors in the germination of spore-bearing aerobes—the effects of amino-acids on the germination of Bacillus anthracis, with some observations on the relation of optical form to biological activity. Biochem. J. 45:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hindle, A. A., and E. A. H. Hall. 1999. Dipicolinic acid (DPA) assay revisited and appraised for spore detection. Analyst 124:1599-1604. [DOI] [PubMed] [Google Scholar]
- 13.Hoch, J. A., and R. Losick. 1997. Panspermia, spores and the Bacillus subtilis genome. Nature 390:237-238. [DOI] [PubMed] [Google Scholar]
- 14.Horneck, G., H. Bucker, and G. Reitz. 1994. Long-term survival of bacterial spores in space. Adv. Space Rev. 14:41-45. [DOI] [PubMed] [Google Scholar]
- 15.Janssen, F. W., A. J. Lund, and L. E. Anderson. 1958. Colorimetric assay for dipicolinic acid in bacterial spores. Science 127:26-27. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, and J. L. Gerberding. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston, M. D., S. Lawson, and J. A. Otter. 2005. Evaluation of hydrogen peroxide vapour as a method for the decontamination of surfaces contaminated with Clostridium botulinum spores. J. Microbiol. Methods 60:403-411. [DOI] [PubMed] [Google Scholar]
- 18.Jones, G., and V. I. Vullev. 2002. Medium effects on the stability of terbium(III) complexes with pyridine-2,6-dicarboxylate. J. Phys. Chem. 106:8213-8222. [DOI] [PubMed] [Google Scholar]
- 19.Kanemitsu, K., T. Imasaka, S. Ishikawa, H. Kunishima, H. Harigae, K. Ueno, H. Takemura, Y. Hirayama, and M. Kaku. 2005. A comparative study of ethylene oxide gas, hydrogen peroxide gas plasma, and low-temperature steam formaldehyde sterilization. Infect. Control Hosp. Epidemiol. 26:486-489. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy, M. J., S. L. Reader, and L. M. Swierczynski. 1994. Preservation records of microorganisms—evidence of the tenacity of life. Microbiology (Reading, England) 140:2513-2529. [DOI] [PubMed] [Google Scholar]
- 21.Kort, R., A. C. O'Brien, I. H. M. van Stokkum, S. Oomes, W. Crielaard, K. J. Hellingwerf, and S. Brul. 2005. Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl. Environ. Microbiol. 71:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lester, E. D., G. Bearman, and A. Ponce. 2004. A second-generation anthrax “smoke detector.” IEEE Eng. Med. Biol. 23:130-135. [DOI] [PubMed] [Google Scholar]
- 23.Lester, E. D., and A. Ponce. 2002. An anthrax “smoke” detector: online monitoring of aerosolized bacterial spores. IEEE Eng. Med. Biol. 21:38-42. [DOI] [PubMed] [Google Scholar]
- 24.Lukasova, J., J. Vyhnalkova, and Z. Pacova. 2001. Bacillus species in raw milk and in the farm environment. Milchwissenschaft 56:609-611. [Google Scholar]
- 25.Mamane-Gravetz, H., and K. G. Linden. 2004. UV disinfection of indigenous aerobic spores: implications for UV reactor validation in unfiltered waters. Water Res. 38:2898-2906. [DOI] [PubMed] [Google Scholar]
- 26.McFarland, L. V. 1995. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am. J. Infect. Control 23:295-305. [DOI] [PubMed] [Google Scholar]
- 27.McFarland, L. V., M. E. Mulligan, R. Y. Y. Kwok, and W. E. Stamm. 1989. Nosocomial acquisition of Clostridium-difficile infection. N. Engl. J. Med. 320:204-210. [DOI] [PubMed] [Google Scholar]
- 28.Murray, P., E. Baron, M. Pfaller, J. Jorgensen, and R. Yolken (ed.). 2003. Manual of clinical microbiology, 8th ed., vol. 1. ASM Press, Washington, D.C.
- 29.Murty, G. G. K., and H. O. Halvorson. 1957. Effect of duration of heating, l-alanine and spore concentration on the oxidation of glucose by spores of Bacillus cereus var. terminalis. J. Bacteriol. 73:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson, W. L., and B. Galeano. 2003. UV resistance of Bacillus anthracis spores revisited: validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis Sterne. Appl. Environ. Microbiol. 69:1327-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons, P. 1996. Exobiology—dusting off panspermia. Nature 383:221-222. [DOI] [PubMed] [Google Scholar]
- 33.Pellegrino, P. M., N. F. Fell, D. L. Rosen, and J. B. Gillespie. 1998. Bacterial endospore detection using terbium dipicolinate photoluminescence in the presence of chemical and biological materials. Anal. Chem. 70:1755-1760. [DOI] [PubMed] [Google Scholar]
- 34.Perry, J. J., and J. W. Foster. 1956. Monoethyl ester of dipicolinic acid from bacterial spores. J. Bacteriol. 72:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potts, M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell, J. F. 1953. Isolation of dipicolinic acid (pyridine-2-6-dicarboxylic acid) from spores of Bacillus megatherium. Biochem. J. 54:210-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice, J. K., and M. Ewell. 2001. Examination of peak power dependence in the UV inactivation of bacterial spores. Appl. Environ. Microbiol. 67:5830-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers, S. O., V. Theraisnathan, L. J. Ma, Y. Zhao, G. Zhang, S. G. Shin, J. D. Castello, and W. T. Starmer. 2004. Comparisons of protocols for decontamination of environmental ice samples for biological and molecular examinations. Appl. Environ. Microbiol. 70:2540-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen, D. L., C. Sharpless, and L. B. McGown. 1997. Bacterial spore detection and determination by use of terbium dipicolinate photoluminescence. Anal. Chem. 69:1082-1085. [Google Scholar]
- 40.Roszak, D., and R. Colwell. 1987. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 51:365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacks, L. E. 1990. Chemical germination of native and cation-exchanged bacterial spores with trifluoperazine. Appl. Environ. Microbiol. 56:1185-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanderson, W. T., R. R. Stoddard, A. S. Echt, C. A. Piacitelli, D. Kim, J. Horan, M. M. Davies, R. E. McCleery, P. Muller, T. M. Schnorr, E. M. Ward, and T. R. Hales. 2004. Bacillus anthracis contamination and inhalational anthrax in a mail processing and distribution center. J. Appl. Microbiol. 96:1048-1056. [DOI] [PubMed] [Google Scholar]
- 43.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637-648. [DOI] [PubMed] [Google Scholar]
- 44.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 45.Slieman, T. A., and W. L. Nicholson. 2001. Role of dipicolinic acid in survival of Bacillus subtilis spores exposed to artificial and solar UV radiation. Appl. Environ. Microbiol. 67:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sneath, P. 1962. Longevity of micro-organisms. Nature 195:643-646. [DOI] [PubMed] [Google Scholar]
- 47.Vreeland, R. H., W. D. Rosenzweig, and D. W. Powers. 2000. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407:897-900. [DOI] [PubMed] [Google Scholar]
- 48.Weis, C. P., A. J. Intrepido, A. K. Miller, P. G. Cowin, M. A. Durno, J. S. Gebhardt, and R. Bull. 2002. Secondary aerosolization of viable Bacillus anthracis spores in a contaminated US Senate Office. JAMA 288:2853-2858. [DOI] [PubMed] [Google Scholar]
- 49.Woese, C., and H. J. Morowitz. 1958. Kinetics of the release of dipicolinic acid from spores of Bacillus subtilis. J. Bacteriol. 76:81-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zink, D. L. 1997. The impact of consumer demands and trends on food processing. Emerg. Infect. Dis. 3:467-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.