Abstract
Resveratrol has been shown to have anticarcinogenic activity. We previously found that resveratrol inhibited growth and induced apoptosis in 2 human melanoma cell lines. In this study we determined whether resveratrol would inhibit human melanoma xenograft growth. Athymic mice received control diets or diets containing 110 μmol/L or 263 μmol/L resveratrol, 2 wk prior to subcutaneous injection of the tumor cells. Tumor growth was measured during a 3-wk period. Metabolism of resveratrol was assayed by bolus gavage of 75 mg/kg resveratrol in tumor-bearing and nontumor-bearing mice. Pellets containing 10–100 mg resveratrol were implanted into the mice, next to newly palpated tumors, and tumor growth determined. We also determined the effect of a major resveratrol metabolite, piceatannol, on experimental lung metastasis. Resveratrol, at any concentration tested, did not have a statistically significant effect on tumor growth. The higher levels of resveratrol tested (0.006% in food or 100 mg in slow-release pellets) tended to stimulate tumor growth (P = 0.08–0.09). Resveratrol and its major metabolites, resveratrol glucuronide and piceatannol, were found in serum, liver, skin, and tumor tissue. Piceatannol did not affect the in vitro growth of a murine melanoma cell line, but significantly stimulated the number of lung metastases when these melanoma cells were directly injected into the tail vein of the mouse. These results suggest that resveratrol is not likely to be useful in the treatment of melanoma and that the effects of phytochemicals on cell cultures may not translate to the whole animal system.
Introduction
Resveratrol is a polyphenol found in high concentration in red grapes, red wine, peanuts, and pines (1). In many plants, resveratrol is synthesized in response to a stress condition, such as an infection, and thus can be considered to be a phytoalexin (2). Resveratrol has estrogenic activity in mammals (3,4) and therefore is classified as a phytoestrogen. Epidemiological studies indicate an inverse correlation between red wine consumption and the incidence of cardiovascular disease and that resveratrol might be the active principle in red wine. Indeed, resveratrol inhibits platelet aggregation (1) and has a vasorelaxing effect (5). Resveratrol also inhibits the oxidation of low-density lipoproteins (6), most likely because of its general antioxidant activity (7). All of these properties of resveratrol are consistent with its protective effect against cardiovascular disease.
In the past few years it has been discovered that resveratrol also has antitumor activity (8). Resveratrol has been shown to inhibit proliferation and induce apoptosis in a variety of human cancer cell lines, including breast, leukemia, prostate, and colon (9–12). Induction of in vivo apoptosis in tumor cells in rats as the result of resveratrol administration has also been reported (13). The exact mechanism by which resveratrol can inhibit the various steps in carcinogenesis (initiation, promotion, and progression) is not known.
Human melanoma is a tumor whose frequency is increasing at an alarming rate (14). If detected early and surgically excised, the 5-y survival rate is favorable. However, later stages of the disease are difficult to treat and long-term survival is low. Clinical evidence suggests that acute sun exposure and frequent sunburns during childhood could give rise, decades later, to melanoma. There are only a few reports on the effect of resveratrol on melanoma. Caltagirone et al. (15) found that resveratrol inhibited the growth of the mouse melanoma B16-BL6, but did not decrease its metastatic or invasive potential. We previously reported (16) that resveratrol inhibited growth and induced apoptosis in 2 human melanoma cell lines. In contrast, Yang and Meyskens (17) found that resveratrol inhibited anchorage-independent growth of several human melanoma cell lines, but did not induce apoptosis or inhibit anchorage-dependent growth. The purpose of our study was to determine whether resveratrol could inhibit the growth of the cell line that we found most sensitive to resveratrol-induced apoptosis when grown as a xenograft in athymic mice.
Material and Methods
Cell lines
A375, SK-mel-28, and SB2 melanoma cells were obtained from the American Type Tissue Collection. MeWo melanoma cells were provided by Dr. Menashe Bar-Eli, the University of Texas MD Anderson Cancer Center, Houston. B16BL6 melanoma cells were originally obtained from the Mason Research Institute, Worcester, MA. Cells were maintained as previously described (16,18). The A375 cells were grown until they were 70% confluent. They were then harvested by trypsinization (0.5% trypsin/2.6 mmol/L EDTA), and washed with PBS and 2 × 106 cells injected subcutaneously (s.c.) in the hind flank of athymic mice.
Mice and experimental design
Four-week old male mice, Nu/Nu-nuRB, an out-bred strain, were purchased from Charles River Laboratories. Mice were housed in individual ventilated cages, 5/cage. After a 1-wk acclimation period, mice received 0.005%, 0.01% resveratrol (Sigma), or 0.1% ethanol in their drinking water. Each control and experimental group had 10 mice. The amount of drinking water consumed was measured every second day. Two weeks after adding resveratrol or ethanol in the drinking water, all mice were injected s.c. in the right flank with 2 × 106 A375 human melanoma cells. Mice weight, water, and food intake were determined every second day. At these times, mice were palpated and the presence of a mass noted (latency time). After tumor formation, its volume (V = 1 × w2/2) was measured every second day using digital calipers.
For feeding experiments, mice were acclimated for 1 wk and then fed the following diets: 5LG4 NIH-31 R&M/6F irradiated (19) (control diet); 5LG4 NIH-31 R&M/6F irradiated, and containing 0.0025% (110 umol/L) resveratrol; 5LG4 NIH-31 R&M/6F irradiated, and containing 0.006% (263 umol/L) resveratrol. All diets were purchased from Purina Mills. After mice consumed the diets for 2 wk (10 mice/diet), they were injected s.c. in the right flank with 2 × 106 A375 human melanoma cells. Weight, drinking water, and food consumed were measured every other day. Mice were also palpated every second day and the presence of a mass (latency time) recorded. After tumor formation, its volume was measured every other day, as described above.
We also treated the Nu/Nu mice with slow-release resveratrol pellets. They were acclimated for 3 wk and then injected s.c. in the right flank with 2 × 105 A375 human melanoma cells. Once a mass was detected (approximately 1 wk after injection), mice were anesthetized using Halothane, and pellets were implanted next to the mass by making an incision equal to the diameter of the pellet. A small pocket was created and the pellet implanted with forceps. The incision was closed using Derma bond, and Neosporin was applied to the wound. All procedures were performed in a laminar flow hood to ensure sterility. Pellets (21-d release) were purchased from Innovative Research of America and contained 0, 10, 25, 50, or 100 mg of resveratrol. Because the pellets were different sizes, the control group was implanted with the largest size of placebo pellet. Each group contained 10 mice (50 mice for the entire experiment). After the surgery, mice were weighed and their water and food intake was measured every other day. Tumor volume was also recorded every second day. At the end of 21 d, mice were killed by CO2 inhalation, tumors were removed and weighed, and the size of the implanted pellets was recorded. Samples of blood, skin, kidney, and liver were taken to determine amounts of resveratrol and its metabolites. The animal facilities and protocols reported were approved by the Marshall University Institutional Animal Care and Use Committee.
Resveratrol metabolism studies
General treatment
Naïve or tumor-bearing male mice (28–33 g body weight) were administered resveratrol (75 mg/kg; 10 mL/kg) dissolved in 50% ethanol (in 0.9% saline) orally via a feeding needle (20 gauge). The tumor-bearing mice used for these studies were obtained from the control group at the end of the resveratrol feeding studies. Mice were anesthetized with CO2 and blood was collected by cardiac puncture using heparinized 1 mL syringes at 5 min post-treatment. Tissues (liver, skin, and tumors) were removed immediately following cardiac puncture, placed in liquid nitrogen, and stored at −75°C until assayed.
Sample preparation
Plasma was obtained by centrifugation (5400 × g; 10 min) of blood samples. To 50 μL of plasma, 945 μL 50% methanol and 5 μL internal standard solution (5-sulfosalicylic acid, 500 ng/μL) were added. The sample was centrifuged (9000 × g; 10 min, 4°C) and the supernatant filtered through a 0.45 μm syringe filter into a 1.5 mL plastic microcentrifuge tube and stored at −75°C prior to HPLC analysis. Tissue (liver, skin, and tumor) samples (200–300 mg) were homogenized (Tekmar Tissuemizer) in 500 μL cold acetone. The probe was rinsed with 500 μL acetone and the rinse added to the homogenized sample. The combined sample and rinse were then centrifuged (9000 × g; 10 min, 4°C). The supernatant was collected and the acetone evaporated under a stream of nitrogen. The residue was suspended in 995 μL 50% methanol and 5 μL internal standard solution. The suspension was mixed for 10 min at room temperature using a vortex mixer and the supernatant filtered through a 0.45 μm syringe filter into a 1.5 mL plastic microcentrifuge tube and stored at −75°C prior to HPLC analysis.
HPLC methods
Resveratrol and piceatannol concentrations were determined using a Beckmann HPLC system consisting of a Beckman System Gold 126 solvent module linked to Beckman Nouveau System Gold software (version 1.7) for analysis, a Model 166 variable wave-length detector, and a Waters Nova-Pak C18 4.6 × 250 mm cartridge column (4 μm particle size) fitted with a Nova-Pak Sentry guard column (3.9 × 20 mm). A detection wavelength of 306 nm and ambient temperature were used for all assays. The mobile phase was adopted from Kuhnle et al. (20) and consisted of aqueous methanol (20%) in hydrochloric acid (0.1%) and acetonitrile. The flow rate for all runs was 1.0 mL/min, and the gradient system was (min/% acetonitrile): 5/5, 10/15, 15/25, 20/35, 25/25, and 30/5. A 5-min equilibration period was used at the end of each 35-min run. Initially, standard curves were constructed for resveratrol and piceatannol (0.5–8.0 μg/μL) with a correlation coefficient >0.99. The limit of detection for each compound was ~1 ng/100 μL. For each sample run, 100 μL of sample with internal standard added as described above was injected for analysis. The method of Hong and Rankin (21) was used to determine the concentration of resveratrol glucuronides.
Food analysis
To determine the content of resveratrol in food, pellets (~1.0 g) were crushed into powder, and a portion of the powder (200 mg) was ground to a fine powder with a mortar and pestle. A mixture of the fine powder (100 mg) and internal standard solution (5 μL) was brought to 1.0 mL with acetone. The resulting suspension was mixed on a vortex mixture (1 min) and centrifuged (5400 × g; 10 min). The supernatant was removed, and the acetone evaporated under a stream of nitrogen. The residue was reconstituted into 1 mL 50% methanol and centrifuged (5400 × g; 10 min). The resulting supernatant was filtered via syringe filter and analyzed using the HPLC method, described above.
Determination of experimental metastasis after piceatannol treatment
Ten-wk old C57BL/6 male mice (syngeneic with the B16BL6 melanoma cells) were randomly divided into 2 groups and fed a fully characterized nutritionally complete normal crystalline amino acid diet (BioServ) that supports growth and reproduction (18). After 7 d the mice were inoculated with 1.25 × 104 B16BL6 melanoma cells into the lateral tail vein. One day later the treatment group (n = 15) received 1 daily injection of 50 mg/kg piceatannol intraperitoneally for 9 d. Piceatannol, isolated from the seeds of Euphorbia lagascae as previously described (22), was administered in a vehicle containing 10% v:v of ethanol in 0.9% saline. Tumor-bearing control mice (n = 14) received equivalent daily injections in the vehicle of 10% v:v alcohol in 0.9% saline. The injection volume was equal to 1% of the body weight. Body weight and food consumption were determined daily for 3 d before tumor inoculation, and each day thereafter. Because food intake decreased during treatment with piceatannol during the treatment period, the amount of the food given to the control group was restricted to the amount of reduced diet consumed by the treated mice on the preceding day. All mice were killed by CO2 on d 21 following tumor inoculation and examined for pulmonary and extrapulmonary metastases according to previously described methods (23).
Statistical analysis
Data for tumor growth curves are presented as means ± SEM. Differences among groups were analyzed by 1-way ANOVA, followed by least significant difference post-hoc test. We used a P < 0.05 as the cutoff for significant difference among groups. Data analysis of the effect of piceatannol on B16 BL6 tumor metastases was analyzed by the Student’s t test, with P < 0.05 considered significant. Values are presented as means ± SEM.
Results
Effect of resveratrol containing diets on human melanoma xenograft growth
We initially administered resveratrol in the drinking water at 0.001 and 0.05% concentrations. We encountered 2 problems: the higher concentration of resveratrol was difficult to dissolve and we documented substantial oxidation of the resveratrol. Despite our best efforts, we could not prevent the oxidation of resveratrol; therefore, we abandoned this method of administering resveratrol to the mice.
Resveratrol at 110 μmol/L and 263 μmol/L concentrations (0.0025 and 0.006%, respectively) was incorporated into 5LG4 NIH-31 R&M/6F research mouse diets (Purina) and irradiated. These concentrations of resveratrol were chosen based on previous literature reports (24,25). A 2-wk prefeeding period was used to simulate potential chemoprevention. Stability of resveratrol in the mouse semipurified diet for the duration of these experiments was confirmed by HPLC analysis (data not shown). Despite the 2-wk feeding of resveratrol in their semipurified diet, the mice in this group did not differ in tumor latency time compared with mice receiving the nonresveratrol supplemented semipurified diet (control, 7.6 ± 3.1 d; 0.025% resveratrol, 7.6 ± 0.5 d; 0.006%, resveratrol 7.3 ± 0.6 d). Mice fed the diet containing the highest amount of resveratrol had melanoma xenograft tumors that tended (P = 0.09) to grow faster than mice fed the control diet (Fig. 1A). This experiment was repeated a second time with similar results.
Figure 1.

Effect of a resveratrol-containing diet (A) and slow-release resveratrol-containing pellets (B) on A375 human melanoma tumor xenograft growth in athymic mice. Values are means ± SEM, n = 10. The entire experiment was repeated a second time with similar results.
Resveratrol metabolism in tumor-bearing and nontumor-bearing athymic mice
Part of the explanation for these negative effects of resveratrol on tumor growth could be due to the rapid clearance and transformation of resveratrol. Five min after a bolus gavage of 75 mg/kg resveratrol, plasma concentrations of resveratrol were 28.4 ± 36.4 μmol/L (Table 1). We also detected 2 major metabolites of resveratrol in plasma: resveratrol glucuronide, which was present at higher amounts than resveratrol; and piceatannol, which was present at lower amounts than resveratrol. Liver contained higher amounts of resveratrol than either skin or plasma (Table 1). However, in the plasma and liver, the major metabolite, resveratrol glucuronide, was present at equivalent or higher concentrations than resveratrol, whereas in the skin, resveratrol glucuronide concentration was considerably lower than resveratrol (Table 1).
TABLE 1.
Resveratrol and its metabolites in nontumor bearing athymic mice 5 min after a bolus of 75 mg/kg of resveratrol1
| Resveratrol | Resveratrol glucuronide | Piceatannol | |
|---|---|---|---|
| Plasma, μmol/L | 28.37 ± 32.63 | 36.36 ± 39.63 | 5.25 ± 0.99 |
| Skin, nmol/g | 21.15 ± 7.22 | 4.67 ± 2.51 | 2.40 ± 0.54 |
| Liver, nmol/g | 73.04 ± 35.61 | 78.80 ± 66.30 | 11.50 ± 6.68 |
Values are means ± SEM, n = 5 for serum and liver, n = 4 for skin.
A similar experiment was conducted on the tumor-bearing control mice from the experiment described in Fig. 1. Plasma contained more resveratrol than liver, which is different from the experiment with nontumor-bearing mice (Table 2). The key finding is that the tumor contained a measurable amount of resveratrol, but it was only one-third the amount found in the skin.
TABLE 2.
Resveratrol distribution in tumor-bearing athymic mice 5 min after a bolus of 75 mg/kg1
| Plasma, μmol/L | 38.00 ± 25.2 |
| Skin, nmol/g | 9.00 ± 1.00 |
| Liver, nmol/g | 22.82 ± 11.5 |
| Tumor, nmol/g | 3.90 ± 2.50 |
Values are means ± SEM, n = 4.
Effect of resveratrol slow-release pellets on human melanoma xenograft growth
To try and bypass the problem of resveratrol metabolism by the liver, we implanted slow-release resveratrol-containing pellets next to newly developing tumors. In preliminary experiments, we ascertained that pellets containing the highest concentration of resveratrol (100 mg) had no discernable effect on the health of mice over a 4-wk period. Food and water intake as well as weight were similar in control and resveratrol pellet-implanted mice. This method of delivering resveratrol did not inhibit tumor growth (Fig. 1B). As in the resveratrol-feeding experiment, mice receiving the highest concentration of resveratrol tended to have faster growing tumors (0.05 < P < 0.09). This experiment was repeated a second time with similar results. The skin and plasma of mice receiving resveratrol-containing pellets had increased amounts of resveratrol as the concentration of resveratrol in the pellet was increased (Fig. 2).
Figure 2.
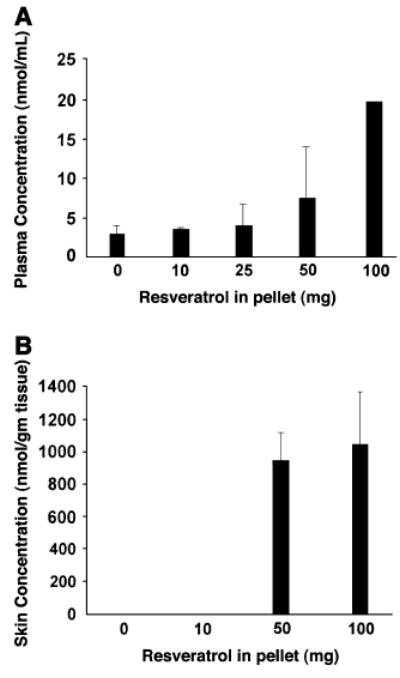
Resveratrol concentrations in plasma (A) and skin (B) from athymic mice treated with resveratrol-containing slow-release pellets. Values are means ± SEM, n = 2–4. In (B) the amount of resveratrol was 2.2 ± 1.09 nmol/g in the mice receiving the 10 mg slow-release pellet. Skin samples from mice receiving the 25 mg slow-release pellet were not suitable for analysis
Piceatannol and experimental melanoma metastsis
Because piceatannol was a major metabolite of resveratrol, we determined whether this compound had any antimelanoma activity. For these experiments, we measured the effect of this compound on the in vitro growth of several human melanoma cell lines. Piceatannol treatment (5–40 μmol/L) for 48 h did not inhibit growth of A375, MeWo, SK-mel-28, or SB2 melanoma cells (data not shown). We then examined whether piceatannol would have any effect on experimental metastasis. For these experiments, we switched to the B16-BL6 highly metastatic murine melanoma cells and C57BL/6 mice. Nine days of treatment with 50 mg/kg piceatannol significantly increased the number of lung tumor colonies following intravenous injection of B16 BL6 melanoma cells (Table 3). No extrapulmonary metastases were found during the biopsy of these mice. Piceatannol treatment decreased diet intake by 67% (P = 0.05) during the first 4 d of therapy, and this resulted in a 10% loss of body weight during this period. Diet intake gradually increased from d 5 and was the same as the control group between d 10 and 21.
TABLE 3.
Piceatannol treatment increases B16BL6 melanoma lung colony formation in C57/BL6 mice
| n | Means ± SD | Median | Range | |
|---|---|---|---|---|
| Colonies, n | ||||
| Control | 14 | 98 ± 47 | 86 | 48–237 |
| Treated | 13 | 149 ± 52* | 139 | 87–237 |
Different from controls, P = 0.01 by Student’s t test.
Discussion
In a previous study, we found that resveratrol treatment of 2 human melanoma cell lines resulted in the inhibition of growth and a large increase in apoptosis (16). We chose the most resveratrol-sensitive human melanoma cell line (A375) for the studies reported here. In our initial animal studies, we found that resveratrol was unstable in solution, probably because of rapid oxidation, and we therefore abandoned oral administration. In contrast, when resveratrol was incorporated into defined research diets, it was stable (data not shown). The results of the resveratrol feeding study were quite surprising in that there was a nonsignificant trend for faster-growing human melanoma xenografts in the mice fed the highest concentration of resveratrol. One possible reason for the lack of inhibition of tumor growth could be our finding that resveratrol is rapidly cleared from the athymic mice and very little of it reaches the skin and tumor. Rapid clearance of resveratrol has been reported for rabbits (24), rats (25), and mice (26).
To circumvent the metabolism problem, we used slow-release resveratrol pellets implanted under the skin next to newly palpated tumors. However, the results of these experiments were similar to the feeding studies insofar as there was a nonstatistically significant trend for faster growing tumors in mice receiving the pellets containing the highest amount of resveratrol. Analysis of resveratrol levels in skin samples from these experiments indicate an association between mice receiving increasing amounts of resveratrol in the pellets and the amount of resveratrol in their skin at the end of the experiment. These data confirm that the pellets released resveratrol in the immediate vicinity of the tumor.
One possible explanation for the potential stimulatory effect of resveratrol on human melanoma xenograft growth is the presence of piceatannol in the skin of the mice (Table 2). We found that piceatannol stimulated the formation of metastasis in B16BL6 cells in the lung of C57/BL6 mice. It is possible that this resulted from enhanced growth of micrometastases in the lung because the tumor cells were directly injected into the circulatory system, thus bypassing the steps of tumor invasion and extravasation.
There are several other reports that found resveratrol ineffective in inhibiting tumor growth in animal models despite its ability to inhibit cancer cell growth in vitro. The in vitro growth of 32Dp210 mouse myeloid leukemia cells was inhibited by resveratrol. However, when these cells were grown subcutaneously in mice, resveratrol administration did not alter the growth of the tumor (27). Resveratrol also did not inhibit tumorigenesis in a chemically induced lung tumor model (28) or in a mouse model of intestinal tumorigenesis (29). Although we used a 2-wk prefeeding period to mimic chemoprevention, the xenograft system could not adequately model spontaneous tumor development. Therefore it is still possible that resveratrol may inhibit early steps in melanoma formation.
In summary, despite the ability of resveratrol to inhibit human melanoma cell growth and induce apoptosis in cell culture, it was ineffective and may be stimulatory for the same cell line when grown as a xenograft in athymic mice. One reason for the lack of growth inhibition in vivo may be the rapid clearance of resveratrol, so that biologically effective concentrations cannot reach the tumor. Another possibility is the presence of the resveratrol metabolite piceatannol in the skin. We found that this compound can stimulate lung metastasis of a mouse melanoma cell line B16-BL6 when cells were injected into the tail vein of the syngeneic mice. We speculate that this resveratrol metabolite may also stimulate the in vivo growth of human melanoma cells either directly or by causing the release of growth factors from nontumor cells in the microenvironment of the melanoma.
Acknowledgments
We thank Margaret McFarland for assistance with preparation of illustrations and John Ball for the HPLC studies.
Footnotes
This work was supported in part by a grant (RO3 CA101045) from the NCI.
References
- 1.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–19. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 2.Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11:287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–67. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 4.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–43. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–6. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 6.Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol. 1997;53:1347–55. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell S, Cruickshank A, Thorpe G. Red wine and antioxidant activity in serum. Lancet. 1994;344:193–4. doi: 10.1016/s0140-6736(94)92795-2. [DOI] [PubMed] [Google Scholar]
- 8.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 9.Schneider Y, Vincent F, Duranton B, Badolo L, Gosse F, Bergmann C, Seiler N, Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000;158:85–91. doi: 10.1016/s0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
- 10.Dorrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731–39. [PubMed] [Google Scholar]
- 11.Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429–41. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–15. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 13.Carbo N, Costelli P, Baccino FM, Lopez-Soriano FJ, Argiles JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999;254:739–43. doi: 10.1006/bbrc.1998.9916. [DOI] [PubMed] [Google Scholar]
- 14.Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–36. doi: 10.3322/canjclin.50.4.215. [DOI] [PubMed] [Google Scholar]
- 15.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157–63. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Meyskens FL. Alterations in activating protein 1 composition correlate with phenotypic differentiation changes induced by resveratrol in human melanoma. Mol Pharmacol. 2005;67:298–308. doi: 10.1124/mol.104.006023. [DOI] [PubMed] [Google Scholar]
- 18.Meadows GG, Pierson HF, Abdallah RM, Desai PR. Dietary influence of tyrosine and phenylalanine on the response of B16 melanoma to carbidopa-levodopa methyl ester chemotherapy. Cancer Res. 1982;42:3056–63. [PubMed] [Google Scholar]
- 19.Rader JI, Wolnik KA, Gaston CM, Fricke FL, Fox MR. Purified reference diets for weanling rats: effects of biotin and cellulose. J Nutr. 1986;116:1777–88. doi: 10.1093/jn/116.9.1777. [DOI] [PubMed] [Google Scholar]
- 20.Kuhnle G, Spencer JP, Chowrimootoo G, Schroeter H, Debnam ES, Srai SK, Rice-Evans C, Hahn U. Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochem Biophys Res Commun. 2000;272:212–7. doi: 10.1006/bbrc.2000.2750. [DOI] [PubMed] [Google Scholar]
- 21.Hong SK, Rankin GO. Biotransformation of 2-chloroaniline in Fischer 344 rat: identification of urinary metabolites. Xenobiotica. 1998;28:985–94. doi: 10.1080/004982598239047. [DOI] [PubMed] [Google Scholar]
- 22.Ferrigni NR, McLaughlin JL, Powell RG, Smith CR., Jr Use of potato disc and brine shrimp bioassays to detect activity and isolate piceatannol as the antileukemic principle from the seeds of Euphorbia Lagascae. J Nat Prod. 1984;47:347–52. doi: 10.1021/np50032a019. [DOI] [PubMed] [Google Scholar]
- 23.Elstad CA, Meadows GG, Abdallah RM. Specificity of the suppression of metastatic phenotype by tyrosine and phenylalanine restriction. Clin Exp Metastasis. 1990;8:393–416. doi: 10.1007/BF00058152. [DOI] [PubMed] [Google Scholar]
- 24.Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–98. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 25.Bertelli AA, Giovannini L, Stradi R, Bertelli A, Tillement JP. Plasma, urine and tissue levels of trans- and cis-resveratrol (3,4′, 5-trihydroxystilbene) after short-term or prolonged administration of red wine to rats. Int J Tissue React. 1996;18:67–71. [PubMed] [Google Scholar]
- 26.Sale S, Verschoyle RD, Boocook D, Jones DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemo-preventive agent resveratrol and the synthetic analogue trans 3, 4, 5, 4′ –tetramethoxystilbene. Br J Cancer. 2004;90:736–44. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–81. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo [a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–30. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler CC, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in Apc (Min/+) mice. J Nutr. 2004;134:5–10. doi: 10.1093/jn/134.1.5. [DOI] [PubMed] [Google Scholar]


