Abstract
Purpose
To demonstrate that the murine corneal stroma is inhabited by heterogeneous cell populations that include cells expressing nestin.
Methods
Collagenase-isolated corneal stroma cells obtained from newborn and adult mice (2nd and 12th postnatal weeks, respectively), were seeded at low (5 cells/mm2), intermediate (50 cells/mm2), and high (500 cells/mm2) densities in DMEM/F12 containing insulin, transferrin, selenium, and 1% nonessential amino acids. Corneal stroma cells cultured at 500 cells/mm2 were treated with 10 ng/mL human recombinant transforming growth factor (TGF)-β1 for 5 days. Cell morphology and expression of α-smooth muscle actin, choline acetyltransferase, CD45, glial fibrillary acidic protein (GFAP), keratocan, nestin, neurofilaments, protein gene product 9.5, tyrosine hydroxylase, and vimentin were examined.
Results
Phase-contrast microscopy demonstrated that freshly isolated corneal stromal cells are heterogeneous in morphology and include dendritic, stellate, neuronal, and small polyhedral cells. Immunostaining of primary cultures of 2- and 12-week-old mice, 24 hours after seeding at the intermediate density, showed that 100% of cells expressed vimentin and 97.7% ± 2.7% expressed keratocan. α-Smooth muscle actin was expressed by 0.2% ± 0.05% of cells in the 2-week-old group and 0.1% ± 0.07% in 12-week-old group. Neurofilament was expressed by 0.5% ± 0.03% and 0.7% ± 0.03% of cells in the 2- and 12-week-old groups, respectively. No cell expressed GFAP or nestin. After 5 days in culture, cells seeded at high density aggregated as clusters that were immunoreactive to nestin in both groups. Cell clusters and migrating cells reacted to pgp 9.5, and migrating cells, but not the cell clusters, reacted to tyrosine hydroxylase. Cell cluster formation and nestin expression were abolished by culturing in the presence of TGF-β1.
Conclusions
Normal murine corneal stroma contains heterogeneous cell populations including cells with the potential to form clusters and express the progenitor marker nestin. This potential is disrupted by the addition of TGF-β1 to the culture medium.
Corneal keratocytes, a neural crest (NC)– derived cell population responsible for corneal stromal arrangement and transparency, have been classically defined as a homogenous population of mitotically quiescent mesenchymal cells with a limited potential for differentiation and cell fate decision.
During facial morphogenesis, multipotent cranial NC cells migrate rostrally to form different facial organs and structures (reviewed in Refs. 1,2). A selected group of cranial NC cells, situated between the forebrain and the third somite, migrate into the corneal region where they differentiate into corneal endothelium and keratocytes3,4 and begin to synthesize extracellular matrix components including collagens5,6 and keratan sulfate-rich proteoglycans7 such as lumican, keratocan, and mimecan.8,9
Nestin, a class VI intermediate filament, has been used to identify cells with progenitor cell properties in developing cortex,10 cultured primary central and peripheral nervous systems,11,12 immortalized cell lines, and tumors.13 It is well documented that expression of nestin reflects the undifferentiated state of neural progenitor cells14 and that the disappearance of nestin reactivity implies the differentiation of neural progenitor cells in the developing central nervous system.14 At the embryonic neurulation stage, neuroepithelial stem cells temporarily express nestin. However, expression of nestin is downregulated when progenitors differentiate into astrocytes or neurons and is replaced by glial fibrillary acidic protein (GFAP) or neurofilaments, respectively.
In this study, we determined the morphologic and phenotypic heterogeneity of murine corneal stromal cells and the effect of TGF-β.
Materials and Method
The tissue culture plastic dishes (96-well) were from BD Biosciences Dickinson (Lincoln Park, NJ). Amphotericin B, Dulbecco’s modified Eagle’s medium (DMEM), gentamicin, Hanks’ balanced salt solutions (HBSS), HEPES-buffer, phosphate-buffered saline (PBS), ITS, and 0.05% trypsin/0.53 mM EDTA were purchased from Invitrogen-Gibco (Grand Island, NY). Dispase II and collagenase A were obtained from Roche (Indianapolis, IN). Sorbitol, Hoechst 33342, propidium iodide, and human recombinant TGF-β1 were from Sigma-Aldrich (St. Louis, MO).
Isolation of Murine Keratocytes
One hundred fifty mice, strain C57BL/6 of the 2nd and 12th postnatal weeks, were obtained from Charles River (Wilmington, MA). Animals were handled according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. After euthanasia, the eyes were enucleated with forceps, washed profusely in PBS and incubated in DMEM containing 20 mM HEPES, 50 mg/mL dispase II (Roche Diagnostics, Indianapolis, IN) and 100 mM sorbitol at 4°C for 18 hours, as reported previously.15–17 This treatment loosened the entire corneal epithelium, which was subsequently removed by vigorous shaking. Under the dissecting microscope, the corneal stroma was separated from the sclera at the corneoscleral limbus by pressing down the more pigmented limbus with the cutting edge of a 27-gauge needle while holding the eye with a 0.12-mm Colibri forceps (see Supplementary Video 1 at http://www.iovs.org/cgi/content/full/46/12/4528/DC1). Isolated corneal stromas were incubated overnight at 37°C in DMEM containing 1.25 mg/mL collagenase A (Roche), 50 μg/mL gentamicin, and 20 mM HEPES in a noncoated plastic dish until the tissue became smeared onto the dish bottom. Digested corneal stromas in collagenase A were centrifuged at 800 g for 5 minutes. Keratocytes were resuspended in DMEM/F12 containing 20 mM HEPES, ITS (5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite), 1% MEM nonessential amino acid solution (NAA), 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin, with or without 10% FBS. This keratocytes-containing cell suspension was then seeded on 96-well dishes or poly-L-lysine (Nalge Nunc International, Naperville, IL)–coated plastic dishes.
Cell Cultures and TGF-β1 Treatment
Freshly isolated keratocytes in suspension were seeded onto poly-L-lysine–coated eight-well chamber slides or 96-well dishes. Cells were seeded in DMEM/F12 containing ITS and 1% NAA, with or without 10% FBS. To observe better the differences in cell morphology, freshly isolated cells were seeded in DMEM/F12 containing ITS and 1% NAA at different densities of 5, 50, and 500 cells/mm2. The culture medium was changed every 2 to 3 days. To assess whether TGF-β1 affects cell morphology and differentiation, 10 ng/mL human recombinant TGF-β1 was added to serum-free DMEM/F12 containing ITS and 1% NAA from day 0.
Immunofluorescence Staining and Counting
To assess protein expression of α-smooth muscle actin (α-SMA), CD45, choline acetyltransferase, GFAP, keratocan, nestin, neurofilaments (NF), protein gene product 9.5 (rabbit polyclonal anti-pgp 9.5, 1:50 dilution; Chemicon, Temecula, CA), tyrosine hydroxylase and vimentin, culture dishes, and 5-μm thick frozen sections were fixed in cold methanol for 10 minutes at −20°C, blocked, and permeabilized as previously described.18 After they were blocked with 1% BSA and 1% goat serum for 30 minutes, cells were incubated overnight with the following antibodies to α-SMA (monoclonal, 1:100 dilution; Dako, Carpinteria, CA), CD45 (rat purified monoclonal, 1:50 dilution; BD PharMingen, San Diego, CA), GFAP (rabbit polyclonal, 1:100; Chemicon), K12 (goat polyclonal antibody, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), NF-68 (monoclonal, clone NR-4; Sigma-Aldrich), NF-150 (rabbit polyclonal; Chemicon), nestin (monoclonal, clone 2Q178; Abcam, Cambridge, MA), tyrosine hydroxylase (Pelfreez-Bio, Rogers, AR), vimentin (monoclonal; Dako) and keratocan (1:50, rabbit antiserum against mouse keratocan N-terminal peptide [AYEIQDPEDWD-VHDDFYC; Invitrogen, Carlsbad, CA]). The latter peptide was conjugated to a purification column (Sulfolink; Pierce, Rockford, IL), which was then used to purify anti-mouse keratocan antibody according to the manufacturer’s instruction. Rhodamine isothiocyanate (RITC)-anti-rabbit, anti-goat and fluorescein isothiocyanate (FITC)-anti-mouse secondary antibodies at 1:100 dilution (Sigma-Aldrich) were used. Cell nuclei were counterstained with 10 μg/mL Hoechst or propidium iodide in PBS.
For quantification of the percentage of cells expressing a given marker, the number of the positive cells was counted in relation to the total number of Hoechst-labeled nuclei under 200× magnification. All data are expressed as mean ± SD. Photographs were taken with a fluorescence microscope (Nikon, Tokyo, Japan) and a confocal laser scanning microscope (LSM 510; Carl Zeiss Meditec, Thornwood, NY).
Results
Morphology of Isolated Mouse Corneal Stromal Cells
Cell suspensions obtained after collagenase digestion yielded 3000 to 5000 cells per mouse cornea. Cells seeded in DMEM/F12 containing ITS and 1% NAA attached uniformly and spread evenly within 24 hours. Although corneal keratocytes have been regarded as a uniform cell population, phase-contrast microscopy showed a heterogeneous morphology in the stromal cells obtained from the 2- and 12-week-old mice. To evaluate further and quantify the differences in cell morphology, we seeded freshly isolated stromal cells at three different densities: 5, 50, and 500 cells/mm2. After 5 days in culture, cells seeded at 5 cells/mm2 showed different morphologies, including dendritic (arrows), stellate, and small polyhedral cells (Fig. 1A). Cells seeded at 50 cells/mm2 also were heterogeneous, exhibiting dendritic, stellate, and polyhedral cells (Fig. 1B). Neuronlike cells (arrows) were noted in cultures seeded at both 5 and 50 cells/mm2 and were calculated to be 1.7% ± 0.9% of the entire population. At a density of 500 cells/mm2, most cells were polyhedral and formed a confluent monolayer, in which there were neuronlike cells (estimated at ~4.7% ± 2.9% in the 2-week-old mice and 5.9% ± 0.7% in the 12-week-old mice; Fig. 1C). These cells characteristically had a centrally located nucleus and long cytoplasmic processes extending like bipolar neurons. There was also heterogeneity in the population of neuronlike cells. Some appeared in a group with a similar morphology and multiple dendritic processes, suggestive of a probable common origin (Fig. 1D), or in a group with different morphologies consisting of both multiple and bipolar dendritic processes (Fig. 1E). Most of the neuronlike cells were found as single cells (Fig. 1F).
Figure 1.
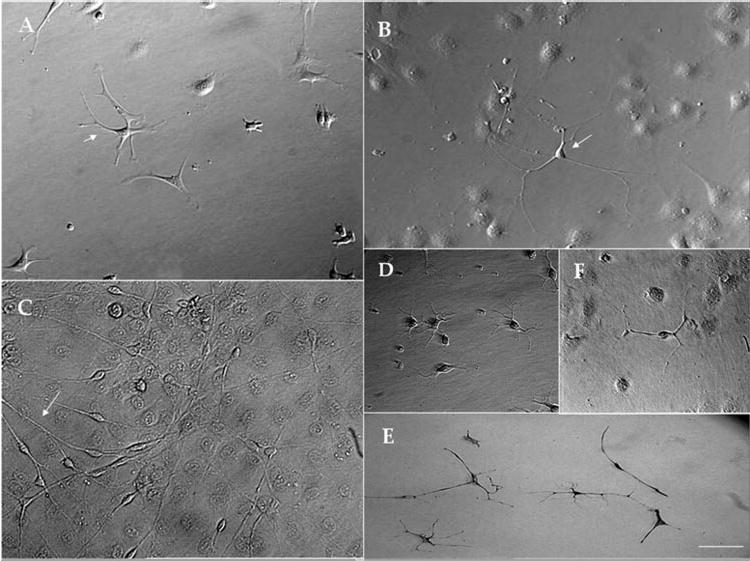
Heterogeneous morphologies of corneal stroma cells seeded at different densities. At 5 cells/mm2, cell morphologies included dendritic (arrows), stellate, small polyhedral, and neuronlike cells (A). A similar finding of neuronlike cells was seen at 50 cells/mm2 (B, arrows). At 500 cells/mm2, cells were polyhedral and formed a confluent monolayer with neuronlike cells (C, arrows). The observed heterogeneity was also seen among neuronlike cells. Cell groups had similar morphologies with multiple dendritic processes that suggested a common origin (D) or different morphologies containing both multiple and bipolar dendritic processes (E). Most of the neuronlike cells were found as single cells (F). Bar, 25 μm.
Heterogeneity of Mouse Corneal Stromal Cells In Vivo and Immediately after Isolation in 2- and 12-Week-Old Mice
The data prompted us to wonder whether such heterogeneity also existed in vivo. As expected, murine corneal keratocytes reacted strongly against vimentin in the entire corneal stroma (data not shown). Keratocan was also expressed in the entire stroma when detected by an affinity-purified antibody against mouse keratocan peptide (Fig. 2A). In contrast, corneal epithelial cells or endothelial cells were not stained. Scarce CD45-positive cells were found in the central corneal stroma with their number increasing in the periphery of the 2- and 12-week-old mice groups (Fig. 2B, marked by arrows). We did not observe immunostaining against α-SMA, GFAP, or nestin (Fig. 2C). Immunostaining against NF-150 detected strong expression at the sclera and posterior peripheral cornea stroma between the corneal lamellae suggesting the presence of corneal nerve stems entering the peripheral corneal and sclera in the 12-week-old mice (Fig. 2D). An intriguing occasional observation was the sporadic linear expression around cell nuclei suggesting the expression of NF-150 by nucleated cells in the superficial central corneal stroma of the 2-week-old (Fig. 2E, marked by arrow) and 12-week-old (Fig. 2F) mice.
Figure 2.
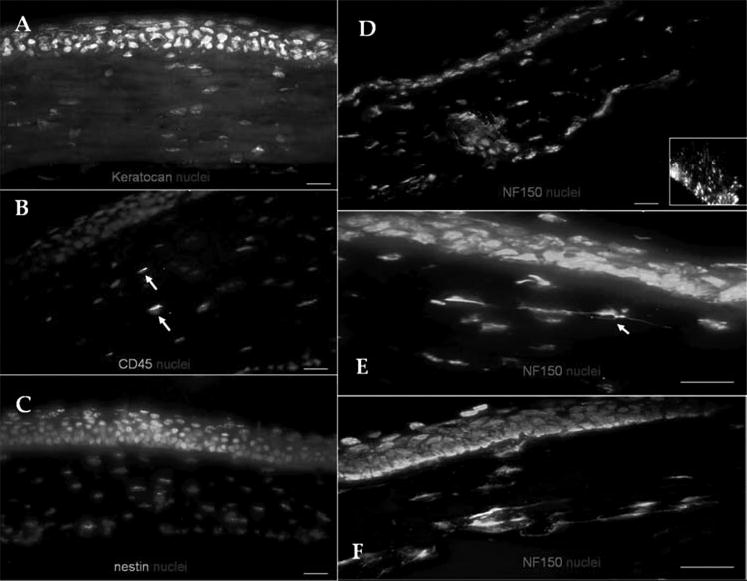
Corneal stroma phenotype in vivo. Noninflamed murine corneas expressed keratocan in the entire stroma (A). Scarce CD45-expressing cells were detected in the stroma (B, arrows). No α-SMA expression was observed (C). NF-150-positive corneal nerve stems entered the posterior sclera and peripheral cornea (D; inset: optic nerve as a positive control). Occasional, nucleated cells expressed NF (arrow) in the 2- (E) and 12- (F) week-old mice. Bar, 25 μm.
To determine whether freshly isolated cell populations from 2- and 12-week-old mice indeed contained heterogeneous phenotypes, we performed immunostaining 24 hours after seeding cells at an intermediate density of 50 cells/mm2 on plastic dishes in DMEM/F12 containing ITS and NAA. The staining to keratin 12, a cornea-specific epithelial marker, was negative, ruling out epithelial contamination during procurement (Fig. 3A). As expected, all cells expressed vimentin (100%) in both groups (Fig. 3B), whereas most, but not all, cells expressed keratocan (97.7% ± 2.7% for 2-week-old mice; Fig. 3C). A small fraction of cells expressed CD45 (1.2% ± 0.5% and 2.1% ± 0.9%, in the 2- and 12-week-old groups, respectively; Fig. 3D, inset shows mouse macrophages as the positive control). To determine whether those cells with a neuronlike morphology were indeed of a neuronal origin, we also performed immunostaining to α-SMA, NFs, GFAP, and nestin. Our results showed that a small fraction of cells expressed α-SMA (0.2% ± 0.05% in 2-week-old mice; Fig. 3E; 0.1% ± 0.07% in 12-week-old mice; Fig. 3H). NF-150 was expressed by 0.5% ± 0.03% of cells in 2-week-old mice (Fig. 3G) and 0.7% ± 0.03% in 12-week-old mice (Fig. 3I). It is known that the low seeding density promotes myofibroblast differentiation in vitro.19 We noted that cells seeded at a high density of 500 cells/mm2 still expressed α-SMA in both groups (Fig. 3F). Nevertheless, we did not find any cells expressing GFAP or nestin. Taken together, these results strongly indicated that freshly isolated corneal stromal cells were not a homogenous population of cells, even before the onset of cell replication and that cell heterogeneity was not affected by the age of mice. Although, most cells belonged to commonly recognized keratocan- and vimentin-expressing keratocytes, a small fraction of cells consisted of NF-expressing neurons and α-SMA-expressing myofi-broblasts in both 2- and 12-week-old mice.
Figure 3.
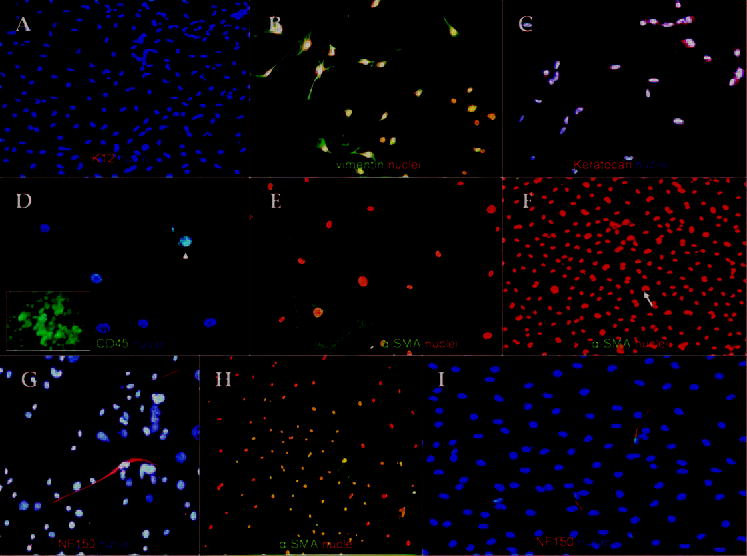
Freshly isolated corneal stroma cells obtained from 2- and 12-week-old mice were heterogeneous in phenotype after 24 hours of culture. Keratin 12–expressing epithelial cells were absent (A). All cells expressed vimentin (B). Most cells expressed keratocan (C). In contrast, few cells expressed CD45 (D; inset: murine macrophages as positive control). α-SMA was observed at intermediate (E) and high densities (F). Elongated and nucleated NF-150-expressing cells were consistently observed (G). The expression of α-SMA (H) and NF-150 (I) expression persisted in 12-week-old mice. Bar, 25 μm.
Presence of Nestin-Positive Cell Clusters at a High Density, but Disappearance after Addition of 10% FBS Addition
Freshly isolated stromal cells from 2-week-old mice were seeded at a high density (500 cells/mm2). Cells attached in 24 hours and became confluent in 2 days in DMEM/F12 containing ITS and NAA. After 3 to 4 days of culturing, we noted the emergence of a cell population of elongated cells resembling bipolar neurons, as mentioned before. Five to 7 days later, cells aggregated as a cluster. These clusters formed domes and later formed structures from which many bipolar neuronlike cells emigrated (Fig. 4A). These cells grew on top of confluent stromal cells (Fig. 4B). As stated earlier, these clusters of neuronlike cells were not observed when stromal cells were seeded at 5 or 50 cells/mm2. Immunostaining of these clusters was negative to NF (Fig. 4C) or GFAP (Fig. 4D), but positive to nestin in the cluster (Figs. 4E, 4F) or after they emigrated onto stromal cells (Fig. 4G).
Figure 4.
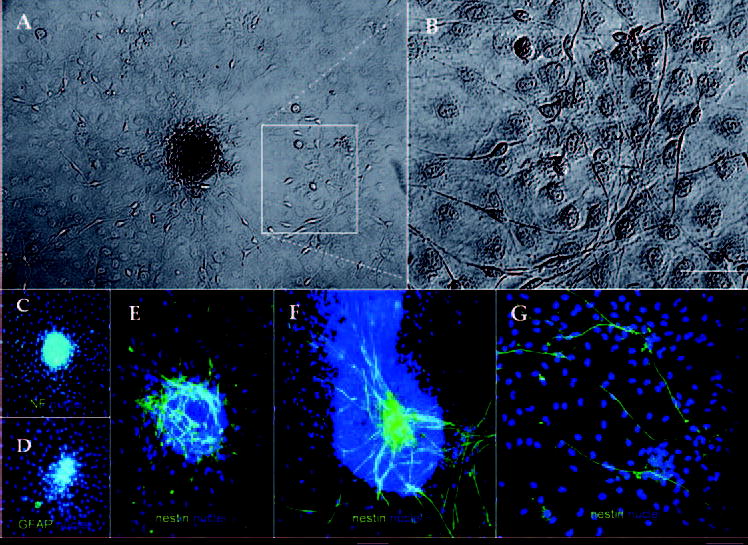
Cell clusters were present in corneal stromal cells isolated from 2-week-old mice. Freshly isolated stromal cells seeded at a 50 cells/mm2 aggregated formed cell clusters, from which many bipolar neuronlike cells emigrated (Fig. 4A, inset). These cells grew on top of confluent stromal cells (Fig. 4B, coming from the inset of Fig. 4A). Immunostaining of these clusters was negative to NF (C) and GFAP (D). In contrast, strong immunoreactivity against nestin was noted in different cell clusters (E, F). Cells migrating out of the cluster also reacted against nestin (G). Bar, 25 μm.
Cell cluster formation and nestin expression were also observed in the adult mice when cultured under the same conditions. Adult corneal stroma cells seeded at a high density (500 cells/mm2) also formed cell clusters (Fig. 5A) and cells that emigrated onto the surrounding stromal cells were also observed. Similarly, cells inside the cluster and those migrating cells also expressed nestin (Figs. 5C, 5D). To prove the neuronal phenotype of these cells, immunostaining to choline acetyltransferase, pgp 9.5, and tyrosine hydroxylase was performed. Cell clusters and migrating cells reacted positively to pgp 9.5 (Fig. 5E). Migrating cells but not the cell clusters reacted to tyrosine hydroxylase (Fig. 5F). No reactivity to choline acetyltransferase appeared (not shown).
Figure 5.
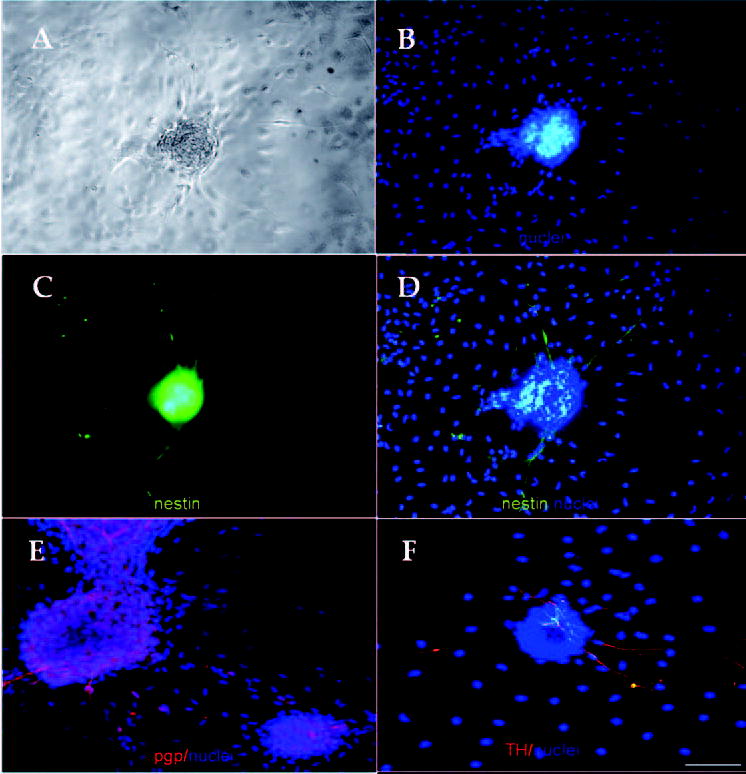
Adult corneal stroma cells also formed nestin-expressing cell clusters. Stromal cells freshly isolated from 12-week-old mice formed similar cell clusters (A). DAPI expression (B). A cell cluster expressing nestin (C). Merged image showing the expression of nestin in the cluster and neuronlike elongated cells. Nestin was not expressed by the stromal cells (D). Cell clusters and migrating cells reacted against pgp 9.5 (E). Migrating cells but not the cell clusters reacted against tyrosine hydroxylase (F). Bar, 25 μm.
To stimulate cell division, we added 10% FBS to DMEM/F12 containing ITS and NAA from the time of seeding. Cells replicated rapidly and became confluent in less than 24 hours. However, we did not observe any clusters of neuronlike cells nor cells expressing nestin during the course of 7 days of observation (data not shown). We then added 10% FBS to the culture containing DMEM/F12 with ITS and NAA, when the cluster of neuronlike cells were already formed (Fig. 6A) or scattered around (Fig. 6B). In 2 days, the cluster began to disintegrate (Fig. 6C) and finally disappeared. At that time, nestin expression was downregulated in the cluster (not shown) or neuronlike cells (Fig. 6D). Hoechst 33342 staining demonstrated highly fluorescent and fragmented nuclei, suggestive of apoptosis in the area (Fig. 6E). No immunoreactivity against GFAP, keratocan, or NFs was observed after 5 days of 10% FBS treatment (data not shown). However, all cells continued to express vimentin (Fig. 6F).
Figure 6.
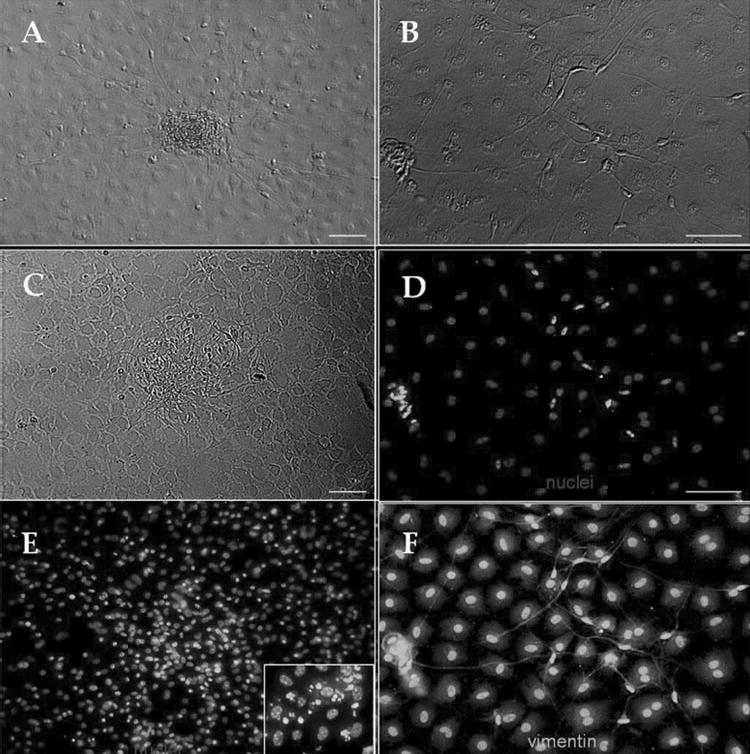
Fetal bovine serum abolished nestin-positive cell clusters. Already formed cell clusters (A) or neuronlike cells (B) in DMEM/F12/ITS were challenged by adding 10% FBS. In 2 days, the cluster began to disintegrate (C), and nestin expression was downregulated (D). Hoechst 33342 staining demonstrated highly fluorescent and fragmented nuclei (E, inset shows the higher magnification). Cells continued to express vimentin (F). Bar, 25 μm.
Effect of TGF-β1 Treatment on Cell Cluster Formation and Nestin Expression
To test whether TGF-β1, a known FBS component and a potent modulator of differentiating corneal keratocyte into myofibroblasts,20,21 was capable of abolishing the cells with a neuron morphology, we added 10 ng/mL TGF-β1 to the culture seeded with corneal stromal cells at 500 cells/mm2 in DMEM/F12 containing ITS and NAA. Cells attached within 24 hours and formed a confluent monolayer. After 3 days in culture, cells started to aggregate but never formed any cluster of cells or a single neuronlike cell (Fig. 7A). After 5 days in culture, this cell monolayer contracted to form a mass of cells in the center of the dish (Fig. 7B). Immunostaining showed that this cell mass was negative to nestin (not shown), whereas all cells were strongly positive to α-SMA (Fig. 7C).
Figure 7.
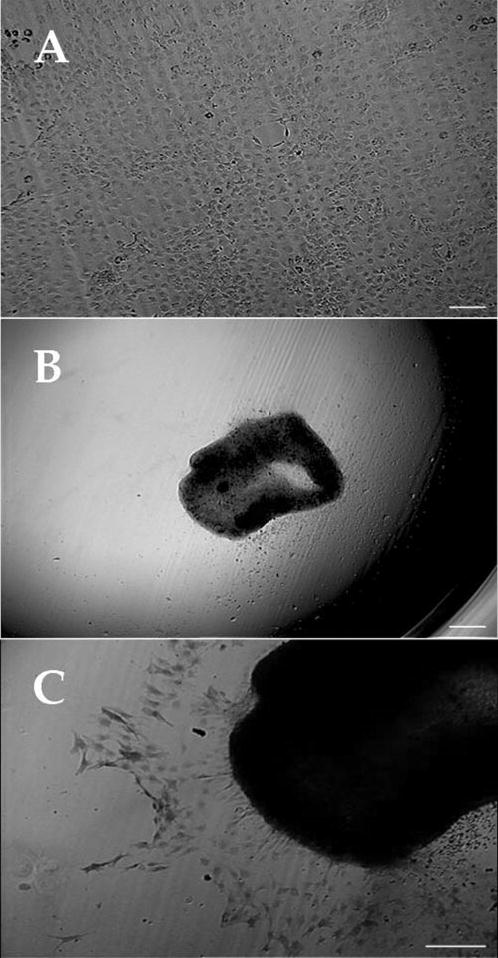
TGF-β1 abolishes cell cluster formation. Corneal keratocyte seeded with corneal stromal cells at 500 cells/mm2 in DMEM/F12 containing ITS and supplemented with 10 ng/mL TGF-β1 formed a confluent monolayer but no cluster nor single neuronlike cells (A). After 5 days in culture, this monolayer contracted and formed a mass of cells in the center of the dish (B). Immunostaining showed that cells in this structure were strongly positive to α-SMA (C). Bar, 25 μm.
Discussion
The results presented herein indicate that the noninflamed murine corneal stroma obtained from 2-week-old and adult mice harbors heterogeneous cell populations that at least include cells expressing keratocan, NF, and α-SMA. In addition, there were a small number of cells with the potential to develop into cell clusters expressing the progenitor marker, nestin, under a serum-free culturing condition. These findings collectively challenge the traditional view that corneal stroma cells are a homogeneous population of highly differentiated NC-derived mesenchymal cells, termed keratocytes.
Our finding of α-SMA expressing myofibroblasts in corneal stromal cultures within 24 hours after seeding at low and high cell densities is surprising because the time was too short to allow keratocytes to differentiate into myofibroblasts, especially in the absence of serum. Previously, Maltseva et al.22 also described the occasional presence of spontaneous myofibroblasts in rabbit keratocyte cultures under serum-containing conditions. They attributed their finding to an unintentional exposure to TGF-β during isolation. Clearly, one cannot rule out the possibility that myofibroblasts may be derived from prior corneal injuries before death or during in vitro manipulation. Because we noted a similar finding in human corneal stroma cultures under serum-free conditions (Espana EM, Tseng SCG, unpublished data, 2003), we wonder whether this phenomenon might not be a haphazard situation and might warrant future studies to determine their pathophysiological significance.
Another striking finding was that of NF150-expressing elongated nucleated cells in the corneal stromal tissue sections and in cells seeded within 24 hours in culture. It is noteworthy that counts of neuronal cells under phase-contrast microscopy and those cells stained with NF150 were slightly different. We ascribe this difference to the possibility that some of these cells with a neuronal morphology were either more undifferentiated or of a different cell type that did not react to NF. Because neurons and myofibroblasts are potential lineages derived from NC, one hypothetical explanation is that both myofibroblasts and NF150-expressing neurons may result from an incomplete task of NC cells in performing tissue-specific differentiation after migrating into corneal stroma.
Recent immunostaining data revealed that bone-marrow–derived cells reside in the normal noninflamed central corneal stroma.23,24 These cells were also found in peripheral and central corneal stroma after injection of lin (−) Sca (+) enhanced green fluorescent protein (EGFP)– expressing cells in a recipient mouse.25 Several studies have shown a tremendous degree of plasticity in bone marrow– derived hematopoietic and mesenchymal stem cells in vitro, suggesting an almost unlimited differentiation potential. However, this view has been challenged by some in vivo studies26,27 and by others showing that the transdifferentiation potential is related to cell fusion.28 We detected CD45 expression in central and peripheral stroma corneal sections (Fig. 2F) and in low percentages after 1 day of culture in vitro (Fig. 3D). Because we did not detect CD45 expression in the cell clusters or neuronlike cells (not shown), we believe that they were not derived from the bone marrow. Previous reports have shown nestin expression in human limbal tissue in vivo29 and after limbal explants cultures with serum containing medium.30 Rat limbal explants containing epithelial cells were cultured under serum-free conditions and formed neurospheres that expressed nestin and other neuronal markers.31 However, none of these authors described the expression of nestin in cells of the corneal stroma. Finally, we noted that NGFRp75, a neural crest stem cell marker, was exclusively expressed in the corneal epithelium and not in the stromal section or in cultures (Espana EM, Tseng SCG, unpublished observation, 2003). We have observed a similar finding in the human corneal stroma (Espana EM, Tseng SCG, unpublished observation, 2003).
Future studies are needed to delineate the origin of these nestin-expressing cells in the murine and human corneal stroma. There is a possibility that these nestin-expressing clusters are derived from Schwann cells. However, this affirmation is merely speculative because to our knowledge no report has ever demonstrated the expansion of Schwann cells from the cornea and because Schwann cells do not express nestin but instead other differentiation markers including GFAP. Because we have not observed any spontaneous differentiation of these nestin-expressing cells into keratocan-expressing corneal keratocytes, new studies are also needed to look into the possible inducible role of extracellular cue(s) in the corneal stroma to allow these nestin-expressing cells to differentiate into keratocytes.
It should be emphasized that nestin-expressing cells developed only when a high seeding density was used, which might function as a feeder layer (Figs. 4A, 5A). One plausible mechanism to explain why nestin-positive cells grew in a serum-free condition without undergoing floating in the medium may be due to the presence of a feeder layer of corneal stromal cells. This concept has been demonstrated in a recent study showing that the only method to expand nestin-positive neural stem cells is to coculture them with a feeder layer of vascular endothelial cells.32
However, it is clear that the formation of cell clusters vanished or disintegrated when cultured in the presence of FBS added either from the beginning or later on after being formed (Fig. 6C). This detrimental effect against cell cluster formation and nestin expression was also reproducible by exposing stromal cells to 10 ng/mL TGF-β1 (Fig. 7B). TGF-β, a known serum component, has been identified as a potent factor in the differentiation of neural crest stem cell lines into myofibroblasts.33 Our experiments showed that TGF-β not only compromised cell growth as monolayers with differentiation into myofibroblasts, but also abolished the formation of cell clusters, suggesting that TGF-β is detrimental for the maintenance of cell clusters and nestin expression.
Myofibroblasts appearing in the corneal stroma are associated with matrix deposition, scarring, and visual loss. The presence of neurons and nestin-expressing cell clusters and their propensity of differentiation into myofibroblasts on TGF-β1 stimulation had a strong impact on our current understanding of corneal wound healing and variety of nerve-related corneal pathologies such as herpetic and neurotrophic keratitis.
Acknowledgments
The authors thank Brigitte Shaw (University of Miami, Miller School of Medicine, Analytical Imaging Core Facility) for her technical assistance.
Footnotes
Disclosure: E.M. Espana, TissueTech, Inc. (E); T. Kawakita, TissueTech, Inc. (E); M.A. Di Pascuale, TissueTech, Inc. (E); W. Li, None; L.-K. Yeh, None; J.-M. Parel, None; C.-Y. Liu, None; S.C.G. Tseng, TissueTech, Inc. (E, P)
Supported by a research grant from TissueTech, Inc. and in part by an unrestricted grant from the Ocular Surface Research and Education Foundation (Miami, FL); by National Eye Institute Grant R01 EY06819 (SCGT) and EY12486 (C-YL); National Eye Institute center grant P30 EY014801 (University of Miami); and an unrestricted grant from Research to Prevent Blindness. C-YL is a Research to Prevent Blindness Olga Keith Wiess Scholar.
References
- 1.Chambers D, McGonnell IM. Neural crest: facing the facts of head development. Trends Genet. 2002;18:381–384. doi: 10.1016/s0168-9525(02)02733-6. [DOI] [PubMed] [Google Scholar]
- 2.Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- 3.Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- 4.Johnston MC, Noden DM, Hazelton RD, et al. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29:27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- 5.Birk DE, Lande MA, Fernandez-Madrid FR. Collagen and glycosaminoglycan synthesis in aging human keratocyte cultures. Exp Eye Res. 1981;32:331–339. doi: 10.1016/0014-4835(81)90038-5. [DOI] [PubMed] [Google Scholar]
- 6.Cintron C, Hong BS. Heterogeneity of collagens in rabbit cornea: type VI collagen. Invest Ophthalmol Vis Sci. 1988;29:760–766. [PubMed] [Google Scholar]
- 7.Hart GW. Glycosaminoglycan sulfotransferases of the developing chick cornea. J Biol Chem. 1978;253:347–353. [PubMed] [Google Scholar]
- 8.Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW. Unique glycosylation of three keratan sulfate proteoglycan isoforms. J Biol Chem. 1991;266:14226–14231. [PubMed] [Google Scholar]
- 9.Funderburgh JL, Funderburgh ML, Brown SJ, et al. Sequence and structural implications of a bovine corneal keratan sulfate proteoglycan core protein. Protein 37B represents bovine lumican and proteins 37A and 25 are unique. J Biol Chem. 1993;268:11874–11880. [PubMed] [Google Scholar]
- 10.Fishell G, Mason CA, Hatten ME. Dispersion of neural progenitors within the germinal zones of the forebrain. Nature. 1993;362:636–638. doi: 10.1038/362636a0. [DOI] [PubMed] [Google Scholar]
- 11.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 13.Frederiksen K, Jat PS, Valtz N, et al. Immortalization of precursor cells from the mammalian CNS. Neuron. 1988;1:439–448. doi: 10.1016/0896-6273(88)90175-4. [DOI] [PubMed] [Google Scholar]
- 14.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 15.Espana EM, Romano AC, Kawakita T, et al. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281. doi: 10.1167/iovs.03-0089. [DOI] [PubMed] [Google Scholar]
- 16.Espana EM, Kawakita T, Romano A, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- 17.Kawakita T, Espana EM, He H, et al. Calcium induced abnormal epidermal-like differentiation in culture of mouse corneal/limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- 18.Espana EM, Kawakita T, Liu C-Y, Tseng SCG. CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-β1. Invest Ophthalmol Vis Sci. 2004;45:2985–2991. doi: 10.1167/iovs.04-0201. [DOI] [PubMed] [Google Scholar]
- 19.Masur SK, Dewal HS, Dinh TT, et al. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of α-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 21.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 22.Maltseva O, Folger P, Zekaria D, et al. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 23.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 24.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Ishikawa F, Sonoda KH, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 26.Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 27.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, De Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seigel GM, Sun W, Salvi R, et al. Human corneal stem cells display functional neuronal properties. Mol Vis. 2003;9:159–163. [PubMed] [Google Scholar]
- 31.Zhao X, Das AV, Thoreson WB, et al. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev Biol. 2002;250:317–331. [PubMed] [Google Scholar]
- 32.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DJ. Cellular and molecular biology of neural crest cell lineage determination. Trends Genet. 1997;13:276–280. doi: 10.1016/s0168-9525(97)01187-6. [DOI] [PubMed] [Google Scholar]


