Abstract
Five potentially probiotic canine fecal lactic acid bacterium (LAB) strains, Lactobacillus fermentum LAB8, Lactobacillus salivarius LAB9, Weissella confusa LAB10, Lactobacillus rhamnosus LAB11, and Lactobacillus mucosae LAB12, were fed to five permanently fistulated beagles for 7 days. The survival of the strains and their potential effects on the indigenous intestinal LAB microbiota were monitored for 17 days. Denaturing gradient gel electrophoresis (DGGE) demonstrated that the five fed LAB strains survived in the upper gastrointestinal tract and modified the dominant preexisting indigenous jejunal LAB microbiota of the dogs. When the LAB supplementation was ceased, DGGE analysis of jejunal chyme showed that all the fed LAB strains were undetectable after 7 days. However, the diversity of the intestinal indigenous microbiota of the dogs, as characterized from jejunal chyme plated on Lactobacillus selective medium without acetic acid, was reduced and did not return to the original level during the study period. In all but one dog, an indigenous Lactobacillus acidophilus strain emerged as the dominant LAB strain. In conclusion, strains LAB8 to LAB12 have potential as probiotic strains for dogs as they survive in and dominate the jejunal LAB microbiota during feeding and have the ability to modify the intestinal microbiota.
In the past, the study of lactic acid bacteria (LAB) has been associated with food, feed, and fermentation. However, today the health-promoting effects of LAB are receiving increased attention. Many strains have been considered probiotics as they have been reported to have positive effects on the gastrointestinal well-being of humans (11, 13, 14, 15, 28, 30, 33).
Most of the commercially available probiotics are LAB (5, 7, 10, 24, 34). Some strains have been documented to have beneficial effects on the health of dogs (2, 4, 16). However, our knowledge about the canine intestinal microbiota is still limited. Bacteria are believed to be associated with clinical canine gastrointestinal disorders, such as inflammatory bowel disease and small-intestinal bacterial overgrowth, leading to chronic diarrhea (8, 9, 17, 25). These diseases are often treated with weeks of antibiotic therapy, but prolonged use of broad-spectrum antimicrobials can lead to increasing antimicrobial resistance, resulting in the need for alternative therapies, such as probiotics (29).
Bile and acid tolerance and survival in the gastrointestinal tract are required characteristics of successful probiotics (26, 27). As there are additional antibacterial factors in the intestine, it is recommended that in vitro exposure to jejunal chyme should be used to assess the ability of health-promoting bacteria to survive in the small intestine (23). One important criterion for selection of a probiotic is host species specificity, which is regarded as a prerequisite for showing the beneficial characteristics of the probiotic (10, 22). However, most of the commercial probiotic strains for dogs do not have a canine origin. In addition, many canine probiotic products contain Enterococcus faecium, whose safety has been questioned due to its antibiotic resistance genes and pathogenic characteristics (12, 24). Therefore, probiotic bacteria with fewer potential health risks and greater specificity for dogs should be considered. Exclusion of pathogens by LAB has been demonstrated for dogs (24, 31), but the effects of fed LAB on the indigenous LAB microbiota of the host have not been studied and are poorly understood.
We previously isolated and characterized five canine fecal LAB strains, Lactobacillus fermentum LAB8, Lactobacillus salivarius LAB9, Weissella confusa LAB10, Lactobacillus rhamnosus LAB11, and Lactobacillus mucosae LAB12, as candidate probiotics for canines. These bacteria were dominant, acid tolerant, active antimicrobially, and able to grow to high densities both aerobically and anaerobically (3). In this study, we examined the in vitro tolerance of these five candidate probiotic LAB strains to canine jejunal chyme and fed them as a mixture to five permanently fistulated beagles. Denaturing gradient gel electrophoresis (DGGE) analysis showed that the fed strains survived in the canine gastrointestinal tract and altered the indigenous LAB microbiota in vivo.
MATERIALS AND METHODS
Bacterial culture conditions.
Five LAB strains, L. fermentum LAB8, L. salivarius LAB9, W. confusa LAB10, L. rhamnosus LAB11, and L. mucosae LAB12, which were isolated from dog feces (3), were grown on Lactobacillus selective medium (LBS) (BBL, Becton Dickinson Microbiology Systems, Cockeysville, MD) without acetic acid (mLBS) for 24 to 48 h at 37°C. When strains LAB8 to LAB12 were isolated, acetic acid was left out of the medium to reduce its selectivity as no bacteria grew on the original LBS plates (3). Even with the reduced selectivity of mLBS plates, all the bacteria from mLBS plates characterized were LAB species (3).
Animals.
Five permanently fistulated beagles (one female and four castrated males) from the experimental animal colony unit at the University of Helsinki were selected for the study. All of the dogs had permanent jejunum nipple valve fistulas in the proximal jejunum, located distally 60 cm from the pylorus. The operations were performed 3 to 6 years before this study took place using the method described previously (32). The dogs had been used only for sampling of jejunal chyme and had never been treated with antimicrobial agents. Jejunal chyme was collected via the nipple valve fistulas using a sterile plastic tube. At the time of this study, the dogs were 4 to 8 years old. They were fed dry commercial balanced dog food containing cereal, meat, animal by-products, oils, fats, fish, fish derivatives, minerals, yeast, and vegetable derivatives. The composition of this dog food was as follows: protein, 23%; fat, 13%; fiber, 2.5%; ashes, 8%; calcium, 16 g/kg; phosphorus, 12 g/kg; and moisture content, 8%. Dog food pellets and the jejunal chyme of each dog were examined for LAB by mLBS (BBL) plating before strains LAB8 to LAB12 were fed to the dogs. The study was approved by the University of Helsinki ethics committee.
Resistance of strains to canine jejunal chyme.
The survival and growth of strains LAB8 to LAB12 in jejunal chyme of the dogs were tested by adding 200 μl of each strain (106 to 107 CFU/ml) to 1,800 μl of a fresh, heat-treated (80°C, 15 min) jejunal chyme pool prepared by mixing 400 μl of jejunal chyme from each dog together. The mixture was incubated for 24 h at 37°C without shaking. For determination of viable counts (CFU/ml), serial dilutions of samples (0, 4, 8, and 24 h) were plated on mLBS and incubated for 24 h at 37°C.
Preparing the supplement containing strains LAB8 to LAB12.
Bacterial strains LAB8 to LAB12 were grown separately in mLBS broth for 48 h; cells were then harvested by centrifugation at 4,424 × g (10 min, 4°C; Beckman, Palo Alto, CA), washed twice with sterilized water, and resuspended in sterile water with 0.9% NaCl and 20% glycerol. The viable counts of the suspensions were determined (LAB8, 5.8 × 107 CFU/ml; LAB9, 3.6 × 107 CFU/ml; LAB10, 7.0 × 106 CFU/ml; LAB11, 8.0 × 106 CFU/ml; and LAB12, 3.9 × 107 CFU/ml). Suspensions of the strains were pooled and stored at −20°C until feeding. The viable count of the pool was determined daily during the feeding period and was between 1.4 × 107 and 5.9 × 107 CFU/ml. The pooled bacterial suspension was mixed with the canine feed and given twice a day for 7 days.
Sampling the jejunal chyme.
Approximately 5 ml of jejunal chyme was removed daily within 2 h after morning feeding using a sterile Falcon tube (Greiner Bio-One GmbH, Essen, Germany). Samples were collected during the feeding of strains LAB8 to LAB12 and for an additional 10 days. For determination of the aerobic LAB microbiota and the stability of strains LAB8 to LAB12 in the canine intestine, jejunal contents were plated daily on mLBS (BBL) at dilutions of 10−2 to 10−10, and the plates were incubated aerobically for 48 h at 37°C. After the colonies were counted, 2 ml of LBS broth was added on top of the plates and spread evenly. The bacterial suspension was mixed with 0.5 ml of 87% glycerol and frozen at −20°C before isolation of the chromosomal DNA for DGGE analysis.
Isolation and amplification of total DNA.
Total DNA was isolated from a 2-ml bacterial suspension obtained as described above. The isolation method was the method described by Anderson and McKay (1), except that lysozyme (Sigma-Aldrich, St. Louis, MO) was used at concentration of 100 mg/ml and proteinase K (Finnzymes, Espoo Finland) was used at a concentration of 20 mg/ml for 1 h at 37°C. DNA was extracted with chloroform-isoamyl alcohol (24:1). Samples were also dipped in liquid nitrogen before lysozyme treatment, and 0.1 mg/ml RNase (Sigma-Aldrich Chemie GmbH, Steinheim Germany) was added at the end of the procedure. The chromosomal DNA was amplified by PCR by using the following PCR program: 94°C for 3 min, followed by 35 cycles consisting of 94°C for 1 min, 51°C for 1 min and 72°C 1 min and then a final 10-min extension step at 72°C. Eubacterial 16S rRNA gene primers F-968-GC (5′-CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGGAACGCG AAGAACCTTAC-3′) and R-1401 (5′-CGGTGTGTACAAGACCC-3′) (21, 35) were used to PCR amplify 433-bp products for DGGE. Each 50-μl PCR mixture contained 1 M betaine (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 1× Dynazyme buffer (Finnzymes, Espoo, Finland), each deoxynucleoside triphosphate (Promega, Madison, WI) at a concentration of 400 μM, each primer (Oligomer, Helsinki, Finland) at a concentration of 0.4 μM, 0.4 U Dynazyme (Finnzymes, Espoo, Finland), 2 μl DNA template, and sterile water for adjustment of the volume.
DGGE.
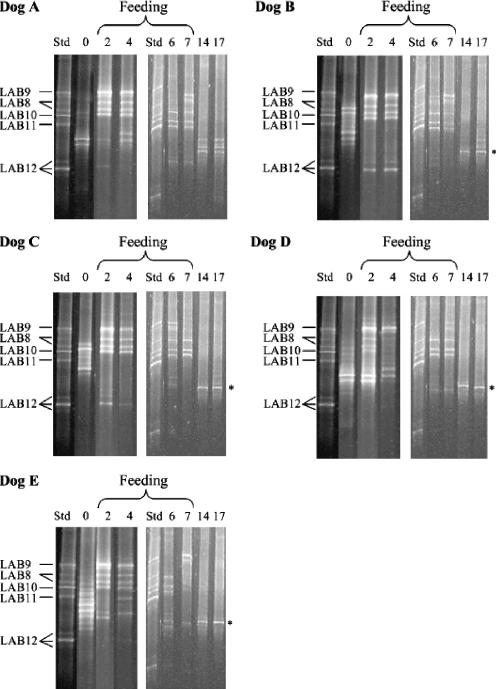
PCR products were separated in a 6% polyacrylamide gel in 0.5× TAE (20 mM Tris-acetate, 10 mM acetate, 0.5 mM Na2EDTA) with a 35 to 55% urea formamide denaturing gradient. A peristaltic pump system together with a Gradient Maker (Amersham Pharmacia Biotech) was used to cast the gel, which was left to polymerize overnight. A 20-μl aliquot of amplified DNA and 10 μl of loading dye were combined and loaded into the gel. DGGE was performed using reagents from a DCode electrophoresis reagent kit (Bio-Rad, Hercules, CA). For 20 μl of DGGE standard, the chromosomal DNA of strains LAB8 to LAB12 was amplified by individual PCRs, and then 4-μl portions of the PCR products were combined. To determine the bands corresponding to strains LAB8 to LAB12 in DGGE standard lanes, the separate PCR products of each LAB strain were electrophoresed in a DGGE gel (separate bands are not shown in Fig. 2). Double and triple bands were present in the DGGE standard lane. These bands were most likely due to sequence heterogeneity in ribosomal operons (20, 21). DGGE gels were electrophoresed at 150 V for 4 h 30 min at 60°C in a Dcode apparatus (Bio-Rad, Hercules, CA) containing a magnetic stirrer. The bands were visualized by fluorescent staining (Gelstar; FMC BioProducts, Rockland, ME) for 40 min, and the gels were photographed under UV light using the Kodak 1D image analysis software (Kodak Company, Rochester, NY).
FIG. 2.
PCR-amplified partial 16S rRNA gene fragments subjected to DGGE. DNA was isolated from bacteria obtained from jejunal chyme of five dogs (dogs A to E) plated on mLBS before (zero time [lane 0]), during (2, 4, and 7 days [lanes 2, 4, and 7, respectively]), and after (14 and 17 days [lanes 14 and 17, respectively]) feeding of strains LAB8 to LAB12. Lane Std contained L. fermentum LAB8, L. salivarius LAB9, W. confusa LAB10, L. rhamnosus LAB11, and L. mucosae LAB12. L. acidophilus (asterisk) was detected after the feeding of strains LAB8 to LAB12 was finished.
Sequencing.
The bands for the dominant nonfed bacteria in a DGGE gel were sequenced. These separate bands were cut out from the DGGE gel and then transferred into a tube containing 50 μl of sterile water and kept overnight at 4°C. Eluted DNA (2 μl) was reamplified with primers F-968 (F-968-GC without the GC clamp) and R-1401. The DNA Synthesis and Sequencing Laboratory (Institute of Biotechnology, Helsinki, Finland) purified these PCR products and sequenced them using primer R-1401. The quality of the sequences obtained was ensured using the Staden Package software (version 1.5.3), and then the sequences were compared with the National Center for Biotechnology Industry (NCBI) BLAST Library (http://www.ncbi.nlm.nih.gov). A level of identity greater than 98% was used to define a species.
RESULTS
Resistance of lactic acid bacterium strains LAB8 to LAB12 to canine jejunal chyme in vitro.
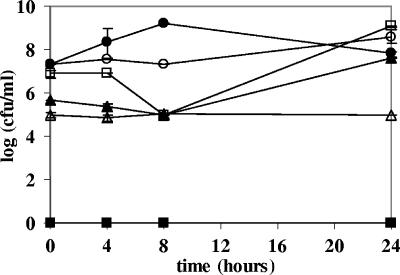
Strains LAB8 to LAB12 all survived in the heat-treated jejunal chyme (Fig. 1). LAB9 and LAB10 were able to grow after 8 h of exposure to the jejunal chyme.
FIG. 1.
Tolerance of lactic acid bacterium strains LAB8 to LAB12 to a mixture of heat-treated jejunal chyme from five fistulated beagles. The viable counts are means for duplicate mLBS plates incubated aerobically. ○, L. fermentum LAB8; □, L. salivarius LAB9; ▴, W. confusa LAB10; ▵, L. rhamnosus LAB11; •, L. mucosae LAB12; ▪, jejunal chyme.
In vivo persistence of strains LAB8 to LAB12 in the jejunum.
The appearance of strains LAB8 to LAB12 in the jejunum of five dogs was dependent on the dog and the stage of feeding. L. fermentum LAB8, L. salivarius LAB9, W. confusa LAB10, and L. rhamnosus LAB11 exhibited the best persistence in the intestine during feeding, whereas L. mucosae LAB12 was detected only in two dogs at the beginning of the supplementation period. Seven days after the LAB supplementation ended strains LAB8 to LAB12 were not detected by DGGE.
Effect of feeding strains LAB8 to LAB12 on the indigenous LAB microbiota of the dogs.
The diversities of the indigenous dominant LAB microbiota of dogs B to E were reduced during the feeding trial and were not completely reestablished after LAB supplementation ended (Fig. 2). In these dogs, a dominant single band appeared in the DGGE gel after the feeding of strains LAB8 to LAB12 had ceased. This band represented the only indigenous LAB that was present at high levels after strains LAB8 to LAB12 had been cleared from the jejunum. The four DGGE bands were sequenced due to their dominance and were identified as Lactobacillus acidophilus bands (NCBI accession number AY773947).
Enumeration of lactic acid bacteria.
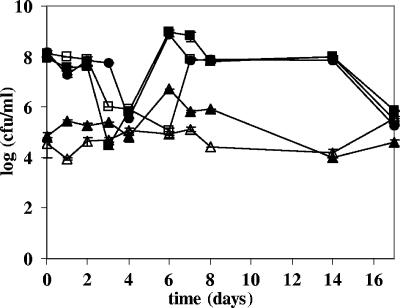
Clearly, feeding of strains LAB8 to LAB12 had a minor effect on the total number of jejunal LAB capable of growing on mLBS aerobically (Fig. 3). Based on this finding, together with the results of the DGGE analysis, which showed that the fed strains were the dominant LAB strains during the feeding period, we concluded that the fed strains displaced the indigenous LAB microbiota capable of growing on mLBS. Dog food pellets did not contain LAB.
FIG. 3.
Viable counts for the jejunal chyme of five dogs (dogs A to E) plated on mLBS before (zero time), during (1 to 7 days), and after (14 and 17 days) feeding of L. fermentum LAB8, L. salivarius LAB9, W. confusa LAB10, L. rhamnosus LAB11, and L. mucosae LAB12. The viable counts are means for duplicate mLBS plates incubated aerobically. ▪, dog A; □, dog B; ▴, dog C; ▴, dog D; •, dog E.
DISCUSSION
Feeding of potentially probiotic strains resulted in alterations in the indigenous jejunal LAB microbiota of five fistulated beagles. During the feeding period strains LAB8 to LAB12 were dominant in the jejunal chyme, but after the supplementation ceased, this dominance disappeared and the organisms were replaced by indigenous LAB. However, the indigenous LAB microbiota was not completely restored to its original composition in four dogs. Remarkably, indigenous L. acidophilus emerged as the dominant LAB. This organism colonized the jejunal chyme rapidly and multiplied to high levels, suggesting that supplementation with strains LAB8 to LAB12 resulted in alteration of the indigenous LAB microbiota culturable on mLBS. We suggest that strains LAB8 to LAB12 could have contributed to the enhanced prevalence of L. acidophilus. This is a new effect of fed LAB, resulting in a selective advantage for specific indigenous LAB species. These properties of strains LAB8 to LAB12 need to be studied further. The L. acidophilus strain isolated might be a promising host-specific probiotic for canines due to its capacity to take over the jejunal niche after the feeding of strains LAB8 to LAB12 has ceased. This colonization efficacy of L. acidophilus may be explained by nutritional factors and adherence in the jejunum.
The numbers of mLBS-culturable LAB in the jejunal chyme did not increase during the feeding period, although strains LAB8 to LAB12 were dominant in the jejunum at this time. This can be explained by the limited number of host-specific colonization sites in the mucosa. In addition, depletion of available nutrients, as well as alterations in the transit time, may have had an effect on the LAB count. However, the dominance of strains LAB8 to LAB12 during the feeding period suggests that these strains were metabolically active in the jejunum and competed successfully in the same ecological niche as the putative lactobacilli of the indigenous microbiota. Intestinal LAB may adhere to the enterocytes or the mucus layer covering them (19), both of which are constantly renewed. The competitive properties of strains LAB8 to LAB12 are likely related to adherence to epithelial cells or the mucus layer. However, as these strains were rapidly cleared from the intestine after the supplementation period, temporary colonization was dependent on constant bacterial supplementation.
The DGGE bands corresponding to the fed strains were not detected in the postsupplementation samples. This showed that the fed strains did not permanently colonize the intestine of the dogs. This is in accordance with previous studies where probiotic strains were not able to permanently colonize individuals having a preexisting intestinal microbiota (2, 6, 18). The dogs did not show any obvious adverse effects during and after the strain LAB8 to LAB12 feeding period.
In conclusion, this study showed that the supplemented LAB had an effect on the hosts' indigenous LAB microbiota. In addition, the candidate probiotics, strains LAB8 to LAB12, were able to survive in the canine upper gastrointestinal tract during the supplementation period. Further clinical studies are warranted to evaluate the potential beneficial health effects of the LAB tested for stimulation of immune functions and competitive exclusion of pathogens.
Acknowledgments
This study was funded by Academy of Finland project number 177321 and by the Faculty of Veterinary Medicine, University of Helsinki.
Eija Kolmonen and Leila Sihvonen are warmly thanked for guidance and assistance with the DGGE method. Janetta Hakovirta is acknowledged for valuable comments on the manuscript.
REFERENCES
- 1.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillon, M. L., Z. V. Marshall-Jones, and R. F. Butterwick. 2004. Effects of probiotic Lactobacillus acidophilus strain DSM13241 in healthy adult dogs. Am. J. Vet. Res. 65:338-343. [DOI] [PubMed] [Google Scholar]
- 3.Beasley, S. S., T. J. K. Manninen, and P.-E. J. Saris. 2006. Lactic acid bacteria isolated from canine faeces. J. Appl. Microbiol. 101:131-138. [DOI] [PubMed] [Google Scholar]
- 4.Benyacoub, J., G. L. Czarnecki, C. Cavadini, T. Sauthier, R. E. Anderson, E. J. Schiffrin, and T. von der Weid. 2003. Supplementation of food with Enterococcus faecium (SF68) stimulated immune functions in young dogs. J. Nutr. 133:1158-1162. [DOI] [PubMed] [Google Scholar]
- 5.Berg, R. D. 1998. Probiotics, prebiotics or ‘conbiotics’? Trends Microbiol. 6:89-92. [DOI] [PubMed] [Google Scholar]
- 6.Biourge, V., C. Vallet, A. Levesque, R. Sergheraert, S. Chevalier, and J.-L. Roberton. 1998. The use of probiotics in the diet of dogs. J. Nutr. 128:2730-2732. [DOI] [PubMed] [Google Scholar]
- 7.Culpin, P. A. 1986. Treatment of canine colitis. Vet. Rec. 119:311-312. [DOI] [PubMed] [Google Scholar]
- 8.Delles, E. K., M. D. Willard, R. B Simpson, T. W. Fossum, M. Slater, D. Kolp, G. E. Lees, R. Helman, and G. Reinhart. 1994. Comparison of species and numbers of bacteria in concurrently cultured samples of proximal small intestinal fluid and endoscopically obtained duodenal mucosa in dogs with intestinal bacterial overgrowth. Am. J. Vet. Res. 55:957-964. [PubMed] [Google Scholar]
- 9.Dotan, I., and D. Rachmilewitz. 2005. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr. Opin. Gastroenterol. 21:426-430. [PubMed] [Google Scholar]
- 10.Dunne, C., L. Murphy, S. Flynn, L. O'Mahonny, S. O'Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly, B. Kiely, E. M. M. Quigley, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 1999. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Leeuwenhoek 76:279-292. [PubMed] [Google Scholar]
- 11.Fooks, L. J., R. Fuller, and G. R. Gibson. 1999. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 9:53-61. [Google Scholar]
- 12.Franz, C. M. A. P., W. H. Holzapfel, and M. E. Stiles. 1999. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 47:1-24. [DOI] [PubMed] [Google Scholar]
- 13.Gotz, V., J. A. Romankiewicz, J. Moss, and H. W. Murray. 1979. Prophylaxis against ampicillin-associated diarrhea with a lactobacillus preparation. Am. J. Hosp. Pharm. 36:754-757. [PubMed] [Google Scholar]
- 14.Isolauri, E., M. Juntunen, T. Rautanen, P. Sillanaukee, and T. Koivula. 1991. A human Lactobacillus strain (Lactobacillus casei strain GG) promotes recovery from acute diarrhea in children. Pediatrics 88:90-97. [PubMed] [Google Scholar]
- 15.Jin, L. Z., Y. W. Ho, N. Abdullah, and S. Jalaludin. 1998. Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett. Appl. Microbiol. 27:183-185. [DOI] [PubMed] [Google Scholar]
- 16.Kanasugi, H., T. Hasegawa, Y. Goto, H. Ohtsuka, S. Makimura, and T. Yamamoto. 1997. Single administration of enterococcal preparation (FK-23) augments non-specific immune responses in healthy dogs. Int. J. Immunopharmacol. 19:655-659. [DOI] [PubMed] [Google Scholar]
- 17.Kanauchi, O., Y. Matsumoto, M. Matsumura, M Fukuoka, and T. Bamba. 2005. The beneficial effects of microflora, especially obligate anaerobes, and their products on the colonic environment in inflammatory bowel disease. Curr. Pharm. Des. 11:1047-1053. [DOI] [PubMed] [Google Scholar]
- 18.Kullen, M. J., M. M. Amann, and M. J. O'Shaughnessy. 1997. Differentiation of ingested and endogenous bifidobacteria by fingerprinting demonstrates the survival of an unmodified strain the gastrointestinal tract of humans. J. Nutr. 127:89-94. [DOI] [PubMed] [Google Scholar]
- 19.Mikelsaar, M., R. Mändar, and E. Sepp. 1998. Lactic acid microflora in the human microbial ecosystem and its development, p. 211-253. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria. Microbiology and functional aspects, 2nd ed. Marcel Dekker Inc., New York, N.Y.
- 20.Muyzer, G., and K. Smalla. 1998. Application of denaturing gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 73:127-141. [DOI] [PubMed] [Google Scholar]
- 21.Nübel, U., B. Engelen, A. Felske, J. Snaidr, A. Wieshuber, R. I. Amann, W. Ludwig, and H. Backhaus. 1996. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 178:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouwehand, A. C., S. Salminen, and E. Isolauri. 2002. Probiotics: an overview of beneficial effects. Antonie Leeuwenhoek 82:279-289. [PubMed] [Google Scholar]
- 23.Rinkinen, M., J. Mättö, S. Salminen, E. Westermarck, and A. C. Ouwehand. 2000. In vitro adhesion of lactic acid bacteria to canine small intestinal mucus. J. Anim. Physiol. Anim. Nutr. 84:43-47. [Google Scholar]
- 24.Rinkinen, M., K. Jalava, E. Westermarck, S. Salminen, and A. C. Ouwehand. 2003. Interaction between probiotic LAB and canine enteric pathogens: a risk factor for intestinal Enterococcus faecium colonization? Vet. Microbiol. 92:111-119. [DOI] [PubMed] [Google Scholar]
- 25.Rutgers, H. C., R. M. Batt, C. M. Elwood, and A. Lamport. 1995. Small intestinal bacterial overgrowth in dogs with chronic intestinal disease. J. Am. Vet. Med. Assoc. 206:187-193. [PubMed] [Google Scholar]
- 26.Saarela, M., G. Mogensen, R. Fonde'n, J. Mättö, and T. Mattila-Sandholm. 2000. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 84:197-215. [DOI] [PubMed] [Google Scholar]
- 27.Salminen, S., A. C. Ouwehand, and E. Isolauri. 1998. Clinical applications of probiotic bacteria. Int. Dairy J. 8:563-572. [Google Scholar]
- 28.Saxelin, M., S. Tynkkynen, T. Mattila-Sandholm, and W. de Vos. 2005. Probiotic and other functional microbes: from markets to mechanisms. Curr. Opin. Biotechnol. 16:204-211. [DOI] [PubMed] [Google Scholar]
- 29.Shanahan, F. 2004. Probiotics in inflammatory bowel disease—therapeutic rationale and role. Adv. Drug Delivery Rev. 56:809-818. [DOI] [PubMed] [Google Scholar]
- 30.Tuohy, K. M., H. M. Probert, C. W. Smejkal, and G. R. Gibson. 2003. Using probiotics and prebiotics to improve gut health. Drug Discov. Today 8:692-700. [DOI] [PubMed] [Google Scholar]
- 31.Vahjen, W., and K. Männer. 2003. The effect of a probiotic Enterococcus faecium product in diets of healthy dogs in bacteriological counts of Salmonella spp., Campylobacter spp. and Clostridium spp. in faeces. Arch. Anim. Nutr. 57:229-233. [DOI] [PubMed] [Google Scholar]
- 32.Wilsson-Rahmberg, M., and O. Jonsson. 1997. Method for long-term intestinal access in the dog. Lab. Anim. 31:231-240. [DOI] [PubMed] [Google Scholar]
- 33.Wunderlich, P. F., L. Braun, I. Fumagalli, V. D'Apuzzo, F. Heim, M. Karly, R. Lodi, G. Politta, F. Vonbank, and L. Zeltner. 1989. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J. Int. Med. Res. 17:333-338. [DOI] [PubMed] [Google Scholar]
- 34.Yeung, P. S. M., and M. E. Sanders. 2002. Species specific identification of commercial probiotic strains. J. Dairy Sci. 85:1039-1051. [DOI] [PubMed] [Google Scholar]
- 35.Zoetendal, E. G., A. von Wright, T. Vilpponen-Salmela, K. Ben-Amor, A. D. Akkermans, and W. M. de Vos. 2002. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl. Environ. Microbiol. 68:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]