Abstract
The aim of this study was to compare the effects of the mixture of Lactobacillus delbrueckii subsp. rhamnosus strain GG, Bifidobacterium lactis Bb12, and inulin on intestinal populations of lactobacilli, bifidobacteria, and enterobacteria in adult and elderly rats fed the same (in quality and quantity) diet. The portal plasma levels of two neuropeptides, neuropeptide Y (NPY) and peptide YY (PYY), were also evaluated to assess the physiological consequences of the synbiotic treatment for the gastrointestinal (GI) tracts of rats of different ages. Adult (n = 24) and elderly (n = 24) male rats were fed the AIN-93 M maintenance diet. After 2 weeks of adaptation, the diet of 12 rats of each age group was supplemented with 8% inulin and with strains GG and Bb12 to provide 2.2 × 109 CFU of each strain g−1 of the diet. Blood and different regions of the GI tract were sampled from all rats after 21 days of the treatment. Treatment with the mixture of strain GG, strain BB12, and inulin induced significantly different changes in the numbers of lactobacilli, bifidobacteria, and enterobacteria of the stomach, small intestine, cecum, and colon microflora. Moreover, the GG, BB12, and inulin mixture increased the concentrations of NPY and PYY for adult rats. For the elderly animals, the PYY concentration was not changed, while the NPY concentration was decreased by treatment with the GG, BB12, and inulin mixture. The results of the present study indicate that the physiological status of the GI tract, and not just diet, has a major role in the regulation of important groups of the GI bacteria community, since even the outcome of the dietary modification with synbiotics depends on the ages of the animals.
The composition of the gastrointestinal (GI) microflora changes with increasing age of the host (34). These changes involve a decrease in the number and diversity of bifidobacteria and bacteroides and an increase in the number of enterobacteria, clostridia, streptococci, and enterococci in elderly humans (17, 20, 51) and in older animals (5).
The age-related changes in the GI bacterial community in elderly humans are likely related to changes in nutritional habits, since it is well recognized that diet/dietary components codetermine the spectrum of intestinal microflora (2, 18, 28, 33, 37). In particular, aging is associated with the decrease in daily fiber intake (27) related to the prolongation of mastication (42), the decline in olfactorial and gustatorial sensitivity (24), and a decrease in cognition (36). However, aging is also associated with alterations of GI physiology and function, such as gastric hypochlorhydria, alterations in intestinal motility, and decreased colonic transit time (23, 31, 44). Changes in GI tract physiology during the aging process may allow specific bacterial populations to take advantage of novel ecological niches, thereby altering the composition of the GI microbiota, in turn affecting intestinal homeostasis and function (22, 28).
Pro- and prebiotics and their combination (synbiotics) have been shown to modify the bacterial community structure in the elderly (4). However, apart from studies on constipation and transit time, where some reports indicate beneficial effects of pro- and prebiotics (30, 38, 41, 49), the usefulness of such supplements has been investigated only with healthy younger adults or with patients with specific pathological conditions (6, 7, 50). It is likely that the net effect of pro- and prebiotics on the composition of the GI microflora and ensuing GI function in the elderly differs from that observed with younger adults due to the specific status of the aged GI tract.
Therefore, the aim of this study was first to compare the initial bacterial composition of different sections of the GI tract with respect to lactobacilli, bifidobacteria, enterobacteria, and total anaerobes of healthy adult and elderly conventional rats under identical nutritional conditions and second to compare the effects of a pro- and prebiotic mixture (synbiotic) on these bacterial populations. The portal plasma levels of two neuropeptides, neuropeptide Y (NPY) and peptide YY (PYY), were also evaluated to assess the physiological consequences of the synbiotic treatment on the GI tract. Circulatory NPY and PYY display a profound inhibitory effect on gastric emptying and acid secretion, gut motility, and exocrine pancreatic secretion (52). The probiotic strains used in this study, i.e., Lactobacillus delbrueckii subsp. rhamnosus strain GG and Bifidobacterium lactis Bb12, have previously been shown to grow in each other's presence in a three-stage gut model in vitro (11). The prebiotic used in this study, Synergy 1, is a 1/1 mixture of long-chain and short-chain fractions of inulin and has been shown in vitro to stimulate the survival and growth of both of these probiotic bacteria (11). It has also been shown that the pro- and prebiotics used in this study, when applied together using the rat model, interact in an additive manner and are more effective in reducing the occurrence of colon carcinogenesis than when applied as individual components (11).
MATERIALS AND METHODS
Diets, supplements, and bacterial strains.
The control diet, the AIN-93 M synthetic meal rodent maintenance diet, was purchased from Lillico (Betchworth, United Kingdom). The prebiotic used was an oligofructose-enriched inulin (Raftilose Synergy 1), provided by ORAFTI (Tienen, Belgium). Raftilose Synergy 1 is a 1/1 mixture of long-chain and short-chain fractions of inulin, a β(2-1)-fructan extracted from chicory roots (Cichorium intybus) as described elsewhere (11). Lactobacillus delbrueckii subsp. rhamnosus strain GG and Bifidobacterium lactis Bb12 were provided by Valio (Helsinki, Finland) and purchased from Chr. Hansen (Horsholm, Denmark), respectively. These strains were supplied as a freeze-dried powder that contained 1 × 1013 to 2 × 1013 CFU g−1 in the case of GG and ∼5 × 1011 CFU g−1 in the case of Bb12. For the purpose of the experiment, the concentrations of GG and Bb12 of different batches were evaluated by dissolving 0.1 g of the powder in 10 ml of maximum recovery diluent (MRD) (Oxoid, Basingstoke, Hampshire, United Kingdom). Serial dilutions were prepared using the same diluent and plating them on Rogosa agar (Oxoid) containing glacial acetic acid (1.32 ml liter−1) for lactobacilli and a selective medium for bifidobacteria (BSM) (Rogosa agar containing 0.05% [wt/vol] cysteine hydrochloride [Sigma, Gillingham, United Kingdom] and 50 mg liter−1 of Mupirocin [Oxoid]) as described by Leuschner et al. (29). Plates were incubated in the anaerobic cabinet at 37°C: Rogosa agar plates for 48 h and BSM plates for 72 h. When the two strains were plated together (Wilkins Chalgren agar; Oxoid), they were easily recognizable, since GG forms large colonies, whereas those of Bb12 are pinpoints in size.
Animals and experimental protocol.
All management and experimental procedures conducted during this study were performed in accordance to the United Kingdom regulations concerning the welfare of experimental animals, i.e., Animals (Scientific Procedures) Act 1986.
Adult (6 months old; n = 24) and elderly (16 months old; n = 24) conventional Sprague-Dawley, male rats (Harlan, United Kingdom), weighing 451 g (± 8.5) or 554 g (± 9.6), respectively, were housed individually and kept in a temperature-controlled environment (22 ± 2°C) with a 12-h light-dark cycle. All rats were fed with AIN-93 M rodent maintenance (control) diet (4 g 100 g−1 of live body weight 24 h−1). The daily amount of the diet (g rat−1) was estimated based on the body weight of the rats, taken every week. After 2 weeks of adaptation, the diet of 12 randomly chosen rats of each age was supplemented with 8% Raftilose Synergy 1 and with GG and Bb12 to provide 2.2 × 109 CFU of each strain g−1 of the diet. The required amount of Raftilose Synergy 1, GG, and Bb12 was mixed with the daily diet allowance for each animal. The remaining rats were fed AIN-93 M without supplements. All rats had free access to distilled water at all times. Food consumption was monitored daily, and body weight was monitored weekly.
Sampling.
After 21 days of the experimental treatment, food but not water was removed for 12 h. Rats were then anesthetized with 2% (vol/vol) isoflurane (Baxter A/S, Allerød, Denmark) in a gas mixture of O2 and NO2 (at the ratio of 2:3), and an abdominal incision was made through the linea alba into the abdominal cavity. Blood was collected from the portal vein into chilled heparinized tubes. Blood samples were centrifuged (800 × g, 10 min, 4°C) and the plasma frozen at −80°C for the subsequent immunoassay. The animals were then killed by isoflurane overdose and exsanguination. At death, the stomach, two specimens of the small intestine, i.e., a jejunal sample (10 to 20 cm from the pylorus) and an ileal sample (10-cm segment ending 2 cm proximal to the cecum), the cecum, and the colon were removed for isolation and enumeration of bacteria.
Isolation and enumeration of bacteria.
GI tract samples were weighed and homogenized in diluent on ice with a Janke-Kunkel Ultra-Turrax T25 homogenizer (Ascher, The Netherlands) at 20,000 rpm in MRD (Oxoid) to provide a 100-g liter−1 suspension and serially diluted to 10−8 with the same diluent. Dilutions of the samples were then inoculated in duplicate onto selective and nonselective agars: Columbia agar (Oxoid) containing 5% (vol/vol) defibrinated horse blood (for total anaerobic bacteria); Rogosa agar (Oxoid) containing glacial acetic acid (1.32 ml liter−1) (for lactobacilli); and MacConkey agar no. 3 (for enterobacteria). Plates for anaerobic incubation had been stored in an anaerobic environment for 48 h. Two selective media were used for bifidobacterium enumeration: Wilkins Chalgren agar (Oxoid) containing acetic acid (1 ml liter−1) and mupirocin (100 mg liter−1; Oxoid), as described by Rada et al. (43), and BSM (29). Inoculated agar plates were incubated in an anaerobic cabinet (Don Whitley Scientific, Shipley, United Kingdom) at 37°C (Columbia agar and Rogosa agar plates for 48 h, both bifidobacterium selective medium plates for 72 h), except for MacConkey agar plates, which were incubated aerobically (at 37°C for 18 h).
The numbers of total anaerobic bacteria, lactobacilli, enterobacteria, and bifidobacteria in the random samples of the control and experimental diets, taken on a weekly basis, were evaluated by dissolving 1 g of the diet in 100 ml of MRD (Oxoid). Serial dilutions were prepared by using the same diluent and plating them in duplicate on selective and nonselective agars as described above.
Extraction of peptides from plasma and immunoassay.
Plasma was acidified with an equal amount of 1% trifluoroacetic acid (TFA) (high-performance liquid chromatography grade; Phoenix Pharmaceuticals, Belmont, CA), mixed, and centrifuged at 10,000 × g for 20 min at 4°C. A Sep column containing 200 mg of C18 was equilibrated by washing with 60% acetonitrile in a 1%-TFA solution (Phoenix Pharmaceuticals, Belmont, CA), followed by three washes with a 1%-TFA solution. Acidified plasma was loaded onto the C18 Sep column, washed with 1% TFA, and eluted with 60% acetonitrile in a 1%-TFA solution. The eluent was evaporated to dryness in a centrifugal concentrator. The residue was dissolved in enzyme immunoassay (EIA) buffer, and concentrations NPY and PYY were measured according to the EIA protocol provided by the manufacturer (NPY and PYY EIA kit; Phoenix Pharmaceuticals, Belmont, CA).
Statistics.
Statistical analyses were performed using the SPSS statistics package (version 11.0.1 for Windows; Woking, United Kingdom). The effects of age and diet on bacterial counts and on the plasma concentration of PYY and NPY were analyzed using the following model:
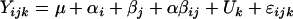 |
where Yijkl is the dependent variable, μ is the overall mean, αi is the effect of diet (nonsupplemented versus supplemented), βj is the effect of age (6 versus 16 months), αβij is the interaction between diet and age, Uk is the random effect of the animal, k = 1,… 12, and ɛijk represents the unexplained random error.
Where appropriate, the Tukey posthoc test was used to compare the dietary effects for each of the two ages studied. The level of statistical significance was assigned as a P value of <0.05 in all performed analyses. Data are expressed as mean values with standard errors.
RESULTS
Animals.
All rats appeared clinically healthy throughout the experiment. The mean food intake was 4.0 g (± 0.1 g) 100 g−1 of live body weight, and there were no differences in food consumption between or within dietary/age groups.
Microbiological studies.
Table 1 shows the counts of total anaerobes, lactobacilli, bifidobacteria, and enterobacteria in the stomach, jejunum, ileum, cecum, and colon in 6- and 16-month-old rats fed a control or synbiotic-supplemented diet.
TABLE 1.
Bacterial counts of different regions of GI tract tissue and content from 6- and 16-month-old rats fed a control or experimental diet
| Organ and bacterial group | Bacterial count (log10 CFU g−1 wet weight) by patient age and dieta
|
Significance (P value)
|
|||||
|---|---|---|---|---|---|---|---|
| 6 mo old
|
16 mo old
|
||||||
| C | E | C | E | Age | Diet | Age × dietc | |
| Stomach | |||||||
| Total anaerobes | 4.9 ± 0.2 | 5.6 ± 0.2* | 5.0 ± 0.3 | 5.4 ± 0.6 | NSb | <0.05 | NS |
| Lactobacilli | 3.9 ± 0.5 | 4.7 ± 0.7 | 3.7 ± 0.2 | 4.0 ± 0.3 | NS | NS | NS |
| Bifidobacteria | 2.5 ± 0.1 | 2.7 ± 0.6 | 2.5 ± 0.4 | 4.5 ± 0.5* | NS | <0.01 | NS |
| Enterobacteria | 3.9 ± 0.4 | 4.6 ± 0.5 | 2.2 ± 0.2 | 2.1 ± 0.3 | <0.01 | NS | <0.05 |
| Jejunum | |||||||
| Total anaerobes | 5.0 ± 0.2 | 6.6 ± 0.4* | 4.3 ± 0.2 | 5.0 ± 0.4 | NS | <0.001 | NS |
| Lactobacilli | 4.2 ± 0.3 | 5.7 ± 0.4* | 4.0 ± 0.2 | 4.6 ± 0.4 | NS | <0.01 | NS |
| Bifidobacteria | 2.9 ± 0.3 | 3.8 ± 0.4 | 2.0 ± 0.3 | 4.2 ± 0.6** | NS | <0.001 | NS |
| Enterobacteria | 4.3 ± 0.4 | 5.7 ± 0.4 | 2.6 ± 0.2 | 1.4 ± 0.2* | <0.01 | <0.05 | <0.001 |
| Ileum | |||||||
| Total anaerobes | 5.1 ± 0.4 | 6.4 ± 0.4* | 4.7 ± 0.4 | 5.7 ± 0.4 | NS | <0.01 | NS |
| Lactobacilli | 4.5 ± 0.3 | 5.6 ± 0.3* | 4.3 ± 0.3 | 5.0 ± 0.2 | NS | <0.05 | NS |
| Bifidobacteria | 3.6 ± 0.4 | 4.2 ± 0.6 | 2.4 ± 0.4 | 5.8 ± 0.3** | NS | <0.001 | NS |
| Enterobacteria | 4.8 ± 0.5 | 5.9 ± 0.7 | 3.3 ± 0.2 | 1.7 ± 0.2* | <0.001 | <0.05 | <0.001 |
| Cecum | |||||||
| Total anaerobes | 8.4 ± 0.1 | 8.5 ± 0.4 | 6.1 ± 0.1 | 7.2 ± 0.1* | <0.001 | <0.05 | NS |
| Lactobacilli | 7.1 ± 0.2 | 7.5 ± 0.6 | 5.1 ± 0.2 | 6.2 ± 0.1* | <0.05 | <0.05 | NS |
| Bifidobacteria | 5.4 ± 0.3 | 6.6 ± 0.5** | 4.3 ± 0.3 | 6.7 ± 0.2** | <0.01 | <0.001 | NS |
| Enterobacteria | 8.0 ± 0.1 | 8.2 ± 0.2 | 5.2 ± 0.2 | 4.3 ± 0.3*** | <0.001 | <0.001 | <0.01 |
| Colon | |||||||
| Total anaerobes | 8.6 ± 0.2 | 9.1 ± 0.1* | 6.8 ± 0.3 | 7.6 ± 0.2** | <0.001 | <0.01 | NS |
| Lactobacilli | 7.2 ± 0.3 | 8.3 ± 0.2* | 4.0 ± 0.2 | 5.4 ± 0.2* | <0.001 | <0.05 | NS |
| Bifidobacteria | 5.1 ± 0.4 | 6.9 ± 0.7** | 4.0 ± 0.4 | 6.5 ± 0.3*** | <0.01 | <0.001 | NS |
| Enterobacteria | 8.2 ± 0.1 | 8.7 ± 0.1** | 4.9 ± 0.1 | 4.3 ± 0.1** | <0.01 | <0.01 | <0.01 |
Data are presented as means with standard errors. Values marked with an asterisk are significantly different from those for the control (C) diet (AIN-93M) within an age group at the respective gastrointestinal region (*, P < 0.05; **, P < 0.01; ***, P < 0.001). E, experimental (AIN-93M supplemented with synbiotics) diet.
NS, not significant.
Interaction between diet and age.
Stomach.
There were no differences in the numbers of total anaerobe, lactobacilli, and bifidobacteria in the stomach samples from 6- and 16-month-old rats.
Supplementation of the diet with synbiotics increased the number of total anaerobes in rats of both age groups, but this increase was significant only for 6-month-old rats (P < 0.05). Dietary supplementation significantly increased (P < 0.01) the number of bifidobacteria in 16-month-old rats but not in 6-month-old rats (P > 0.05). Neither age nor dietary supplementation had any significant effect on the number of lactobacilli in the stomach.
The numbers of enterobacteria in the stomachs of 16-month-old rats were significantly lower (P < 0.01) than those observed for 6-month-old rats. Dietary supplementation did not have any significant effect on the numbers of enterobacteria in the stomachs of either 6- or 16-month-old rats.
Small intestine.
There were no differences in the numbers of total anaerobes, lactobacilli, and bifidobacteria in the jejuna and ilea of 6- or 16-month-old rats.
Supplementation of the diet with synbiotics increased the numbers of total anaerobes in jejuna and ilea of rats of both age groups. However, this increase was significant (P < 0.01 for jejunum and ileum) only in 6-month-old rats. Similarly, dietary supplementation led to a significant increase in the numbers of lactobacilli in jejuna (P < 0.01) and ilea (P < 0.05) of 6-month-old rats, but the increase for 16-month-old rats was not significant.
Treatment with synbiotics increased the numbers of bifidobacteria in jejuna and ilea of rats of both ages. However, the increase in bifidobacteria numbers in the jejuna and ilea was significant (P < 0.001 for both jejunum and ileum) only for 16-month-old rats.
In both jejuna and ilea, the number of enterobacteria for 16-month-old rats was significantly lower (P < 0.01 and P < 0.001 for jejunum and ileum, respectively) than that observed in 6-month-old rats. Furthermore, for both parts of the small intestine, the effects of age and diet were codependent (P < 0.001 for both jejunum and ileum), and the number of enterobacteria was increased for 6-month-old rats, while it was decreased (P < 0.05 for both jejunum and ileum) for 16-months-old rats by the synbiotic treatment.
Large intestine.
In the cecum and colon, the numbers of total anaerobes, lactobacilli, and bifidobacteria were significantly lower for the 16-month-old rats than for 6-month-old rats. In the cecum and colon, supplementation of the diet with synbiotics significantly increased the numbers of total anaerobes, lactobacilli, and bifidobacteria for rats of both ages (Table 1).
In both the cecum and the colon, the numbers of enterobacteria for 16-month-old rats were significantly lower (P < 0.001 and P < 0.01 for the cecum and the colon, respectively) than those observed for 6-months-old rats. Furthermore, for both the cecum and the colon, the effects of age and diet were codependent (P < 0.01 for both cecum and colon), and the number of enterobacteria was increased for 6-month-old rats, while it was significantly decreased (P < 0.001 and P < 0.01 for cecum and colon, respectively) for 16-month-old rats by the synbiotic treatment.
NPY and PYY.
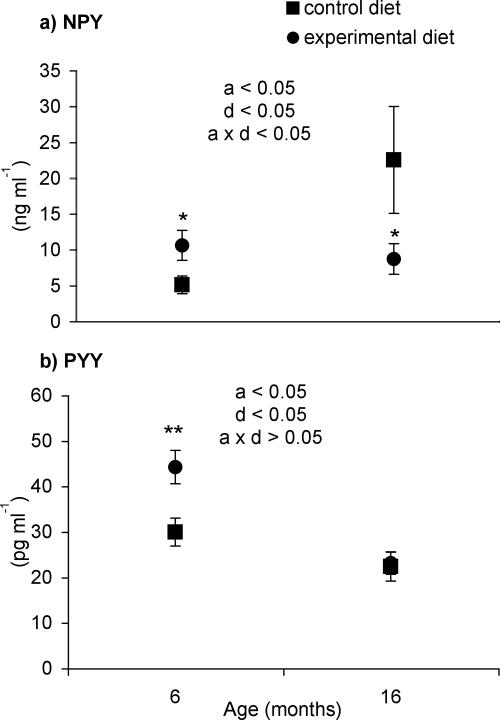
Figure 1 shows the plasma concentrations of NPY and PYY. NPY was significantly higher for older control rats than for their younger counterparts. The effects of age and dietary treatment on the plasma concentration of NPY were codependent (P < 0.05), and the NPY concentration for 6-month-old rats was increased (P < 0.05), while in 16-month-old rats it was decreased (P < 0.05) by the synbiotic treatment (Fig. 1a).
FIG. 1.
Interdigestive plasma concentrations of NPY and PYY of 6- or 16-month-old rats fed a control (AIN-93 M) or experimental (AIN-93 M supplemented with synbiotics) diet. a, d, and axd are P values for the effect of age, diet, or the interaction between age and diet, respectively. Values marked with an asterisk are significantly different from those for the control diet within an age group (*, P < 0.05; **, P < 0.01).
The concentration of PYY in plasma was significantly lower (P < 0.05) for 16-month-old rats than with 6-month-old animals. For 6-month-old rats, but not for older animals, the synbiotic treatment significantly increased (P < 0.05) the plasma concentration of PYY (Fig. 1B).
DISCUSSION
This study shows that dietary treatment with the pro- and prebiotic mixture (synbiotic) produced age-dependent changes in the numbers of total anaerobes, bifidobacteria, lactobacilli, and enterobacteria in the GI tracts of rats.
The composition of these groups in the large intestine from the elderly control rats from the present study was different from that observed for adult animals, which has been reported in earlier studies with both animals and humans (5, 17, 51). Since control adult and elderly animals in the present study consumed the same diet in quality and quantity, the results suggest that the observed differences in microflora in the large intestine of control rats of different ages were related to the differences in the physiological status of animals rather than age-related dietary changes. Furthermore, the NPY plasma level for elderly animals from the present study was higher than that observed for adult rats. It has been previously shown that the frequency of NPY-containing cells increases with age in the rat colon (47) and that the number of NPY-containing cells is also significantly increased in constipated patients (45). A higher level of portal plasma NPY may suggest alterations in GI motility and transit time of digesta, since NPY has been shown to alter the contraction of the gastrointestinal smooth muscles (1, 48) and the GI motility pattern (12). A high NPY level can also affect digestion due to the possible inhibition of pancreatic enzyme secretion (21). Thus, this may suggest that the differences in the initial composition of the GI microflora in adult and elderly rats from the present study were related to physiological status of the GI tract, including differences in the GI motility and transit time of digesta.
In the present study, the 21-day supplementation of the diet with synbiotics induced quantitative changes in specific bacterial numbers in the GI tracts of both adult and elderly animals. Since the pro- and prebiotics were fed together and a suitable (molecular) marker was not available, it was not possible to deduce whether the increase in intestinal lactobacilli and bifidobacteria was due to the given probiotics or the presence of the prebiotic inducing a stimulation of the normal population of these groups. Changes in the composition of the GI tract bacterial community after consumption of pro- and prebiotics have been well documented in both rat (8, 28, 35) and human studies (14, 15, 26). However, results of this study show that consumption of the equal quality and quantity of synbiotics by animals of different ages produced divergent changes in the microflora along the GI tract. In the upper part of the GI tract, i.e., the stomach and small intestine, of the rats from the present study, the most profound differences were observed with regard to bifidobacteria versus lactobacilli. For the adult rats, the number of lactobacilli was increased by synbiotic treatment, whereas for the elderly rats, the same treatment increased the number of bifidobacteria. In the large intestine, the differences in the synbiotic effect were less profound. Synbiotics increased all anaerobic bacterium groups, regardless of age. However, the observed increase was more evident for adult than for elderly rats. There are numerous factors, including local immune mechanisms, interactions between different microbial species, the substrates supplied by the mucosa, initial digesta transit time, pH, and the local supply of oxygen (25, 46), that could have an influence on the GI microbial composition and thus regulate the outcome of the synbiotic supplementation on the GI microflora in the present study. Since most of the changes in GI tract characteristics appear to be age dependent, it suggests that the age of the subject may have a significant impact on the effect of synbiotics on the GI microflora composition.
There were differences between adult and elderly rats in the responses of NPY and PYY to synbiotic treatment. Synbiotic treatment elevated plasma PYY in adult rats, but the same treatment did not affect portal plasma PYY in the elderly rats from the present study. Furthermore, the portal plasma concentration of NPY was decreased by synbiotics for the elderly, while it was elevated for adult animals from the present study. For the adult rats, our results are consistent with those of previous studies (10, 13), but we are not aware of any corresponding data for older animals. The results may suggest that the outcome of dietary treatment with synbiotics depends on the age of animals, although this would not appear to be a consequence of age-related differences in the microflora response to synbiotics, since there were increases in lactobacilli and bifidobacteria for both age groups.
NPY and PYY have diverse and profound physiological functions, including regulation of GI motility, gastric emptying, gastric acid secretion, pancreatic secretion, and free water absorption in the jejunum and colon (3, 9, 16, 19, 30, 39, 40). Morphological studies using a rat model have demonstrated that the numbers of colonic cells containing NPY and PYY increase with age (47). It has also been shown with human subjects that the number of colonic cells containing NPY and PYY is increased in constipated patients (45). Inhibition of GI motility, together with an increase in the absorption of free water due to an elevated level of both NPY and PYY, may cause slow-transit constipation, which is especially frequent in the elderly (32). Results from the present study suggest that the same synbiotic supplement may exacerbate or alleviate certain GI conditions, e.g., slow-transit constipation, due to the initial status of the GI tract.
In conclusion, the results of the present study indicate that the physiological status of the GI tract and not just diet has a major role in the regulation of important groups of the GI bacterial community, since even the outcome of the dietary modification with synbiotics depends on the age of the animals.
Acknowledgments
This work was funded by the European Commission (Marie Curie Individual Fellowship, contract no. CT-2001-51915).
REFERENCES
- 1.Allen, J. M., J. Hughes, and S. R. Bloom. 1987. Presence, distribution, and pharmacological effects of neuropeptide Y in mammalian gastrointestinal tract. Dig. Dis. Sci. 32:506-512. [DOI] [PubMed] [Google Scholar]
- 2.Apajalahti, J. H., A. Kettunen, M. R. Bedford, and W. E. Holben. 2001. Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens. Appl. Environ. Microbiol. 67:5656-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballantyne, G. H., J. R. Goldenring, F. X. Fleming, S. Rush, J. S. Flint, L. P. Fielding, H. J. Binder, and I. M. Modlin. 1993. Inhibition of VIP-stimulated ion transport by a novel Y-receptor phenotype in rabbit distal colon. Am. J. Physiol. 264:G848-G854. [DOI] [PubMed] [Google Scholar]
- 4.Bartosch, S., E. J. Woodmansey, J. C. Paterson, M. E. McMurdo, and G. T. Macfarlane. 2005. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin. Infect. Dis. 40:28-37. [DOI] [PubMed] [Google Scholar]
- 5.Benno, Y., H. Nakao, K. Uchida, and T. Mitsuoka. 1992. Impact of the advances in age on the gastrointestinal microflora of beagle dogs. J. Vet. Med. Sci. 54:703-706. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi-Salvadori, B., R. Vesely, A. Ferrari, E. Canzi, C. Casiraghi, and F. Brighenti. 2001. Behaviour of the pharmaceutical probiotic preparation VSL#3 in human ileostomy effluent containing its own natural elements. New Microbiol. 24:23-33. [PubMed] [Google Scholar]
- 7.Brighenti, F., M. C. Casiraghi, E. Canzi, and A. Ferrari. 1999. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur. J. Clin. Nutr. 53:726-733. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, J. M., G. C. Fahey, Jr., and B. W. Wolf. 1997. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 127:130-136. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. H., R. L. Stephens, Jr., and R. C. Rogers. 1997. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol. Motil. 9:109-116. [DOI] [PubMed] [Google Scholar]
- 10.Delzenne, N. M., P. D. Cani, C. Daubioul, and A. M. Neyrinck. 2005. Impact of inulin and oligofructose on gastrointestinal peptides. Br. J. Nutr. 93(Suppl. 1):S157-S161. [DOI] [PubMed] [Google Scholar]
- 11.Femia, A. P., C. Luceri, P. Dolara, A. Giannini, A. Biggeri, M. Salvadori, Y. Clune, K. J. Collins, M. Paglierani, and G. Caderni. 2002. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesi s 23:1953-1960. [DOI] [PubMed] [Google Scholar]
- 12.Fujimiya, M., and A. Inui. 2000. Peptidergic regulation of gastrointestinal motility in rodents. Peptides 21:1565-1582. [DOI] [PubMed] [Google Scholar]
- 13.Ghatei, M. A., B. Ratcliffe, S. R. Bloom, and R. A. Goodlad. 1997. Fermentable dietary fibre, intestinal microflora and plasma hormones in the rat. Clin. Sci. (London) 93:109-112. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, G. R. 1999. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J. Nutr. 129:1438S-1441S. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, G. R., E. R. Beatty, X. Wang, and J. H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975-982. [DOI] [PubMed] [Google Scholar]
- 16.Guo, Y. S., P. Singh, G. Gomez, G. H. Greeley, Jr., and J. C. Thompson. 1987. Effect of peptide YY on cephalic, gastric, and intestinal phases of gastric acid secretion and on the release of gastrointestinal hormones. Gastroenterology 92:1202-1208. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, H., M. Sakamoto, M. Kitahara, and Y. Benno. 2003. Molecular analysis of fecal microbiota in elderly individuals using 16S rDNA library and T-RFLP. Microbiol. Immunol. 47:557-570. [DOI] [PubMed] [Google Scholar]
- 18.Hedemann, M. S., L. L. Mikkelsen, P. J. Naughton, and B. B. Jensen. 2005. Effect of feed particle size and feed processing on morphological characteristics in the small and large intestine of pigs and on adhesion of Salmonella enterica serovar Typhimurium DT12 in the ileum in vitro. J. Anim. Sci. 83:1554-1562. [DOI] [PubMed] [Google Scholar]
- 19.Holzer-Petsche, U., W. Petritsch, T. Hinterleitner, A. Eherer, G. Sperk, and G. J. Krejs. 1991. Effect of neuropeptide Y on jejunal water and ion transport in humans. Gastroenterology 101:325-330. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins, M. J., R. Sharp, and G. T. Macfarlane. 2001. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, S. C., and M. F. Tsai. 1994. Receptors for peptide YY and neuropeptide Y on guinea pig pancreatic acini. Peptides 15:405-410. [DOI] [PubMed] [Google Scholar]
- 22.Husebye, E., P. M. Hellström, F. Sundler, J. Chen, and T. Midtvedt. 2001. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G368-G380. [DOI] [PubMed] [Google Scholar]
- 23.Johanson, J. F., A. Sonnenberg, and T. R. Koch. 1990. Clinical epidemiology of chronic constipation. J. Clin. Gastroenterol. 12:478-479. [DOI] [PubMed] [Google Scholar]
- 24.Kaneda, H., K. Maeshima, N. Goto, T. Kobayakawa, S. Ayabe-Kanamura, and S. Saito. 2000. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem. Senses 25:331-337. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, D., R. Begbie, and T. King. 1994. Nutritional influences on interactions between bacteria and the small intestinal mucosa. Nutr. Res. Rev. 7:233-257. [DOI] [PubMed] [Google Scholar]
- 26.Kruse, H. P., B. Kleessen, and M. Blaut. 1999. Effects of inulin on faecal bifidobacteria in human subjects. Br. J. Nutr. 82:375-382. [DOI] [PubMed] [Google Scholar]
- 27.Kwok, T., C. N. Yu, H. W. Hui, M. Kwan, and V. Chan. 2004. Association between functional dental state and dietary intake of Chinese vegetarian old age home residents. Gerodontology 21:161-166. [DOI] [PubMed] [Google Scholar]
- 28.Lesniewska, V., I. Rowland, H. N. Laerke, G. Grant, and P. J. Naughton. 2006. Relationship between dietary-induced changes in intestinal commensal microflora and duodenojejunal myoelectric activity monitored by radiotelemetry in the rat in vivo. Exp. Physiol. 91:229-237. [DOI] [PubMed] [Google Scholar]
- 29.Leuschner, R. G. K., J. Bew, P. Simpson, P. R. Ross, and C. Stanton. 2003. A collaborative study of a method for enumeration of probiotic bifidobacteria in animal feed. Int. J. Food Microbiol. 83:161-170. [DOI] [PubMed] [Google Scholar]
- 30.Ling, W. H., O. Hanninen, H. Mykkanen, M. Heikura, S. Salminen, and A. Von Wright. 1992. Colonization and fecal enzyme activities after oral Lactobacillus GG administration in elderly nursing home residents. Ann. Nutr. Metab. 36:162-166. [DOI] [PubMed] [Google Scholar]
- 31.Madsen, J. L., and J. Graff. 2004. Effects of ageing on gastrointestinal motor function. Age Ageing 33:154-159. [DOI] [PubMed] [Google Scholar]
- 32.Merkel, I. S., J. Locher, K. Burgio, A. Towers, and A. Wald. 1993. Physiologic and psychologic characteristics of an elderly population with chronic constipation. Am. J. Gastroenterol. 88:1854-1859. [PubMed] [Google Scholar]
- 33.Mikkelsen, L. L., P. J. Naughton, M. S. Hedemann, and B. B. Jensen. 2004. Effects of physical properties of feed on microbial ecology and survival of Salmonella enterica serovar Typhimurium in the pig gastrointestinal tract. Appl. Environ. Microbiol. 70:3485-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitsuoka, T. 1992. Intestinal flora and aging. Nutr. Rev. 50:438-446. [DOI] [PubMed] [Google Scholar]
- 35.Montesi, A., R. Garcia-Albiach, M. J. Pozuelo, C. Pintado, I. Goni, and R. Rotger. 2005. Molecular and microbiological analysis of caecal microbiota in rats fed with diets supplemented either with prebiotics or probiotics. Int. J. Food Microbiol. 98:281-289. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, C., S. Nordin, and L. Acosta. 1997. Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology 11:126-137. [DOI] [PubMed] [Google Scholar]
- 37.Naughton, P. J., L. L. Mikkelsen, and B. B. Jensen. 2001. Effects of nondigestible oligosaccharides on Salmonella enterica serovar Typhimurium and nonpathogenic Escherichia coli in the pig small intestine in vitro. Appl. Environ. Microbiol. 67:3391-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouwehand, A. C., H. Lagstrom, T. Suomalainen, and S. Salminen. 2002. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann. Nutr. Metab. 46:159-162. [DOI] [PubMed] [Google Scholar]
- 39.Pappas, T. N., H. T. Debas, A. M. Chang, and I. L. Taylor. 1986. Peptide YY release by fatty acids is sufficient to inhibit gastric emptying in dogs. Gastroenterology 91:1386-1389. [DOI] [PubMed] [Google Scholar]
- 40.Pappas, T. N., H. T. Debas, and I. L. Taylor. 1985. Peptide YY: metabolism and effect on pancreatic secretion in dogs. Gastroenterology 89:1387-1392. [DOI] [PubMed] [Google Scholar]
- 41.Pathmakanthan, S., S. Meance, and C. A. Edwards. 2000. Probiotics: a review of human studies to date and methodological approaches. Microb. Ecol. Health Dis. 12(Suppl. 2):10-30. [Google Scholar]
- 42.Peyron, M. A., O. Blanc, J. P. Lund, and A. Woda. 2004. Influence of age on adaptability of human mastication. J. Neurophysiol. 92:773-779. [DOI] [PubMed] [Google Scholar]
- 43.Rada, V., K. Sirotek, and J. Peter. 1999. Evaluation of selective media for bifidobacteria in poultry and rabbit caecal samples. Zentbl. Vet. B 46:369-373. [DOI] [PubMed] [Google Scholar]
- 44.Russell, R. M. 1992. Changes in gastrointestinal function attributed to aging. Am. J. Clin. Nutr. 55:1203S-1207S. [DOI] [PubMed] [Google Scholar]
- 45.Sjölund, K., S. Fasth, R. Ekman, L. Hulten, H. Jiborn, S. Nordgren, and F. Sundler. 1997. Neuropeptides in idiopathic chronic constipation (slow transit constipation). Neurogastroenterol. Motil. 9:143-150. [DOI] [PubMed] [Google Scholar]
- 46.Stephen, A. M., H. S. Wiggins, and J. H. Cummings. 1987. Effect of changing transit time on colonic microbial metabolism in man. Gut 28:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweet, M. A., J. A. Ntambi, E. A. Gaumnitz, T. D. Pugh, R. Weindruch, and C. Singaram. 1996. Neuropeptide Y- and peptide YY-containing colonic cells increase with ageing in male rats. Neuropeptides 30:385-390. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi, T., T. Yamamura, and J. Utsunomiya. 1992. Human pancreatic polypeptide, neuropeptide Y and peptide YY reduce the contractile motility by depressing the release of acetylcholine from the myenteric plexus of the guinea pig ileum. Gastroenterol. Jpn. 27:327-333. [DOI] [PubMed] [Google Scholar]
- 49.Teuri, U., and R. Korpela. 1998. Galacto-oligosaccharides relieve constipation in elderly people. Ann. Nutr. Metab. 42:319-327. [DOI] [PubMed] [Google Scholar]
- 50.van Dokkum, W., B. Wezendonk, T. S. Srikumar, and E. G. van den Heuvel. 1999. Effect of nondigestible oligosaccharides on large-bowel functions, blood lipid concentrations and glucose absorption in young healthy male subjects. Eur. J. Clin. Nutr. 53:1-7. [DOI] [PubMed] [Google Scholar]
- 51.Woodmansey, E. J., M. E. McMurdo, G. T. Macfarlane, and S. Macfarlane. 2004. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl. Environ. Microbiol. 70:6113-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, H. 2002. Central and peripheral regulation of gastric acid secretion by peptide YY. Peptides 23:349-358. [DOI] [PubMed] [Google Scholar]