Abstract
Eotaxin-1/CCL11 and its receptor CCR3 are involved in recruitment of eosinophils to diverse tissues, but their role in eosinophil recruitment in pulmonary fibrosis is unclear. The present study examined the pulmonary expression of CCL11 and CCR3 during bleomycin (blm)-induced lung injury and determined their importance in the recruitment of inflammatory cells and the development of lung fibrosis. In mice, blm induced a marked pulmonary expression of CCL11 and CCR3. Immunostaining for CCR3 revealed that this receptor was not only expressed by eosinophils but also by neutrophils. CCL11-deficient (CCL11−/−) mice developed significantly reduced pulmonary fibrosis. Expression of profibrotic cytokines such as transforming growth factor-β1 was diminished in the absence of CCL11. Furthermore, increased lung expression of CCL11 significantly enhanced blm-induced lung fibrosis and production of profibrotic cytokines. These effects were also associated with an increase of eosinophil and neutrophil pulmonary infiltration. In contrast, mice treated with neutralizing CCR3 antibodies developed significantly reduced pulmonary fibrosis, eosinophilia, neutrophilia, and expression of profibrotic cytokines. Together, these data suggest that CCL11 and CCR3 are important in the pulmonary recruitment of granulocytes and play significant pathogenic roles in blm-induced lung fibrosis.
Eosinophil recruitment is often encountered in many fibrotic lesions, and may contribute to the pathogenesis of the fibrotic process. Indeed, several human and experimental studies have implicated eosinophil as an immune/inflammatory cell capable of regulating mesenchymal cell proliferation, myofibroblast differentiation, and exaggerated deposition of matrix proteins, which are hallmark features of fibrosis.1–3 Most recently, congenitally eosinophil-deficient mice are found to have deficient airway wall remodeling in an allergic airway disease model.4 However, the mechanisms by which these cells migrate into the lung interstitium during the establishment of lung fibrosis are not fully understood.
Eosinophil recruitment into injured tissue is a complex process regulated at different levels by different cytokines such as interleukin (IL)-3, IL-4, IL-5, IL-13, GM-CSF, and chemokines, eg, RANTES and CCL11.5 However, only IL-5 and CCL11 appear to selectively regulate eosinophil trafficking in the lung because only double IL-5- and CCL11-deficient mice have a complete defect in the recruitment of eosinophils.6,7 IL-5 is probably the most specific cytokine for eosinophils because it induces growth, differentiation, activation, and survival of this cell type. Additionally, IL-5 has been clearly identified to be necessary, at least in part, for eosinophil-mediated efficient immune response against parasite or pathology, such as allergic asthma.8,9 Recently, we examined the contribution of IL-5 in an experimental model of lung fibrosis induced by bleomycin (blm). The findings show that IL-5 is a key mediator in the recruitment of lung eosinophils, which in turn exacerbates lung fibrosis by secreting profibrotic mediators such as transforming growth factor (TGF)-β, IL-4, and IL-13.3
CCL11 also demonstrates potent and selective ability to recruit eosinophils, and has been implicated in several eosinophil-associated diseases.5,10 Known cellular sources of CCL11 are bronchial epithelial cells, T cells, macrophages, and eosinophils. Its biological effects are mediated via CC chemokine receptor 3 (CCR3) that is highly expressed by eosinophils and other cells including Th2 lymphocytes.11,12
The purposes of the present study were to further delineate the mediators regulating eosinophil accumulation and to specifically examine the role of CCL11 and its receptor CCR3 during blm-induced lung injury and fibrosis in mice. Our data indicated that CCL11 and its receptor, CCR3, not only play an important pathophysiological role by regulating eosinophils but also neutrophil influx, lung collagen deposition, and production of profibrotic cytokines.
Materials and Methods
Murine Model of Lung Fibrosis
Mice were bred and maintained under specific pathogen-free conditions in the University Laboratory Animal Medicine facility at the University of Michigan Medical School before and during experiments. The animal use committee at the University of Michigan approved all experimental procedures involving mice. Blm (Blenoxane, Mead Johnson, Princeton, NJ) was suspended in sterile phosphate-buffered saline (PBS) at 1 U/ml, and administered endotracheally via trans-oral instillation at dose of 0.001 U/g body weight.
Whole Lung Homogenates and Collagen Assays
Nonlavaged whole lungs were excised and homogenized on ice in 2 ml of cold PBS. Collagen deposition was estimated by measuring the hydroxyproline collagen contents of lung homogenates (colorimetric assay previously described).13
CCL11-Deficient Mice
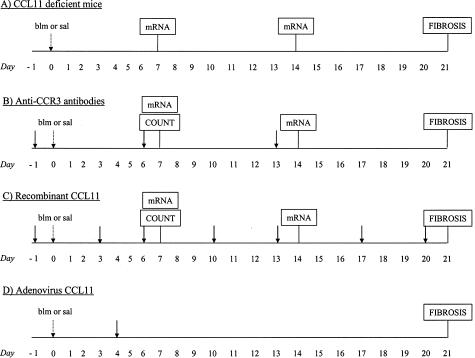
Eotaxin-1/CCL11 knockout (CCL11−/−) and wild-type (CCL11+/+) mice were kindly provided by Dr. Rodrigo Bravo (Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, NJ).14 These mice were of mixed 129 and ICR background. These two murine strains are considered sensitive to blm-induced lung fibrosis.15,16 After saline or blm treatment (0.02 U/mouse; Figure 1A, dotted arrow), lung TGF-β1 and MCP-1/CCL2 mRNA levels were measured on days 7 and 14, whereas the amplitude of lung fibrosis was examined at day 21.
Figure 1.
Experimental protocols used in this study to induce lung fibrosis and to delineate the importance of eotaxin-1/CCL11 and its receptor CCR3. A: Groups of CCL11-deficient or wild-type mice were treated with saline or 0.02 U/mouse of blm (d0, sal or blm; dotted arrow). Lung tissue total RNAs were isolated after blm or saline injection at days 7 and 14 for analysis of TGF-β1 and MCP-1/CCL2 mRNA levels. The amplitude of lung fibrosis was examined 21 days after saline or blm administration. B: CBA/J mice treated with blm or saline were injected with rat anti-mouse CCR3 monoclonal antibodies or irrelevant antibodies (starting at d−1, solid arrows). Pulmonary leukocyte counts in collagenase-digested lungs, and lung tissue TGF-β1 and MCP-1/CCL2 mRNA levels were assessed at day 7 or at days 7 and 14, respectively. Lung fibrosis was analyzed at 21 days after saline or blm treatment. C: Recombinant CCL11 was delivered in saline- or blm (0.02 U/mouse)-treated CBA/J mice by trans-oral instillation at different time points as indicated in the figure by the solid arrows (starting at d−1). The following parameters were assessed in this model: pulmonary leukocyte counts (d7), transcript expression (d7 and 14), and lung fibrosis (d21). D: CCL11 (AdCCL11)-expressing and control (AdCtl) adenoviral constructs were delivered in CBA/J mice by trans-oral instillation 4 days (solid arrow) after saline or blm treatment (0.02 U/mouse, dotted arrow). The intensity of lung fibrosis was estimated at day 21 after saline or blm instillation. Groups of 5 to 10 mice were used in this study for each model.
Administration of Anti-Mouse CCR3 Monoclonal Antibodies
Rat anti-mouse CCR3 monoclonal antibodies (clone 83103) and control IgG were purchased from R&D Systems, Minneapolis, MN. One day before saline or blm treatment (0.02 U/mouse), CBA/J mice (Jackson Laboratory, Bar Harbor, ME) were injected intraperitoneally with a total of 150 μg of anti-CCR3 or IgG as control (3 × 50 μg, every 7 days; Figure 1B, solid arrows). This dosing regimen was previously found to be sufficient for neutralization of CCR3 in mice.17,18 Pulmonary leukocyte counts, transcript expression and lung fibrosis were assessed at the time points indicated in Figure 1B.
Administration of Recombinant CCL11
Recombinant eotaxin-1/CCL11 (400 or 200 ng) (rCCL11; R&D Systems) were diluted in 50 μl of PBS supplemented with 0.1% of CBA/J mouse serum and delivered endotracheally in saline- or blm-treated CBA/J mice by trans-oral instillation two to three times/week (starting 1 day before saline or blm administration, 0.02 U/mouse; Figure 1C, solid arrows). Injection of the highest dose of rCCL11 (400 ng) was associated with a significant mortality after blm administration (mortality rate at 21 days; 80% in rCCL11 group versus only 20% in 0.1% serum group). Because this dose did not allow us to study fibrotic response, we studied more chronic effect of blm by the administration of 200 ng/mouse. Injection of the 200 ng/dose was not accompanied with significant mortality. Pulmonary leukocyte counts, transcript expression, and lung fibrosis were assessed at the time points indicated in the Figure 1C.
Adenoviral Constructs
Eotaxin-1/CCL11 (AdCCL11)-expressing and control (AdCtl) adenoviral constructs were prepared as previously described.19 These two vectors were diluted in 50 μl of PBS at doses of 1.0 or 0.5 × 108 pfu and endotracheally delivered in CBA/J mice by trans-oral instillation 4 days after saline or blm treatment (0.02 U/mouse). This time point was selected because the adenovirus used in this study preferentially infects epithelial cells that are particularly susceptible to blm during the first days after treatment.20 The maximal gene expression allowed by this technique is considered to occur between 7 and 10 days after administration. Thus, on day 14, we verified pulmonary overexpression of CCL11 by measuring the lung contents of this chemokine by enzyme-linked immunosorbent assay (ELISA) (in ng/lung). In mice transfected with 108 pfu AdCCL11, CCL11 levels were 7.1 ± 0.3 versus 1.7 ± 0.1 in control mice transfected with AdCtl; whereas those receiving 0.5 × 108 pfu showed lung contents of 5.8 ± 0.4 versus 1.6 ± 0.2 ng/lung in control lung. In comparison with mice receiving blm (0.02 U/mouse) plus AdCtl, the mortality rate was higher when CCL11 lung expression was increased by AdCCL11 at the dose of 108 pfu. Indeed, at day 21 after blm instillation, cumulative mortality in AdCCL11 groups was 50%, compared with only 20% in the blm/AdCtl groups (minimum 10 mice included in each treatment group). In view of the high mortality induced by 108 pfu of AdCCL11, we lowered the dosages to 0.5 × 108 pfu to allow the animals to survive and enable evaluation of more chronic effects. This low dose of AdCCL11 was not associated with significant mortality in mice treated with blm. After adenoviral treatment (0.5 × 108 pfu), the intensity of lung fibrosis was estimated at day 21 after blm instillation (Figure 1D).
Analysis of Leukocyte Accumulation in Lung Tissue
Lungs from mice were excised, washed in Hanks’ balanced salt solution (HBSS), cut into small pieces, agitated, and digested enzymatically for 80 minutes at 37°C.21 The digestion buffer was composed of collagenase type III (10 mg/lung) and DNase (250 μg/lung) (Worthington Biochemical Corp., Lakewood, NJ) dissolved in HBSS (15 ml/lung) and supplemented with 2% fetal bovine serum, antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml fungizone; Invitrogen Corp., Carlsbad, CA), as well as 350 pg/ml of IL-5 and GM-CSF (R&D Systems). The resulting cell suspension was filtered through 100-μm and 40-μm filters (BD Biosciences, Bedford, MA). After centrifugation (1200 rpm, 10 minutes), cells were washed and resuspended in PBS. Aliquots of the cell suspensions were used to determine total cell numbers and cell differentials. These were done on the cells pelleted onto glass slides by cytocentrifugation and subjected to Diff-Quik staining (Dade Behring, Deerfield, IL). Polymorphonuclear and mononuclear cells were then counted by light microscopy at ×200 magnification (total of 300 cells counted). This technique was performed at 7 days after saline or blm treatment because at this time point inflammation is maximal and lungs are easily and reliably digested by collagenase.
Isolation of Pulmonary Leukocytes and Identification of CCR-Positive Cells by Flow Cytometry
Macrophages, lymphocytes, and granulocytes were isolated from the pulmonary cell suspension obtained as above by density centrifugation in 40% Percoll.22 After centrifugation, the first cellular layer consisted mainly of macrophages and nonimmune cells (eg, fibroblasts and epithelial cells) while the second layer was composed of lymphocytes and granulocytes. Macrophages, eosinophils, neutrophils, and lymphocytes were further separated and isolated using immunomagnetic beads and the magnetic cell separation system (MACS; Miltenyi Biotech, Auburn, CA). Eosinophils were purified from the second Percoll layer by depletion using rat IgG antibodies directed against unwanted cells, eg, erythrocytes (anti-Ter 119; Pharmingen, BD Biosciences, Bedford, MA), lymphocytes (anti-CD3, CD4, CD19; Caltag Laboratories, Burlingame, CA; anti-CD8a, CD90 Thy1.2, NK1.1; Pharmingen, BD Biosciences) and neutrophils (anti GR-1, Pharmingen, BD Biosciences). Goat anti-rat IgG microbeads were used to recognize primary rat IgG antibody. Neutrophils (GR1+ cells) and T lymphocytes (CD3+, CD4+, CD8+, and CD90 Thy1.2+ cells) were obtained during the different steps of eosinophil purification. Macrophages were isolated from the first Percoll layer by positive selection with anti-CD11b magnetic beads (Miltenyi Biotech) after depletion of residual lymphocyte contaminant. The purity of immune cell preparations was routinely >95% as assessed by Diff-Quick staining.
Purified rat IgG2a anti-mouse CCR3 (R-PE)-conjugated monoclonal antibody was used (clone 83103, R&D Systems) to assess CCR3 expression in the different immune cell populations obtained as described above. After staining, purified cells were fixed in paraformaldehyde (1.25%) and 104 cells/sample were analyzed on a FACScan apparatus (BD Biosciences). The control isotype (irrelevant rat IgG2a) was also purchased from R&D Systems. During this study, it was not possible to purify sufficient numbers of pulmonary immune cells from saline-treated animals by this technique.
mRNA Analysis
Northern hybridization analysis was used to analyze TGF-β1 and MCP-1/CCL2 mRNA expression in CCL11-deficient animals as previously described.23,24 Briefly, lungs from mice were suspended in Trizol and RNA was purified following the manufacturer’s protocols (Invitrogen Corp.). Equal amounts of total lung RNA (20 μg/well) were electrophoresed on 1% agarose gels containing formaldehyde. The separated RNAs were then transferred overnight onto a nitrocellulose filter and the blots were confirmed for equal loading by viewing the 28 and 18 S rRNA bands after ethidium bromide staining. Blots were then baked, prehybridized, and hybridized with 32P-labeled oligonucleotide anti-sense probes for TGF-β1, MCP-1/CCL2, or GAPDH. The sequences for the oligonucleotide anti-sense probes were as follows: TGF-β1, 5′-GAACTTGGCATGGTAGCCCTTGGGCTCGTG-3′; MCP-1/CCL2, 5′-TGTCTGGACCCATTCCTTCTTGGGGTCAGC-3′; and GAPDH, 5′-CACCCTGTTTTTGCTGTAG-CCATATTCATTGTC-3′.
After an overnight hybridization at 56°C, the blots were washed, and after exposure to X-ray film, the developed autoradiographs were quantitated using an Ambis Optical Imaging System (Ambis, San Diego, CA). The blots were reprobed with GAPDH to confirm equivalent RNA loading and similarly quantitated for normalization of the cytokine signal.
TaqMan predeveloped assay reagents for mouse gene expression (Applied Biosystems, Foster city, CA) were used to estimate eotaxin-1/CCL11, RANTES/CCL5, CCR3, TGF-β1, and MCP-1/CCL2 transcripts in lungs of blm-treated CBA/J mice (Figure 1, protocols B and C) following the manufacturer’s protocols. Quantification of gene expression was measured using the ABI 7700 sequence detector system (Applied Biosystems) and corresponded to the threshold cycle (CT) at which an increase of reporter fluorescence (ΔRn) can first be detected. Amounts of cytokines or CCR3 mRNA were normalized to GAPDH signals and expressed as 2−ΔΔCT. To normalized the results obtained by Northern hybridization analysis and TaqMan real-time reverse transcriptase-polymerase chain reaction (RT-PCR), TGF-β1 and MCP-1/CCL2 expression in the Figures 3, 4, and 7 were shown as the percentage of the control (saline) values (see also Figure 1; protocols A, B, and C). Transcript analyses were preformed at 7 and 14 days because blm induces overexpression of TGF-β1 and MCP-1/CCL2 with a maximal effect at day 7, and levels of these two profibrogenic cytokines in treated mice return to control values at day 21.23,25
Figure 3.
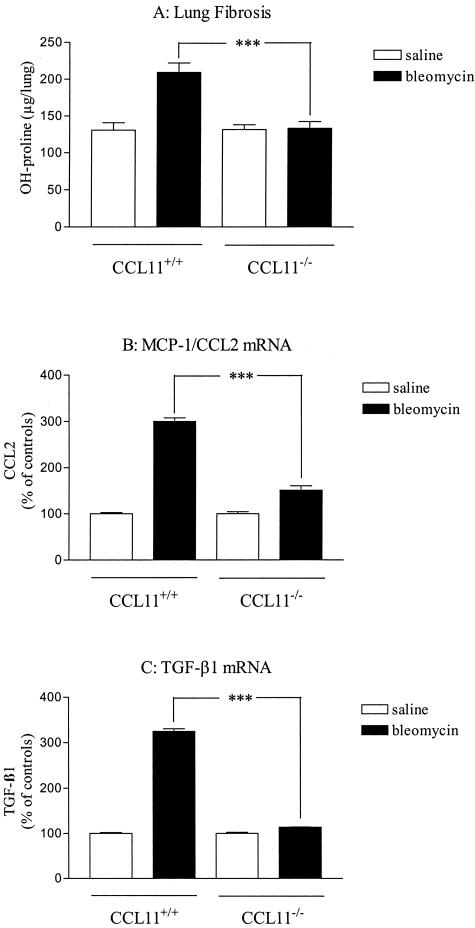
Effect of eotaxin-1/CCL11 deficiency on lung responses to blm. Lungs of saline- or blm (0.02 U/mouse)-treated deficient (CCL11−/−) or wild-type (CCL11−/−) mice were harvested as indicated in the Materials and Methods (Figure 1A) and analyzed for lung hydroxyproline (OH-proline, A), MCP-1/CCL2 (B), and TGF-β1 (C) mRNA (assessed by Northern hybridization analysis). Bars represent means +/− SE (SEM). ***P < 0.001 denotes significant differences in values measured in blm-treated mice compared to their respective control saline-treated mice as determined using the Student-Newman-Keuls multiple comparison test.
Figure 4.
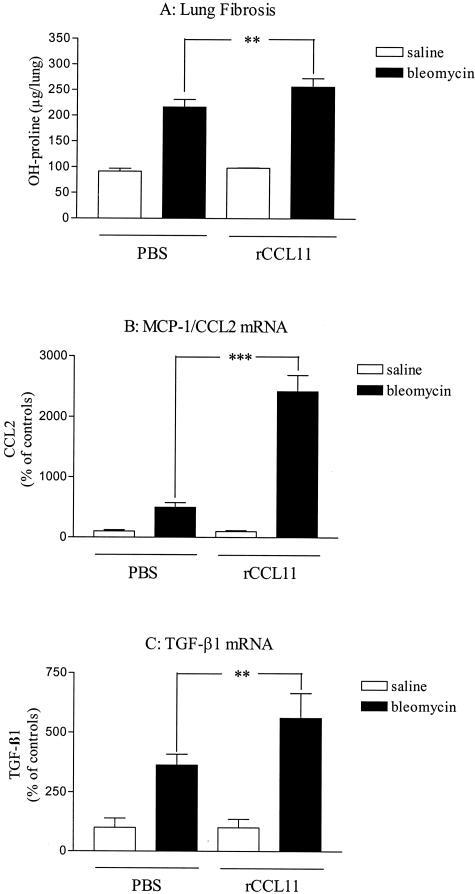
Effects of recombinant eotaxin-1/CCL11 on lung responses to blm. Lungs of saline- or blm (0.02 U/mouse)-treated mice after endotracheal administration of recombinant CCL11 (rCCL11) or saline (PBS supplemented with 0.1% of CBA mouse serum) were harvested as indicated in the Materials and Methods (Figure 1C) and analyzed for lung hydroxyproline (OH-proline, A), MCP-1/CCL2 (B), and TGF-β1 (C) mRNA (assessed by real-time RT-PCR analysis). Bars represent means +/− SE (SEM). **P < 0.01; ***P < 0.001 denote significant differences in values measured in blm-treated mice compared to their respective control saline-treated mice as determined using the Student-Newman-Keuls multiple comparison test.
Figure 7.
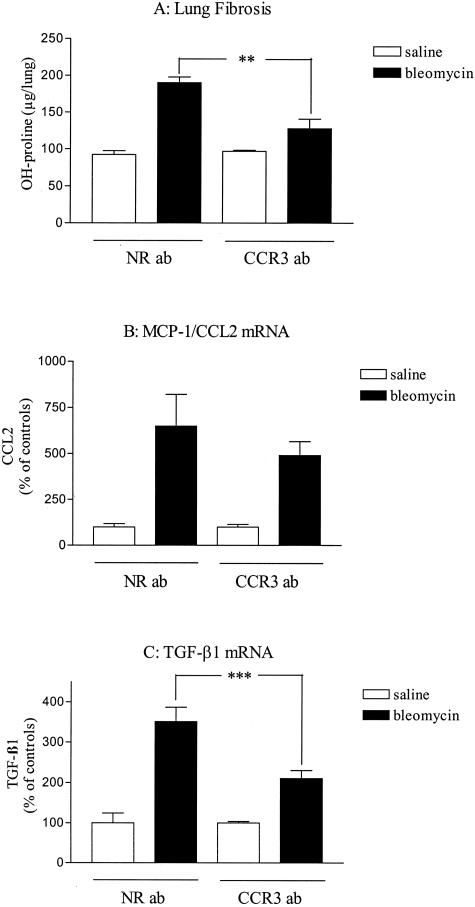
Effects of anti-CCR3 antibody administration on lung responses to blm. Lungs of saline- or blm (0.02 U/mouse)-treated mice after peritoneal administration of neutralizing anti-CCR3 antibodies (CCR3 ab) or control antibodies (irrelevant IgG, NR ab) were harvested as indicated in the Materials and Methods (Figure 1B) and analyzed for lung hydroxyproline (OH-proline, A), MCP-1/CCL2 (B), and TGF-β1 (C) mRNA (assessed by real-time RT-PCR analysis). Bars represent means +/− SE (SEM). **P < 0.01; ***P < 0.001 denote significant differences in values measured in blm-treated mice compared to their respective control saline-treated mice as determined using the Student-Newman-Keuls multiple comparison test.
ELISA Analysis
Lung homogenates (see above) were centrifuged at 4°C (10,000 rpm, 15 minutes) and the supernatants were kept frozen at −80°C until use. Murine eotaxin-1/CCL11 concentrations were measured in lung homogenates by ELISA kits (R&D Systems) following the manufacturer’s protocols.
Histology and Immunohistochemistry
Animals were euthanized and perfused via the right ventricle with saline solution. Lungs were inflated with 1 ml of 10% neutral buffered formalin and fixed overnight. After dehydration in 70% ethanol, the lungs were then processed using standard procedures and embedded in paraffin. Sections were cut, mounted on slides, and stained with hematoxylin and eosin or Masson’s Trichrome.
Paraffin-embedded whole lung samples were also analyzed for immunohistochemical localization of CCR3. Five-μm histological sections were dewaxed with xylene, rehydrated in graded concentrations of ethanol, and blocked with normal serum (Vectastain ABC-AP kit; Vector Laboratories, Burlingame, CA). A solution containing a 1:1 ratio of water to methanol with 3% hydrogen peroxide was added to each slide. Anti-mouse CCR3 Ab and control IgG (R&D Systems) were diluted in PBS to a final concentration of 10 μg/ml. Antibodies were then added to histological sections for 2 hours. After three successive PBS washes, a secondary anti-rat biotinylated Ab (Vector Laboratories) was added to each tissue section and incubated for 60 minutes. Each slide was then thoroughly washed before addition of avidin-conjugated peroxidase (Vector Laboratories). CCR3 localization was then visualized with a peroxidase substrate (diaminobenzidine tetrahydrochloride).
Statistics
Treatment-related differences were evaluated using t-tests or one-way analysis of variance, followed by pairwise comparisons using the Student-Newman-Keuls test, as appropriate. For flow cytometry data, statistical analyses were performed by Mann-Whitney U-test for unpaired values using Instat software (GraphPad Software Inc., San Diego, CA). Statistical significance was considered at P < 0.05.
Results
Up-Regulation of Lung CCL11 Expression after Blm Treatment
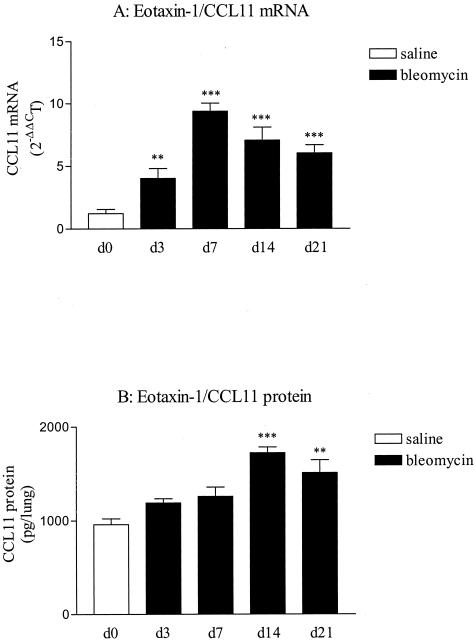
We first determined the expression of CCL11 in lungs of CBA/J mice administered standard doses of blm (0.02 U/mouse). TaqMan real-time RT-PCR analysis revealed a significant increase in CCL11 mRNA content in blm-treated mice in comparison to saline at each time point considered (3 to 21 days) and with a maximal effect after 7 days after treatment (Figure 2A). ELISA revealed an elevated expression at 14 and 21 days of CCL11 in lung homogenates obtained from blm-treated animals compared to saline situation (Figure 2B). Immunohistochemistry revealed that CCL11 was expressed in this model by several pulmonary cell populations including macrophages, lymphocytes, fibroblasts, and epithelial cells (data not shown). We also assessed the expression of another chemokine implicated in the recruitment of eosinophils and recognizing CCR3 receptor.26,27 At each time point considered after blm treatment, no significant modification of the amounts of RANTES transcripts was noted (data not shown). Altogether, these observations demonstrated that CCL11 was significantly up-regulated during blm-induced fibrosis by several pulmonary cells.
Figure 2.
Time course of eotaxin-1/CCL11 mRNA (A) and protein (B) expression in lungs obtained from blm-treated CBA/J mice (0.02 U/mouse of blm). The time points indicated in the x axis refer to days after blm instillation. White bars represent the pooled values obtained for the saline-treated mice at the different time points studied (d3 to d21). Lung tissue total RNAs were isolated after blm or saline injection and subjected to TaqMan real-time RT-PCR. The amounts of CCL11 present in the whole lung homogenates were assessed by ELISA. Bars represent means +/− SE (SEM). **P < 0.01 and ***P < 0.001 denote significant differences in values measured in blm-treated mice compared to saline-treated mice as estimated by Student-Newman-Keuls multiple comparison test.
CCL11-Deficient Mice Developed Limited Lung Fibrosis
To investigate the role of CCL11 in blm-induced lung fibrosis, mice genetically deficient in CCL11 (CCL11−/−) were studied. First, we verified whether the wild-type counterparts of this strain also overproduced CCL11 after blm treatment as observed with CBA/J mice. Our data revealed that blm (0.02 U/animal) also induced expression of CCL11 (mRNA and protein) in this strain of mice (data not shown). As shown in Figure 3A, lung fibrosis in CCL11−/− mice was abrogated compared to their corresponding wild-type counterparts (CCL11+/+) as estimated by the levels of OH-proline. Histological examination (Masson Trichrome stain) confirmed the protection from pulmonary fibrosis in CCL11−/− mice (data not shown). We previously showed that TGF-β1 and MCP-1/CCL2 are key cytokines in the lung fibrotic process induced by blm.23,28,29 We thus investigated whether CCL11 could regulate fibrosis via regulation of fibrogenic cytokine expression. TGF-β1 and MCP-1/CCL2 lung expression were thus assessed in the blm model in CCL11−/− mice. Expression of TGF-β1 and MCP-1 transcripts in deficient mice were significantly inhibited after blm treatment relative to that in wild-type control mice at day 7 (Figure 3, B and C) and day 14 (data not shown). These results indicated that CCL11 is important in the expression of profibrotic factors as well as the establishment of blm-induced lung fibrotic lesions.
Increased CCL11 Lung Expression Exacerbated Pulmonary Fibrosis
To support our observations, we also examined the effects of recombinant CCL11 (rCCL11) on pulmonary responses induced by blm in CBA/J mice. After intratracheal instillation of blm (0.02 U/mouse), mice administrated repeatedly with rCCL11 developed significantly more pulmonary fibrosis than the corresponding control mice (PBS with 0.1% of CBA/J mouse serum as carrier for rCCL11) as estimated by the measurement of hydroxyproline lung content after 21 days (Figure 4A). These observations were confirmed by histological analysis (data not shown). TGF-β1 and MCP-1/CCL2 transcripts were increased in the lung after administration of rCCL11 in blm-treated mice (Figure 4, B and C). No significant effect was observed when saline-treated mice were administered rCCL11.
To further support these observations, we examined the effects of adenoviral CCL11 pulmonary gene transfer treatment on blm-induced pulmonary fibrosis (AdCCL11). The results showed that blm-induced lung fibrosis assessed by OH-proline parameter was significantly increased in CBA/J mice additionally treated with AdCCL11 relative to those treated with AdCtl (Table 1). Pulmonary collagen contents of saline-treated mice were not modified by AdCCL11 administration in comparison to AdCtl. Collectively, these results demonstrated that CCL11 is an important factor regulating the expression of profibrogenic mediators involved in the activation of fibroblasts and collagen deposition.
Table 1.
Levels of OH-Proline in Saline- or Blm-Treated CBA/J Mice (21 Days, 0.02 U of Blm/Mouse) after Adenoviral Construct Administrations
| μg/lung | Sal + AdCtl | Blm + AdCtl | Sal + AdCCL1 | Blm + AdCCL11 |
|---|---|---|---|---|
| 99.0 ± 18.8 | 220.5 ± 22.9 | 94.8 ± 13.4 | 293.2 ± 16.1* |
P < 0.05; denotes significant differences in values measured in AdCtl and blm-treated mice compared to the corresponding AdCCL11 and blm-treated mice, as analyzed by the Student-Newman-Keuls multiple comparison test.
Up-Regulation of Lung CCR3 Expression after Blm Treatment
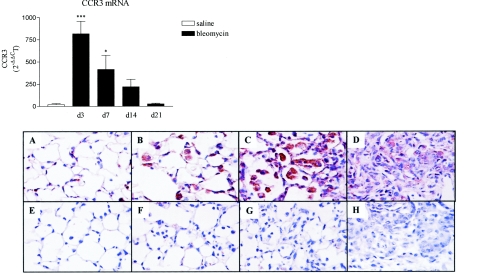
We next determined the expression of the CCL11 receptor CCR3 in lungs of mice administered standard doses of blm (CBA/J, 0.02 U/mouse). TaqMan real-time RT-PCR analysis revealed a significant increase in CCR3 mRNA content in blm-treated mice with a maximal effect after 3 days of treatment in comparison to saline mice (Figure 5, top). At day 21, levels in treated mice returned to control values. To determine which pulmonary cells expressed CCR3, immunohistochemistry and immunofluorescence analyses were performed. After 7 days, significant CCR3 expression was detected by immunohistochemistry in blm-treated mice in comparison to saline-treated animals (Figure 5; A and B, bottom). As shown in Figure 5C, CCR3-staining was maximal at day 14 after blm treatment. At this time, granulocytes and mononuclear cells present in the alveolar spaces were morphologically identified as the major cell types expressing CCR3. At day 21, only a few positive cells present in the fibrotic lesions were observed (Figure 5D). The corresponding sections showed no staining with control nonimmune IgG (Figure 5; E to H).
Figure 5.
Top: Time course of CCR3 mRNA expression in lungs obtained from blm-treated CBA/J mice (0.02 U/mouse of blm). The time points indicated in the x axis refer to days after blm instillation. White bar represents the pooled values obtained for the saline-treated mice at the different time points studied (d3 to d21). Lung tissue total RNAs were isolated after blm or saline injection and subjected to real-time PCR analysis. Bars represent means +/− SE (SEM). *P < 0.05 and ***P < 0.001 denote significant differences in values measured in blm-treated mice compared to saline-treated mice as estimated by Student-Newman-Keuls multiple comparison test. Bottom: Immunohistochemical localization of CCR3 expression in whole lung sections from CBA/J mice treated with 0.02 U/mouse of blm at days 7 (B), 14 (C), and 21 (D) or with saline (A, day 7). Specific staining for CCR3 appears brown in these tissue sections. The corresponding negative staining control sections (nonimmune IgG) for each time point after treatment are depicted in E, F, G, and H. Original magnifications, ×400.
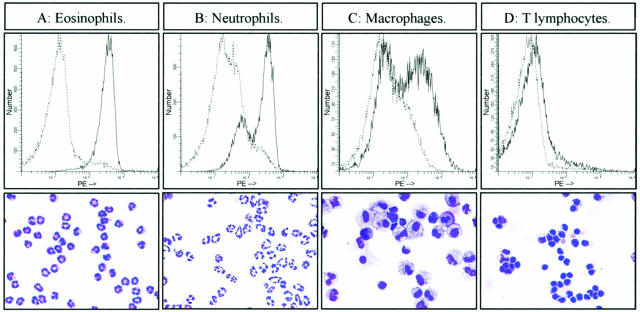
To confirm these observations, we purified by enzymatic digestion the major leukocyte populations recruited in blm-treated lungs at day 7 and stained these cells with anti-CCR3 Ab (see Materials and Methods). Flow cytometric analysis showed that all eosinophils as well as numerous neutrophils (70%) purified from blm-treated mice were strongly positive for CCR3 (Figure 6, A and B). A lower percentage of macrophages expressed CCR3 (Figure 6C), whereas there was no significant staining of T lymphocytes by these anti-CCR3 antibodies (Figure 6D).
Figure 6.
Flow cytometric analysis of CCR3 expression and histological aspect of lung eosinophils (A), neutrophils (B), macrophages (C), and T lymphocytes (D) of blm-treated mice. These different populations were purified from the lungs of blm-treated mice (day 7) as described in the Materials and Methods. Distribution of cells stained with the PE-conjugated CCR3 antibody are shown as underlined lines, while those stained with the isotype-matched control IgG are shown with normal lines. Below each analysis, a picture of the corresponding purified cell population is illustrated (Diff-Quick staining). Original magnifications, ×400.
Neutralizing CCR3 Limited the Lung Fibrotic Response
To investigate the role of CCR3 receptor in the pathogenesis of lung fibrosis induced by blm, we administered blocking CCR3 antibodies to blm-treated CBA/J mice. The anti-CCR3 administration was associated with a substantially less severe lung fibrosis than that in the control group (blm + nonimmune IgG) (Figure 7A). The effects of neutralization of CCR3 on TGF-β1 and MCP-1/CCL2 expression were examined. The expression of these profibrotic mediators was significantly (TGF-β1) and weakly (CCL2) decreased after administration of anti-CCR3 Ab (Figure 7, B and C). No significant effect was observed when saline-treated mice were administered anti-CCR3 Ab. Collectively, these data support key roles for CCR3 in the pathogenesis of blm-induced pulmonary fibrosis.
Pulmonary Immune Cell Populations after rCCL11 or CCR3 Ab Administration
Because we observed that CCL11 and CCR3 were mainly expressed by immune cells, we questioned whether these two factors are important in the pulmonary infiltration of leukocytes during the inflammatory reaction induced by blm. Thus, lung leukocyte cell counts were determined in lung homogenates after collagenase digestion in rCCL11 and Ab CCR3 models. These two models were selected because they were relatively comparable in terms of strain and protocol. On day 7 after blm treatment, pulmonary eosinophil and neutrophil numbers were significantly increased in rCCL11-administered mice (200 ng) in comparison to the corresponding control group (0.1% of serum) (Table 2). In contrast, the numbers of neutrophils and particularly eosinophils diminished significantly after the treatment with antibody directed against CCR3 (Table 2). Similar increases in lung eosinophil and neutrophil infiltration were also observed when AdCCL11 was used to increase lung eotaxin expression in blm-treated CBA/J or BALB/c mice when compared to corresponding AdCtl-treated animals. Thus on day 11 after blm treatment the lung eosinophil counts were 0.7 ± 0.1 × 106 cells/lung in AdCCL11-treated versus 0.1 ± 0.05 × 106 cells/lung in AdCtl-treated control animals, while the neutrophil counts were 9.2 ± 0.8 × 106 versus 3.2 ± 0.3 × 106 cells/lung, respectively. The number of leukocytes in saline-treated mice was not affected by adenoviral CCL11 transfer (data not shown) or recombinant CCL11 instillation (Table 2).
Table 2.
Counts of Macrophages, Lymphocytes, Neutrophils, or Eosinophils in Lungs Obtained from Saline (Sal)- or Bleomycin (Blm)-Treated CBA/J Mice (Day 7) after Administration of Antibodies against CCR3 (CCR3 Ab) or Recombinant Eotaxin-1/ CCL11 (rCCL11)
| 106 Cells/lung | Macrophages | Lymphocytes | Neutrophils | Eosinophils |
|---|---|---|---|---|
| Sal + NR Ab | 2.2 ± 0.6 | 4.2 ± 0.3 | 1.6 ± 0.1 | 0.2 ± 0.1 |
| Sal + CCR3 Ab | 1.9 ± 0.3 | 5.2 ± 1.1 | 1.7 ± 0.7 | 0.3 ± 0.03 |
| Blm + NR Ab | 4.3 ± 0.6 | 4.9 ± 0.6 | 5.8 ± 0.3 | 2.0 ± 0.1 |
| Blm + CCR3 Ab | 2.4 ± 0.7 | 3.0 ± 0.9 | 1.4 ± 0.4† | 0.2 ± 0.04* |
| Sal + 0.1% serum | 2.1 ± 0.9 | 5.7 ± 1.7 | 2.3 ± 1.0 | 0.3 ± 0.1 |
| Sal + rCCL11 200 ng | 2.4 ± 0.6 | 5.4 ± 0.1 | 1.5 ± 0.8 | 0.2 ± 0.03 |
| Blm + 0.1% serum | 4.8 ± 0.7 | 5.3 ± 1.1 | 5.7 ± 0.1 | 1.6 ± 0.1 |
| Blm + rCCL11 200 ng | 5.2 ± 1.3 | 4.5 ± 1.0 | 10.2 ± 0.2‡ | 3.9 ± 0.4* |
P < 0.05; †P < 0.01; ‡P < 0.001; denote significant differences in values measured in blm-treated mice administered anti-CCR3 or rCCL11 compared to corresponding CBA/J treated with blm and nonrelevant antibody (NR ab) or PBS + 0.1% serum, as analyzed by the Student-Newman-Keuls multiple comparison test.
Discussion
In this study, we documented the increased expression of CCL11 and its receptor CCR3 in blm-induced lung injury and fibrosis. We then assessed their role in lung fibrosis by using 1) CCL11-deficient mice, 2) CCR3 neutralizing antibodies, and 3) adenovirus coding for mouse CCL11 or recombinant CCL11. The results showed that either deficiency of CCL11 or neutralization of CCR3 caused a significant reduction in blm-induced lung fibrosis. In contrast, increasing the level of lung CCL11 expression by adenoviral expression vector or by treatment with exogenous recombinant CCL11, resulted in enhancement of fibrosis. Furthermore these effects on fibrosis were paralleled by comparable effects on lung fibrogenic cytokine (TGF-β1, MCP-1/CCL2) expression. Finally, neutralization of CCR3 reduced eosinophil/neutrophil infiltration while administration of rCCL11 promoted granulocyte accumulation. These observations taken together suggest that CCL11/CCR3 are important factors in the pathogenesis of blm-induced lung fibrosis due to their ability to up-regulate lung eosinophil and neutrophil accumulation and to increase the expression of profibrogenic cytokines.
Eosinophils are known to be present in certain fibrotic lesions, including in pulmonary fibrosis and asthmatic airway wall remodeling, which suggests a potential pathogenic role for these cells in the lung fibrotic response. Indeed, several human studies have identified an increase in eosinophil infiltration in association with fibrotic changes.1,30–32 In addition, many experimental models of fibrosis are characterized by a significant accumulation of eosinophils, which represented a key cellular source for expression of profibrogenic mediators such as TGF-β1, MCP-1/CCL2, IL-4, and IL-13.3,23,28,33 Furthermore, recent in vitro studies indicate that purified eosinophils from blood or lung can increase fibroblast proliferation, α-smooth muscle actin, or collagen expression in culture.2,3,34,35 In vivo evidence indicates that eosinophils are essential for airway wall remodeling or fibrosis in asthma and in animal models of allergic airway disease.4,36,37 Thus tissue eosinophilia can potentially play important roles in the fibrotic response by virtue of their ability to elaborate cytokines that could promote fibrosis via indirect or direct recruitment and activation of fibroblasts.
Eosinophil recruitment and movement into tissues are regulated by complex and composite actions of eosinophilotactic chemokines, distinct adhesion pathways, and eosinophilopoietic cytokines. The use of IL-5-,38 CCL11-,39 CCR3-,40,41 or both IL-5- and CCL11-deficient7 mice has benefited research to understand the mechanisms by which eosinophils are recruited into the lung. Such studies and associated ones using specific neutralizing antibodies to these proteins have shown that IL-5 induces eosinophil maturation, emigration, and survival whereas CCL11, by interacting with its receptor CCR3, is responsible for eosinophil migration and sequestration into injured tissues. In our study, recruitment of eosinophils into lung parenchyma after blm treatment was severely impaired in CCL11-deficient mice (data not shown) and in mice treated with anti-CCR3 (Table 2), whereas, in contrast, increased lung CCL11 expression (using either adCCL11 or rCCL11) induced increased pulmonary eosinophil influx in blm mice (Table 2). These observations demonstrated that in addition to IL-5,3 CCL11/CCR3 are additional essential factors in eosinophil accumulation in blm-induced lung injury. It is conceivable that IL-5 produced during blm-induced lung fibrosis42,43 may increase the level of circulating eosinophils and prime these cells to respond to CCL11 induced also by blm. This eosinophil recruitment mediated by CCL11/CCR3 could then contribute to the enhanced pulmonary expression of profibrotic cytokines and thus the subsequent lung fibrosis. However, we cannot exclude the possibility that CCL11 via CCR3 may directly up-regulate the production of profibrotic cytokines by additional leukocytes that may express CCR3. Finally, if it is confirmed that CCL11/CCR3 are among the major factors regulating chemoattraction of eosinophils in lung fibrosis, they could represent potential targets for the treatment of fibrotic disease. Thus recently developed CCR3 chemical antagonists that are successful in abrogating the pathophysiological effects of allergen challenges in mice may also be effective in limiting eosinophil influx and lung fibrosis in the murine model used in this study.44
Manipulation of lung CCL11/CCR3 expression or activity also affected blm-induced lung neutrophil infiltration, thus suggesting that these molecules may also be important in regulating the influx of neutrophils into the injured lung. Interestingly, injection of CCL11 induces a marked influx of neutrophils in the skin.45 There is some controversy regarding the expression of CCR3 by neutrophils. Although there is a suggestion that CCR3 expression by neutrophils may be due to contamination by eosinophils,46 other reports have clearly documented that neutrophils can express CCR3.45,47 In our study, greater than 70% of purified neutrophils (defined as GR1-expressing granulocytes) from blm-treated mice expressed CCR3 (Figure 6), thus indicating that in certain circumstances neutrophils may express this receptor. In addition, the presence of CCR3 on lung neutrophils suggest that increased numbers of these cells after CCL11 treatment may be the result of a direct chemotactic recruitment by this chemokine rather than a secondary effect via the up-regulation of other neutrophil-specific chemokine(s). Paradoxically however, CCL11 has been reported to have anti-inflammatory properties in a separate model of lung inflammation, and inhibit the production of chemokines specifically implicated in neutrophil recruitment such as MIP-2 and CINC.48,49 Nevertheless our data clearly showed that CCL11/CCR3 is important in regulating blm-induced neutrophil accumulation, which in turn may play a pathogenic role by their ability to elaborate toxic metabolites and cytokines, thus accentuating lung injury and subsequent lung fibrosis.50
Macrophages have been identified as a key cell in the pathogenesis of fibrosis because these cells have the high capacity to produce growth factors and profibrogenic cytokines.51 In the blm model, some mononuclear cells, probably macrophages, expressed CCR3 and thus may be a potential target of CCL11. Although the expression of CCR3 has not been previously reported on lung macrophages, CCR3 is known to be expressed on human dendritic cells.52 These latter cells demonstrate consistent chemotactic responses to eotaxin-1 (CCL11) and eotaxin-2 (CCL24). However, it seems that CCL11/CCR3 may not be essential for the recruitment of pulmonary macrophages during the development of experimental lung fibrosis because no significant change in the number of lung macrophages was observed by modulating lung CCL11/CCR3 expression (Table 2). However, we have not ruled out the possibility that CCL11/CCR3 may regulate other macrophage functions, eg, their capacity to produce profibrogenic mediators. This possibility needs to be explored in future investigations.
There is ample evidence documenting fibroblasts as a cellular source of CCL11. For instance, in vitro treatment of fibroblasts with blm induces production of eosinophil-chemotactic factors, including CCL11.53 Interestingly, recent reports indicate that fibroblasts also express the CCL11 receptor CCR3.54,55 This led us to hypothesize that CCL11 may, possibly in an autocrine manner, act directly on fibroblasts to regulate certain functions important for fibrosis. However by studying mouse lung fibroblasts isolated from either normal or fibrotic lungs, we were unable to demonstrate any direct activity of CCL11 on fibroblast proliferation, myofibroblast differentiation, or collagen production (data not shown). Thus these fibroblast functions are unlikely to be regulated by the induction or activation of CCL11/CCR3 signaling during blm-induced lung fibrosis.
A number of factors are known to up-regulate CCL11 and CCR3 expression. It is noteworthy that all cytokines possessing such activities are markedly induced during the development of fibrotic lesions and are considered strong profibrogenic mediators. Thus tumor necrosis factor-α, TGF-β1, IL-4, IL-13, as well as oncostatin can regulate eotaxin expression,56–59 whereas IL-4 and IL-5 have been identified as potent inducers of CCR3 expression.60,61 Thus, we propose that cytokines involved in fibrosis may, in addition to their direct profibrotic functions, modulate CCL11 and CCR3 expression, which can in turn amplify the fibrotic response by increasing influx of granulocytes and their expression of profibrotic mediator synthesis. In our study, pulmonary overexpression of CCL11 had no significant effect in saline-treated mice (Figure 4 and Table 2). These observations suggest that CCL11 seems to exert its deleterious effect in fibrosis in combination with other mediators induced by blm. In this context we can postulate that profibrotic factors such as IL-4, IL-13, and TGF-β may exacerbate the biological activity of eotaxin as demonstrated for IL-5. It is also possible that CCL11 may become functionally important only when its cognate receptor, CCR3, becomes up-regulated such as in inflammatory or other pathological conditions.
CCR3 recognizes a number of chemokines, including eotaxin-1 (CCL11), -2 (CCL24), -3 (CCL26), RANTES, MIP-α, as well as MCP-2, -3, and -4. Among these, eotaxin-2 and -3 and RANTES have been shown to also induce eosinophil migration both in vitro and in vivo in humans and in various animal models.26,27,62,63 In contrast to CCL11, RANTES expression was not significantly up-regulated in blm-induced lung fibrosis. This observation suggests that CCL11 may represent one of the major ligands interacting with CCR3 in this model of lung fibrosis. However, further studies are needed to identify the exact contribution of these eosinophil-related chemokines in comparison to CCL11 in blm-induced pulmonary fibrosis.
In conclusion, in this study we further investigated the mechanism by which deleterious eosinophils can be accumulated during the establishment of blm-induced lung injury and fibrosis. Although the magnitude of the differences as a consequence of the various manipulations (eg, transgenic CCL11 deficiency versus anti-CCR3 treatments) was not identical in all instances, the totality of the findings supports our conclusion that CCL11 and CCR3 are key mediators in the accumulation of eosinophils but also neutrophils, as well as in the production of fibrogenic cytokines. The differing magnitudes in observed effects are likely to be due to incomplete neutralization of CCR3 by antibody instillation versus the complete suppression of CCL11 expression in the transgenic knockout mice.
Acknowledgments
We thank Dr. R. Bravo (Bristol-Myers Squibb Pharmaceutical Research Institute) for the gift of eotaxin knockout mice and Lisa Riggs for her excellent technical assistance.
Footnotes
Address reprint requests to Sem H. Phan, M.D., Ph.D., Department of Pathology, University of Michigan Medical School, Ann Arbor, MI 48109-0602. E-mail: shphan@umich.edu.
Supported by the National Institutes of Health (grants HL28737, HL31963, and HL52285).
During this study F.H. was a research fellow in the Department of Pathology at the University of Michigan, Ann Arbor, MI, and a scientific research worker with the Fonds National de la Recherche Scientifique, Belgium.
References
- Hallgren R, Bjermer L, Lundgren R, Venge P. The eosinophil component of the alveolitis in idiopathic pulmonary fibrosis—signs of eosinophil activation in the lung are related to impaired lung-function. Am Rev Respir Dis. 1989;139:373–377. doi: 10.1164/ajrccm/139.2.373. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, Emonard H, Nagler A, Maquart FA. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta). Proc Natl Acad Sci USA. 1999;96:9660–9665. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F, Liu TJ, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol. 2003;171:5470–5481. doi: 10.4049/jimmunol.171.10.5470. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu CN, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME. Eotaxin—an essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Crit Care Med. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aerollaergens and eosinophils in experimental esophagitis. J Clin Invest Immunol. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie ANJ, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- Devos R, Plaetinck G, Cornelis S, Guisez Y, Vanderheyden J, Tavernier J. Interleukin-5 and its receptor—a drug target for eosinophilia associated with chronic allergic disease. J Leukoc Biol. 1995;57:813–819. doi: 10.1002/jlb.57.6.813. [DOI] [PubMed] [Google Scholar]
- Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today. 2000;6:20–27. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- Ponath PD, Qin SX, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene-expression during pulmonary fibrosis—a combined immunohistochemical and in-situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Loy J, Ryseck RP, Carrasco D, Bravo R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood. 1998;92:3912–3923. [PubMed] [Google Scholar]
- Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA. 2000;97:1778–1783. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HHH, Ochkur SI, McGarry MP, Lee NA, Lee JJ. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol. 2003;284:L169–L178. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- Grimaldi JC, Yu NX, Grunig G, Seymour BWP, Cottrez F, Robinson DS, Hosken N, Ferlin WG, Wu XY, Soto H, O’Garra A, Howard MC, Coffman RL. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3). J Leukoc Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- Wang J, Palmer K, Lotvall J, Milan S, Lei XF, Matthaei KI, Gauldie J, Inman MD, Jordana M, Xing Z. Circulating, but not local lung, IL-5 is required for the development of antigen-induced airways eosinophilia. J Clin Invest. 1998;102:1132–1141. doi: 10.1172/JCI2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 1997;16:91–101. doi: 10.1165/ajrcmb.16.1.8998084. [DOI] [PubMed] [Google Scholar]
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- Cramer R, Dri P, Zabucchi G, Patriarca P. A simple and rapid method for isolation of eosinophilic granulocytes from human blood. J Leukoc Biol. 1992;52:331–336. doi: 10.1002/jlb.52.3.331. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gharaee-Kermani M, Jones ML, Warren JS, Phan SH. Lung monocyte chemoattractant protein-1 gene expression in bleomycin-induced pulmonary fibrosis. J Immunol. 1994;153:4733–4741. [PubMed] [Google Scholar]
- Gharaee-Kermani M, Nozaki Y, Hatano K, Phan SH. Lung interleukin-4 gene expression in a murine model of bleomycin-induced pulmonary fibrosis. Cytokine. 2001;15:138–147. doi: 10.1006/cyto.2001.0903. [DOI] [PubMed] [Google Scholar]
- Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1995;147:352–361. [PMC free article] [PubMed] [Google Scholar]
- Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine Rantes released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe I, Hartnell A, Jopling LA, Bel S, Ponath PD, Pease JE, Collins PD, Williams TJ. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J Immunol. 1999;162:2946–2955. [PubMed] [Google Scholar]
- Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1995;147:352–361. [PMC free article] [PubMed] [Google Scholar]
- Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine. 2003;24:266–276. doi: 10.1016/j.cyto.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kubo K, Yamaguchi S, Honda T, Matsuzawa Y. Eosinophil activation in patients with pulmonary fibrosis. Chest. 1995;108:48–54. doi: 10.1378/chest.108.1.48. [DOI] [PubMed] [Google Scholar]
- Hiwatari N, Shimura S, Sasaki T, Aikawa T, Ando Y, Ishihara H, Sekizawa K, Sasaki H, Takishima T. Prognosis of idiopathic pulmonary fibrosis in patients with mucous hypersecretion. Am Rev Respir Dis. 1991;143:182–185. doi: 10.1164/ajrccm/143.1.182. [DOI] [PubMed] [Google Scholar]
- Peterson MW, Monick M, Hunninghake GW. Prognostic role of eosinophils in pulmonary fibrosis. Chest. 1987;92:51–56. doi: 10.1378/chest.92.1.51. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gharaee Kermani M, McGarry B, Remick D, Phan SH. TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol. 1997;158:954–959. [PubMed] [Google Scholar]
- Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–4612. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- Rochester CL, Ackerman SJ, Zheng T, Elias JA. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol. 1996;156:4449–4456. [PubMed] [Google Scholar]
- Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish MF, Humbles A, Tao H, Finkelstein S, Boyce JA, Gerard C, Friend DS, Austen KF. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol. 2002;168:5730–5736. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- Gharaee Kermani M, Phan SH. Lung interleukin-5 expression in murine bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1997;16:438–447. doi: 10.1165/ajrcmb.16.4.9115755. [DOI] [PubMed] [Google Scholar]
- Gharaee Kermani M, McGarry B, Lukacs N, Huffnagle G, Egan RW, Phan SH. The role of IL-5 in bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1998;64:657–666. doi: 10.1002/jlb.64.5.657. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–448. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies-Gow A, Ying S, Sabroe I, Stubbs VL, Soler D, Williams TJ, Kay AB. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- Hochstetter R, Dobos G, Kimmig D, Dulkys Y, Kapp A, Elsner J. The CC chemokine receptor 3 CCR3 is functionally expressed on eosinophils but not on neutrophils. Eur J Immunol. 2000;30:2759–2764. doi: 10.1002/1521-4141(200010)30:10<2759::AID-IMMU2759>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Cheng SS, Lai JJ, Lukacs NW, Kunkel SL. Granulocyte-macrophage colony stimulating factor up-regulates CCR1 in human neutrophils. J Immunol. 2001;166:1178–1184. doi: 10.4049/jimmunol.166.2.1178. [DOI] [PubMed] [Google Scholar]
- Cheng SS, Lukacs NW, Kunkel SL. Eotaxin/CCL11 is a negative regulator of neutrophil recruitment in a murine model of endotoxemia. Exp Mol Pathol. 2002;73:1–8. doi: 10.1006/exmp.2002.2439. [DOI] [PubMed] [Google Scholar]
- Guo RF, Lentsch AB, Warner RL, Huber-Lang M, Sarma JV, Hlaing T, Shi MM, Lukacs NW, Ward PA. Regulatory effects of eotaxin on acute lung inflammatory injury. J Immunol. 2001;166:5208–5218. doi: 10.4049/jimmunol.166.8.5208. [DOI] [PubMed] [Google Scholar]
- Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- Bitterman PB, Adelberg S, Crystal RG. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983;72:1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu S, Robbiani DF, Du XX, Rodrigues E, Ignatius R, Wei Y, Ponath P, Young JW, Pope M, Steinman RM, Mojsov S. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J Immunol. 2002;169:2925–2936. doi: 10.4049/jimmunol.169.6.2925. [DOI] [PubMed] [Google Scholar]
- Sato E, Koyama S, Robbins RA. Bleomycin stimulates lung fibroblast and epithelial cell lines to release eosinophil chemotactic activity. Eur Respir J. 2000;16:951–958. [PubMed] [Google Scholar]
- Huber MA, Kraut N, Addicks T, Peter RU. Cell-type-dependent induction of eotaxin and CCR3 by ionizing radiation. Biochem Biophys Res Commun. 2000;269:546–552. doi: 10.1006/bbrc.2000.2287. [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL. Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol. 1998;153:1861–1872. doi: 10.1016/S0002-9440(10)65700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy U, Curry SL, de Silanes IL, Shyu AB, Casolaro V, Gorospe M, Stellato C. Regulation of eotaxin gene expression by TNF-alpha and IL-4 through mRNA stabilization: involvement of the RNA-binding protein HuR. J Immunol. 2003;171:4369–4378. doi: 10.4049/jimmunol.171.8.4369. [DOI] [PubMed] [Google Scholar]
- Teran LM, Mochizuki M, Bartels J, Valencia EL, Nakajima T, Hirai K, Schroder JM. Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am J Respir Crit Care Med. 1999;20:777–786. doi: 10.1165/ajrcmb.20.4.3508. [DOI] [PubMed] [Google Scholar]
- Wenzel SE, Trudeau JB, Barnes S, Zhou XX, Cundall M, Westcott JY, McCord K, Chu HW. TGF-beta and IL-13 synergistically increase eotaxin-1 production in human airway fibroblasts. J Immunol. 2002;169:4613–4619. doi: 10.4049/jimmunol.169.8.4613. [DOI] [PubMed] [Google Scholar]
- Langdon C, Kerr C, Tong L, Richards CD. Oncostatin M regulates eotaxin expression in fibroblasts and eosinophilic inflammation in C57BL/6 mice. J Immunol. 2003;170:548–555. doi: 10.4049/jimmunol.170.1.548. [DOI] [PubMed] [Google Scholar]
- Tan JQ, Jacobi HH, Jing C, Millner A, Sten E, Hviid L, Anting L, Ryder LP, Glue C, Skov PS, Jarman E, Lamberth K, Malling HJ, Poulsen LK. CCR3 expression induced by IL-2 and IL-4 functioning as a death receptor for B cells. J Immunol. 2003;171:1722–1731. doi: 10.4049/jimmunol.171.4.1722. [DOI] [PubMed] [Google Scholar]
- Lamkhioued B, Abdelilah SG, Hamid Q, Mansour N, Delespesse G, Renzi PM. The CCR3 receptor is involved in eosinophil differentiation and is up-regulated by Th2 cytokines in CD34(+) progenitor cells. J Immunol. 2003;170:537–547. doi: 10.4049/jimmunol.170.1.537. [DOI] [PubMed] [Google Scholar]
- Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. Rantes and macrophage inflammatory protein 1-alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguccioni M, Loetscher P, Forssmann U, Dewald B, Li HD, Lima SH, Li YL, Kreider B, Garotta G, Thelen M, Baggiolini M. Monocyte chemotactic protein 4 (MCP-4), a novel structural and functional analogue of MCP-3 and eotaxin. J Exp Med. 1996;183:2379–2384. doi: 10.1084/jem.183.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]