Abstract
Cis-platin is an effective anti-neoplastic agent, but it is also highly nephrotoxic. Here, we clearly identify the human organic cation transporter 2 (hOCT2) as the critical transporter for cis-platin nephrotoxicity in isolated human proximal tubules and offer a potential mechanism for reducing nephrotoxicity in clinical practice. Interaction of cis-platin with hOCT2 in kidney or hOCT1 in liver was investigated with the fluorescent cation 4-[4-(dimethyl-amino)styril]-methylpyridinium in stably transfected HEK293 cells and for the first time in tissues physiologically expressing these transporters, human proximal tubules, and human hepatocyte couplets. Cis-platin (100 μmol/L) inhibited transport via hOCT2-HEK293 but not hOCT1-HEK293. In human proximal tubules cis-platin competed with basolateral organic cation transport, whereas it had no effect in tubules from a diabetic kidney or in hepatocytes. In hOCT2-HEK293 cells treated for 15 hours, incubation with cis-platin induced apoptosis, which was completely suppressed by contemporaneous incubation with the hOCT2 substrate cimetidine (100 μmol/L). These findings demonstrate that uptake of cis-platin is mediated by hOCT2 in renal proximal tubules, explaining its organ-specific toxicity. A combination of cis-platin with other substrates that compete for hOCT2 offers an effective option to decrease nephrotoxicity in the clinical setting.
Cis-platin is one of the most effective and potent anti-cancer drugs in the treatment of epithelial malignancies such as lung, head and neck, ovarian, bladder, and testicular cancer.1 Even though the mode of action of cis-platin is still not completely elucidated, it is generally accepted that platinum complexes exert their main anti-tumor activities through binding to DNA, and several specific adducts have been identified.2 The dose of cis-platin that can be administered is limited by its nephrotoxicity, but the mechanism by which cis-platin selectively damages proximal tubule cells has not been fully elucidated.3 To exert its cytotoxic actions, cis-platin has to enter proximal tubular cells. However, its uptake route across plasma membrane is not completely understood. Because cis-platin is actively secreted in renal tubules,4 the existence of a transport system has been suggested. In epithelial cells derived from proximal tubules of opossum (OK cell line) basolateral-to-apical transport of cis-platin was higher than apical-to-basolateral transport.5 Co-incubation of cis-platin with tetraethylammonium (TEA, a model substrate for organic cation transporters, OCT) significantly decreased accumulation and transport of cis-platin from the basolateral medium in OK cell line5 and also in rabbit isolated proximal tubuli.6 Moreover, TEA uptake by NIH3T3 cells stably transfected with rat organic cation transporter 2 was competitively inhibited by cis-platin.7 These results suggest that cis-platin transport might also be mediated by OCTs. Transport mediated by these membrane proteins has been characterized as polyspecific, electrogenic, voltage-dependent, and bi-directional, but pH- and Na+-independent.8 Three isoforms of OCTs have been identified in the rat, mouse, and human: OCT1, OCT2, and OCT3.8 The different isoforms of these transporters have a species- and tissue-specific distribution: for example OCT2 is the main OCT of human kidney,9 whereas in rat kidney the principal OCT is OCT1; in the human OCT1 is the main isoform of the liver.9 In humans, hOCT2 has been shown to be expressed at the basolateral side in all three segments of the proximal tubule.10 Other indications for the importance of OCTs in the uptake of cis-platin by proximal tubule cells come from studies with the clone C7 of Madin-Darby canine kidney cells. These cells have been demonstrated to express the isoform 2 of OCT and to be more sensitive to cis-platin toxicity when cis-platin was added to the basolateral versus luminal side.2 This toxicity could be decreased by incubation with cimetidine, a substrate of OCT.2 Because diabetic rabbits are resistant against cis-platin induced nephrotoxicity11 and diabetic rats have a decreased renal expression of OCTs,12–14 it could be speculated that protection conferred by diabetes could be due to a reduced cis-platin uptake in proximal tubular cells as a consequence of OCT down-regulation. Although these studies open interesting perspectives, cis-platin transport has never been clearly linked to a specific human transport system or has been investigated in human material preparations. Moreover, interaction of a substance with a particular isoform of OCT does not necessarily predict interaction with other OCTs, because the affinity of these transporters for different substrates are species- and isoform-specific.15 The aim of this study was to investigate whether cis-platin specifically interacts with human OCTs. Acute effects of cis-platin and of its less nephrotoxic derivates oxaliplatin and carboplatin on uptake of the fluorescent organic cation 4-[4-(dimethylamino)styryl]-N-methylpyridinium (ASP) were measured in 1) human embryonic kidney cells (HEK293) stably expressing the hepatic or the renal isoform of OCT (hOCT1 or hOCT2); 2) freshly isolated human proximal tubules; and 3) freshly isolated human hepatocyte couplets. Some experiments with human proximal tubules freshly isolated from a diabetic kidney were also performed. To demonstrate that hOCT2 really mediates the uptake of cis-platin, the accumulation of platin in hOCT2-HEK293 and HEK293 cells after 10 minutes of incubation with this anti-cancer drug was also compared. Moreover, induction of apoptosis by incubation of hOCT2-HEK293 cells with cis-platin and its prevention by cimetidine was compared with that observed in nontransfected HEK293 cells. We demonstrate that the transport of cis-platin is isoform- and organ-specific. Moreover, the presence of hOCT2 is necessary for a discrete uptake of cis-platin and to make HEK293 cells sensitive to cis-platin toxicity. These toxic effects are prevented by simultaneous incubation with cimetidine.
Materials and Methods
HEK293 Cells Stably Expressing hOCT1 or hOCT2
The HEK293-cells (human embryonic kidney cortex cells, CRL-1573; American Type Culture Collection, Rockville, MD) stably transfected with hOCT1 (hOCT1-HEK293) or with hOCT2 (hOCT2-HEK293) were prepared as described previously.16,17 Cells were grown at 37°C in 50-ml cell-culture flasks (Greiner, Frickenhausen, Germany) in Dulbecco’s modified Eagle’s medium (Biochrom, Berlin, Germany) containing 3.7 g/L NaHCO3, 1.0 g/L d-glucose, and 2 mmol/L l-glutamine (Gibco BRL/Life Technologies, Eggenstein, Germany) gassed with 8% CO2. To this medium 100,000 U/L penicillin, 100 mg/L streptomycin (Biochrom), 10% fetal calf serum, and 0.8 mg/ml geneticin (Gibco BRL/Life Technologies) were added.
Microdissection of Proximal Tubules
Human proximal tubules were isolated for functional analyses using the procedure customary in our laboratory.18 Human kidneys were obtained from patients undergoing tumor nephrectomy. Pieces of normal kidney tissue distant from the tumor were used. Thin corticomedullary slices were cut from the kidney pieces using a scalpel and immediately transferred into a dissection dish containing minimum Eagle’s culture medium and 5 mmol/L glycine at 4°C. Single proximal tubules (S2/S3 segments) were mechanically isolated using fine forceps under stereo microscopic observation and then transferred into a perfusion chamber with a glass bottom mounted in the focus of the microscope and finally fixed with two holding pipettes.
Isolation of Hepatocyte Couplets
Human hepatocyte couplets were isolated according to Gautam and colleagues19 with modifications. Human livers were obtained from patients undergoing tumor liver resection. Pieces of normal liver tissue with a distance minimum of 5 cm from the tumors were used. Liver tissue was transferred into chilled HCO3−-free phosphate buffer, rinsed free of blood, and tissue was cut into small pieces. The minced tissue was incubated in Dulbecco’s modified Eagle’s medium (Biochrom) containing 3.7 g/L NaHCO3, 1.0 g/L d-glucose, and 0.005% collagenase I (Worthington Biochemical Corp., Freehold, NJ) for 5 minutes at 37°C under shaking. The homogenate was filtered through a metal sieve (pore size, 250 μ) and the filtrate was centrifuged three times at 100 × g for 5 minutes. The pellet was resuspended in Dulbecco’s modified Eagle’s medium containing 3.7 g/L NaHCO3 and 1.0 g/L d-glucose and the suspension was incubated for 8 to 12 hours in Petri dishes at 37°C under 8% CO2 to allow hepatocyte couplets to polarize again.19 For microfluorescence measurements, hepatocyte couplets were picked up with a pipette from the Petri dish and transferred to a perfusion chamber, where they were finally fixed with a holding pipette. All procedures with human material were approved by the ethic commission of the Universitätsklinikum Münster, and written consent was obtained from each patient.
Fluorescence Measurements with ASP
As substrate for OCTs the fluorescent organic cation ASP was used. Fluorescence measurement device and experimental procedures were as customary in our laboratory.18,20,21 Briefly, measurements were performed in the dark with an inverted microscope (Axiovert 135; Zeiss, Oberkochen, Germany) equipped with a ×100 oil immersion objective. Excitation light (450 to 490 nm) was reflected by a dichroic mirror (560 nm) to a perfusion chamber. Cell monolayers, isolated human proximal tubules, or isolated human hepatocyte couplets on coverslips formed the bottom of the chamber. The preparations were superfused at a rate of 10 ml/minute with a HCO3−-free Ringer-like solution containing (in mmol/L): NaCl, 145; K2HPO4,1.6; KH2PO4, 0.4; d-glucose, 5; MgCl2, 1; calcium gluconate, 1.3; and pH adjusted to 7.4 at 37°C. Fluorescence emission (575 to 640 nm) was measured by a photon-counting tube (H 3460-04; Hamamatsu, Herrsching, Germany). The initial slope of fluorescence increase represents directly the ASP uptake across plasma membrane via OCTs and is not significantly influenced by exit of ASP from the cells, intracellular compartmentalization with changes in emission spectrum, and bleaching of the dye.18,21 For this reason, we evaluated the initial linear slope of the first 10 to 30 seconds as transport parameter. For proximal tubules, the cellular fluorescence increase on addition of ASP to perfusion chamber reflected organic cation transport across basolateral membrane of the tubule only because the ends of the tubules were kept inside the holding pipettes and tubules were collapsed.18 For hepatocyte couplets, the field of measurement was adjusted to sinusoidal membrane of hepatocytes, where OCTs are localized.22
Uptake of Cis-Platin by hOCT2-HEK293 and HEK293 Cells
To determine whether the presence of hOCT2 is important for the uptake of cis-platin, the same number of hOCT2-HEK293 and HEK293 cells were incubated for 10 minutes at 37°C with 100 μmol/L cis-platin. In parallel experiments, hOCT2-HEK293 cells were also incubated with 100 μmol/L cis-platin at 4°C. After incubation, cells were washed three times with ice-cold Ringer-like solution and hypoosmotic lysis was induced by addition of water. Lysates were sonicated for 10 minutes and centrifuged 10 minutes at 13,000 rpm. Platin was measured in supernatants and pellets by a validated graphite furnace-flameless atomic absorption spectrometry assay reported previously.23,24 Cell lysates were measured directly without dilution. Cell pellets were dissolved by concentrated nitrogen acid and diluted with water before measurement. Twenty μl of each sample were injected onto a graphite tube and atomized at 2700°C in a SpectrAA-Zeeman 220 System (Varian Inc., Darmstadt, Germany). The obtained absorption signals were evaluated by using peak height and Zeeman background correction. From the mean absorption of standards a calibration curve was generated by linear regression which was used to calculate platinum concentrations in the analyzed samples. Calibration standards and samples were analyzed at least in duplicate. Based on the platinum measurements and the used cell number the total platinum amount per cell was calculated. During each run at least six quality control samples were measured to assure accuracy and precision of measurements.
Quantification of Apoptosis and Necrosis by Flow Cytometry
Apoptosis was determined by detecting annexin V binding using flow cytometry as described previously.25 Evaluation of cell necrosis was performed by simultaneous detection of propidium iodide uptake by nonpermeabilized cells. hOCT2-HEK293 and HEK-293 cells, after 15 hours of incubation with or without 100 μmol/L cis-platin or with 100 μmol/L cis-platin plus 100, 50, 20, or 10 μmol/L cimetidine, were labeled with annexin V-fluorescein isothiocyanate and propidium iodide (5 μg/ml) in staining buffer (containing 1% bovine serum albumin in 50 mmol/L HEPES buffer, pH 7.4) for 15 minutes on ice. Fluorescein isothiocyanate-conjugated murine IgG monoclonal antibodies of unrelated specificities were used as controls. After staining, cells were washed in phosphate-buffered saline and fixed in 4% paraformaldehyde to apply flow cytometry. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Chemicals
Cis-platin [(cis-platinum(II) diamine dichloride)], oxali-platin [(SP-42{1R-trans}]-1,2-cyclohexanediamine-N,N′)-[ethanedioata(2-)-O,O′-platinum], and carboplatin [cisdiamine(1,1-cyclobutanedicarboxylato)-platinum] were obtained from Sigma (Taufkirchen, Germany). ASP was purchased from Molecular Probes (Leiden, The Netherlands). Fluorescein isothiocyanate-labeled annexin V was from Bender Medsystems (Vienna, Austria). All other substances and standard chemicals were obtained from Sigma or Merck (Darmstadt, Germany).
Statistical Analysis
Data are presented as mean values ± SEM, with n referring to the number of monolayers, tubules, or hepatocyte couplets used in fluorescence measurements. EC50 values were obtained by sigmoidal curve fitting using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). Unpaired two-sided Student’s t-test, a Wilcoxon matched pairs test and analysis of variance (with Bonferroni posttest) were used to prove statistical significance of the effects. A P value <0.05 was considered statistically significant.
Results
Interaction of Cis-Platin with hOCT2-HEK293 Cells
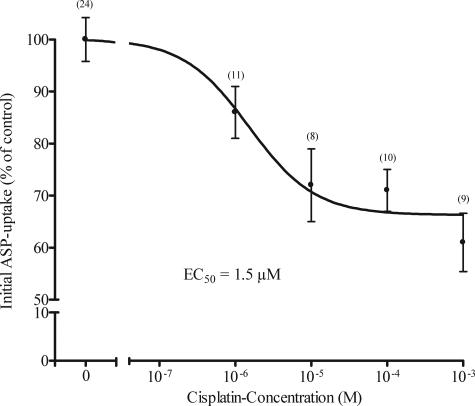
Cisplatin inhibited in a concentration-dependent manner the initial ASP-uptake in hOCT2-HEK293 cells (Figure 1). However, maximal inhibition accounted only for 40% of the initial ASP uptake in absence of cis-platin. Higher concentrations of cis-platin could not be dissolved in superfusion buffer. The EC50 calculated from the inhibition curve was 1.5 μmol/L, indicating an apparent affinity of hOCT2 for cis-platin in the same range as that observed for the typical organic cations tetrapentylammonium, TEA, cimetidine, or quinine (EC50 = 2.7, 35, 36, and 6.7 μmol/L, respectively).17
Figure 1.
Concentration response curve for the inhibition of initial ASP uptake by cis-platin in hOCT2-HEK293 cells. Values are mean ± SEM expressed as percentage change of ASP uptake in the absence of cis-platin. EC50 value determined from this curve was 1.5 μmol/L. In parentheses the number of observations is given.
Interaction of Cis-Platin, Oxaliplatin, and Carboplatin with hOCT2 and of Cis-Platin with hOCT1
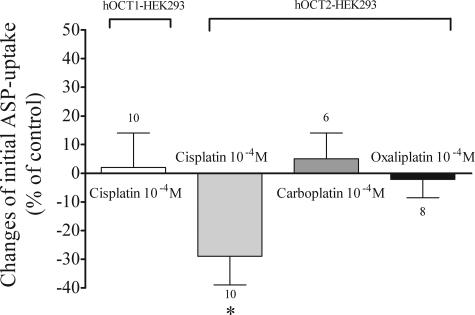
Although 100 μmol/L cis-platin caused a significant inhibition of initial ASP-uptake via hOCT2 (−29 ± 10%, n = 10; Figure 2), the same concentration of the less nephrotoxic derivates oxaliplatin and carboplatin showed no significant interaction with hOCT2 (−2 ± 6%, n = 8 and 5 ± 9%, n = 6, respectively; Figure 2). Cis-platin (100 μmol/L) showed no significant effect on initial ASP uptake via hOCT1 (2 ± 12%, n = 10; Figure 2). These experiments with the isoforms hOCT1 and hOCT2 showed that cis-platin interacts with hOCT2, but not with hOCT1.
Figure 2.
Effects of 100 μmol/L cis-platin on initial ASP uptake in hOCT1- and hOCT2-HEK293 cells. Values are mean ± SEM expressed as percentage change of ASP uptake in the absence of cis-platin. The effects of carboplatin and oxaliplatin on initial ASP uptake in hOCT2-HEK293 cells are also shown. *Statistically significant effect compared to controls (unpaired t-test, P < 0.05). Above the columns are the number of observations.
Interaction of Cis-Platin with OCTs of the Freshly Isolated Human Proximal Tubules and of Human Hepatocyte Couplets
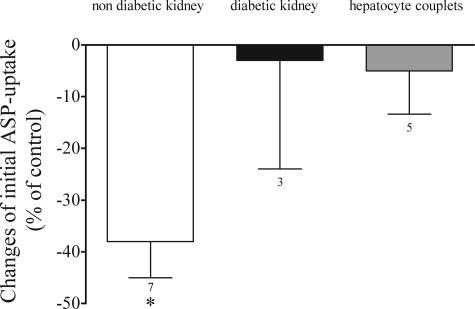
For the first time, we have investigated these transport processes also in human proximal tubules and hepatocyte couplets freshly isolated from tissue of patients undergoing nephrectomy or liver resection. Cis-platin (100 μmol/L) was also able to significantly inhibit the initial ASP uptake across the basolateral membrane of freshly isolated human proximal tubules from patients (−38 ± 7%, n = 7; Figure 3). For the first time we could also perform uptake experiments with proximal tubules from a human diabetic kidney. In three experiments, the prototypical organic cation TEA could not inhibit ASP uptake. Also cis-platin showed no interaction with ASP transport (−3 ± 21%, n = 3; Figure 3). In human hepatocyte couplets initial ASP uptake across basolateral membrane could be inhibited by TEA (−29 ± 3%, n = 5), but not by 100 μmol/L cis-platin (−5 ± 8%, n = 5). To our knowledge, this is the first work in which functional properties of human hepatocyte couplets have been investigated.
Figure 3.
Effects of 100 μmol/L cis-platin on initial ASP uptake in freshly isolated proximal tubules from human nondiabetic and diabetic kidneys. The effects of cis-platin on initial ASP uptake in human hepatocyte couplets are also shown. Values are mean ± SEM expressed as percentage change of ASP uptake in the absence of cis-platin. *Statistically significant effect (P < 0.05, Wilcoxon matched pairs test). Above the columns are the number of observations.
Uptake of Cis-Platin by hOCT2-HEK293 and HEK293 Cells
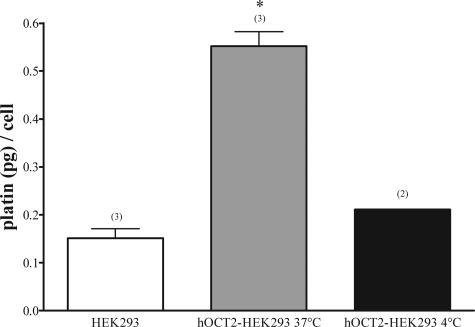
The uptake of cis-platin by hOCT2-HEK293 (0.55 ± 0.03 pg/cell, n = 3) was significantly higher than by HEK293 (0.15 ± 0.02 pg/cell, n = 3) cells (Figure 4). Inhibition of metabolic processes by low temperature (4°C) resulted in a reduction of cis-platin uptake (0.21 pg/cell, n = 2) to values similar to what found in HEK293-cells (Figure 4).
Figure 4.
Cis-platin accumulation in HEK293 (white column) and hOCT2-HEK293 (gray column) cells after 10 minutes incubation with 100 μmol/L cis-platin. Experiments performed with hOCT2-HEK293 cells at 4°C are also shown (black column). Values are mean ± SEM expressed as pg cis-platin per cell. *Statistically significant effect (analysis of variance, P < 0.05). Above the columns are the number of observations.
Apoptosis after Incubation with Cis-Platin
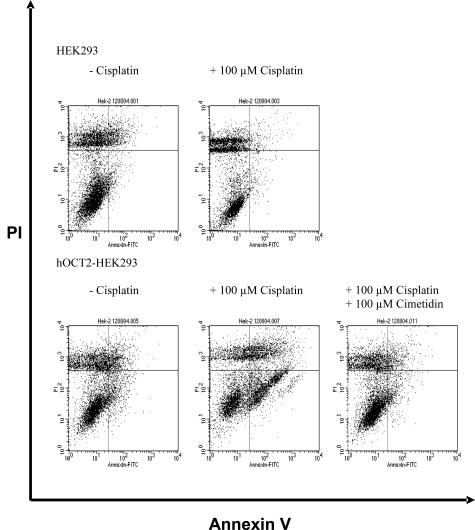
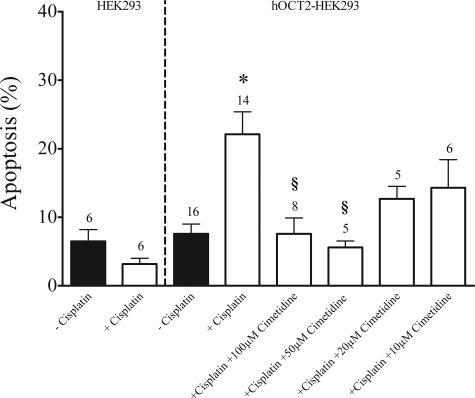
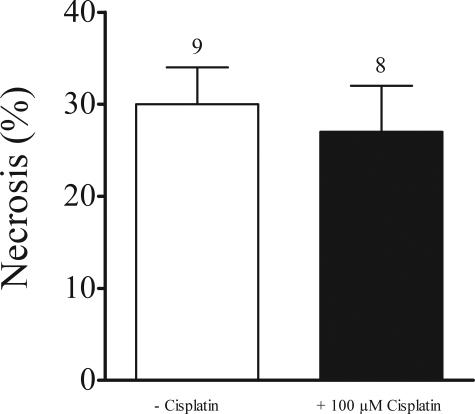
To further demonstrate that the nephrotoxicity of cis-platin is due to uptake via hOCT2 we measured apoptosis induced by cis-platin in HEK293 cells and those transfected with hOCT2 by fluorescence-activated cell sorting analysis. Incubation (24 and 48 hours) with 100 μmol/L cis-platin resulted in an extensive cell death of hOCT2-HEK293 cells, but not of HEK293 cells (not shown). Fifteen hours of incubation with 100 μmol/L cis-platin at 37°C induced a significant increase of apoptosis (expressed in percentage of annexin V-positive, but propidium iodide-negative cells; Figure 5, bottom right quadrants) in hOCT2-HEK293 cells (22 ± 3%, n = 14 with cis-platin, 8 ± 1%, n = 16 without cis-platin), but not in HEK293 cells (3 ± 1%, n = 6 with cis-platin, 7 ± 2%, n = 6 without cis-platin; Figure 6). This induction of apoptosis by cis-platin via hOCT2 was inhibited by the parallel incubation with 100 μmol/L cis-platin and 100 μmol/L of the organic cation cimetidine (8 ± 2%, n = 8). A complete suppression of cis-platin-induced apoptosis could be reached also with 50 μmol/L (6 ± 1%, n = 5), a partial suppression with 20 and 10 μmol/L cimetidine (13 ± 2%, n = 5 and 14 ± 4%, n = 6, respectively). Figure 5 shows a typical experiment and Figure 6 summarizes the results of all experiments. Incubation of hOCT2-HEK293 cells with 100 μmol/L cis-platin did not induce necrosis (27 ± 5%, n = 8 with cis-platin, 30 ± 4%, n = 9 without cis-platin, expressed in percentage of propidium iodide-positive cells; Figure 7).
Figure 5.
Typical fluorescence-activated cell sorting measurement of annexin V (apoptosis marker) and propidium iodide (necrosis marker) in HEK293 cells and hOCT2-HEK293 cells after 15 hours of incubation with or without 100 μmol/L cis-platin or with 100 μmol/L cis-platin + 100 μmol/L cimetidine. After cis-platin incubation a new population of apoptotic cells (bottom right quadrant)appears only in the hOCT2-HEK293 cells.
Figure 6.
Summary of fluorescence-activated cell sorting measurements of annexin V (apoptosis marker) in HEK293 cells and hOCT2-HEK293 cells after 15 hours of incubation with or without 100 μmol/L cis-platin or with 100 μmol/L cis-platin + 100, 50, 20, or 10 μmol/L cimetidine. Values are mean ± SEM expressed as percentage of total cell population. *Statistically significant effect from hOCT2-HEK293 cells without cis-platin; §statistically significant effect from hOCT2-HEK293 cells with cis-platin (analysis of variance, P < 0.05). Above the columns are the number of observations.
Figure 7.
Summary of fluorescence-activated cell sorting measurements of propidium iodide (necrosis marker) in hOCT2-HEK293 cells after 15 hours of incubation with (black column) or without (white column) 100 μmol/L cis-platin. Values are mean ± SEM expressed as percentage of total cell population. Above the columns are the number of observations.
Discussion
Nephrotoxicity is a serious consequence of aggressive therapy with cis-platin that often precludes the use of high-dose treatment. Moreover, the cumulative dose of cis-platin is a strong risk factor for the development of nephrotoxicity in patients who receive high doses of ifosfamide, cis-platin, and etoposide combinations.26 Carboplatin and oxaliplatin are less nephrotoxic analogues of cis-platin.1 In LLC-PK1 cells, a pig renal epithelial cell line, carboplatin was not toxic even at 10-fold the LC50 dose of cis-platin.27
The proximal tubules are maximally exposed to cis-platin during the first 3 hours after administration and toxicity is time- and dose-dependent.3 The first step for cis-platin to develop its toxic action is uptake into the cell. Even though for this process the involvement of the organic cation transport system has been supposed in OK5 and Madin-Darby canine kidney2 renal epithelial cell lines and in isolated rabbit proximal tubules,6 the role of this transport system for transport of cis-platin in humans is not yet known. This seems especially important because expression of specific isoforms of OCT varies between organs and species and substrate specificity is significantly different between the different isoforms also in the same species.17,20,21 In this study we have investigated if and which organic cation transporters are involved in cis-platin uptake in humans in kidney and liver.
The experiments with the isoforms hOCT1 and hOCT2 showed that cis-platin interacts with hOCT2, but not with hOCT1. Because hOCT2 is in humans the renal isoform of OCTs and hOCT1 is the hepatic one,9 these results could explain the organ-specific toxicity of cis-platin with toxic effects in kidney but not in liver. Carboplatin and oxaliplatin, less nephrotoxic analogues of cis-platin, showed no interaction with hOCT2. It can be supposed, that these compounds cannot easily penetrate into proximal tubular cells, because they cannot interact with hOCT2 and therefore are less toxic for the tubule cells. Cis-platin inhibited concentration dependently ASP uptake via hOCT2 with a 40% maximal inhibition. This result can be explained taking into account the very recently proposed structure-function properties of OCTs. These transporters seem to have a big binding pocket with overlapping interaction domains for different substrates.28,29 For example, it has been proposed that rOCT1 contains a large substrate binding region within a large cleft that can bind simultaneously several substrates and/or inhibitors.29 Transport of ASP or cis-platin can therefore require simultaneous or successive substrate binding to two or more binding sites within this large cleft. In this way, even though both cis-platin and ASP are transported by hOCT2, they can bind with different affinities, slightly different domains of the transporter, and a maximal inhibition of ASP uptake by cis-platin cannot be reached. Because a concentration of 100 μmol/L resulted in an almost maximal effect, we have used this concentration for all further experiments. In patients and experimental animals treated with nephrotoxic doses of cis-platin, the serum concentration reached 30 to 50 μmol/L.3 There are no reports on plasma levels in high-dose oxaliplatin treatment so far.2 In patients with high-dose carboplatin therapeutic regimens the platinum complex concentration was 65 μmol/L in plasma and 61 μmol/L in ultrafiltrated plasma.2 These platin concentrations are not far from what was used in this study. Moreover, such concentrations of platinum complexes are commonly used for in vitro studies.2 For the first time, we have investigated these transport processes also in human proximal tubules and hepatocyte couplets. The results of these experiments confirm what was observed with hOCT1 and hOCT2: cis-platin interacts with the transport system of the fluorescent organic cation ASP in proximal tubules, but not in hepatocyte couplets. For the first time we could also perform uptake experiments with proximal tubules from a diabetic kidney. In three experiments, the prototypical organic cation TEA could not inhibit ASP uptake. Also cis-platin showed no interaction with ASP transport. Diabetic rats and rabbits have been shown to be resistant against cis-platin nephrotoxicity.11,12,14 Both short- and long-term diabetes was associated in diabetic rats with reduced gene and protein expression of the renal OCT isotypes.12,14 Therefore, the lack of inhibition of ASP transport observed in this study in freshly isolated human proximal tubules could indicate a down-regulation of OCTs also in diabetic humans. The down-regulation of hOCT2 could protect also diabetic humans from cis-platin nephrotoxicity, as suggested by the lack of interaction of cis-platin with the ASP transport seen in this study. The mechanism by which diabetes can regulate the expression and/or activity of OCTs is not known. However, because hOCT2 is inhibited by G protein activation17 and diabetic patients have an enhanced G protein reactivity,30 this pathway could represent a possible link between diabetes and OCT regulation. Large clinical studies are required to establish if diabetic patients have a protection against cis-platin nephrotoxicity.
The inhibition of ASP uptake by cis-platin does not necessarily imply that cis-platin is transported by hOCT2. However, the experiments in which platin has been measured in lysates of cells incubated with cis-platin confirmed the importance of hOCT2 for its uptake. The uptake of cis-platin by HEK293 cells was of unspecific nature, because it was not different from what was observed in hOCT2-HEK293 cells at 4°C. At this low temperature, specific transport processes are mostly inhibited. Only HEK293 cells expressing hOCT2 responded with increased apoptosis to cis-platin incubation, once more confirming that cis-platin must indeed be taken up by the cells via hOCT2. Apoptosis was suppressed by contemporaneous incubation with cis-platin and cimetidine, a typical organic cation, suggesting a competition for the transport at the transporter and hence a reduction of cellular cis-platin toxicity. Although a concentration of cis-platin in plasma similar to that used in this study can be reached in patients (see above), the mean steady-state cimetidine concentrations on a standard 1000-mg daily dose are ∼4 μmol/L.31 However, after a single 400-mg dose, concentrations up to 18 μmol/L have been measured.32 It must be tested if a cimetidine concentration of 50 μmol/L can be used in clinical practice or if lower concentrations of other substances with higher affinity for OCTs can also effectively compete with cis-platin. In experimental studies in animals it is well demonstrated that organic cations protect from cis-platin nephrotoxicity: quinine and cyanine in hens;33 procaine hydrochloride,34 disopyramide, and verapamil35 in rats. In humans there are few but contradictory results: verapamil and cimetidine protected from cis-platin nephrotoxicity in a group of nine patients with nonseminomatous testicular cancer,36 while ranitidine did not modify renal handling of cis-platin in 10 pediatric patients, in contrast with what was observed from the same group in dogs, where it decreased cis-platin renal clearance.37 The authors speculate that this discrepancy may result from relative insensitivity of the study to detect small differences in the pharmacokinetic parameters in children.37 Interestingly, microarray analysis of rat kidneys after 7 days of cis-platin treatment showed that expression levels of multidrug resistance transporters were increased, whereas that of OCT2 were decreased.38 At that time the significance of these changes to cis-platin toxicity remained elusive. Now we have demonstrated that OCTs play an important role in the tubular uptake of cis-platin. Their decrease would therefore result in a decreased cis-platin uptake and could protect the kidney from further toxicity.
In conclusion, we show that cis-platin interacts with hOCT2 but not with hOCT1. The physiological relevance of this interaction is underlined by the experiments showing that cis-platin interacts with the organic cation transport in freshly isolated human proximal tubules but not with that of human hepatocyte couplets. These findings elucidate the organ-specific toxicity of cis-platin. The interaction with hOCT2 is necessary to induce toxicity, because only cells expressing this transporter became apoptotic after 15 hours of incubation with cis-platin. Apoptosis could be suppressed by contemporaneous incubation with cimetidine, a substrate of OCT, suggesting that administration of a specific competitor for renal OCT could allow the use of high doses of cis-platin in the anti-neoplastic therapy without causing nephrotoxicity or to maintain higher cis-platin concentrations in blood.
Acknowledgments
We thank Ms. K. Beul, Ms. A. Dirks, Ms. U. Kleffner, and Ms. R. Thanos for their excellent technical assistance.
Footnotes
Address reprint requests to Giuliano Ciarimboli, Universitätsklinikum Münster, Medizinische Klinik und Poliklinik D, Experimentelle Nephrologie, Domagkstraβe 3a, D-48149 Münster, Germany. E-mail: gciari@uni-muenster.de.
Supported by the Deutsche Forschungsgemeinschaft (Schl 277/8-3 and 11-1 to E.S. and LU854/2-1 to T.L.) and Innovative Medizinische Forschung (CI 120437 to G.C.).
References
- Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. Oncol Rep. 2003;10:1663–1682. [PubMed] [Google Scholar]
- Ludwig T, Riethmuller C, Gekle M, Schwerdt G, Oberleithner H. Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int. 2004;66:196–202. doi: 10.1111/j.1523-1755.2004.00720.x. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol. 2003;14:1–10. doi: 10.1097/01.asn.0000042803.28024.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece PA, Stafford I, Russell J, Gill PG. Nonlinear renal clearance of ultrafilterable platinum in patients treated with cis-dichlorodiammineplatinum (II). Cancer Chemother Pharmacol. 1985;15:295–299. doi: 10.1007/BF00263904. [DOI] [PubMed] [Google Scholar]
- Endo T, Kimura O, Sakata M. Carrier-mediated uptake of cisplatin by the OK renal epithelial cell line. Toxicology. 2000;146:187–195. doi: 10.1016/s0300-483x(00)00176-1. [DOI] [PubMed] [Google Scholar]
- Kolb RJ, Ghazi AM, Barfuss DW. Inhibition of basolateral transport and cellular accumulation of cDDP and N-acetyl-L-cysteine-cDDP by TEA and PAH in the renal proximal tubule. Cancer Chemother Pharmacol. 2003;51:132–138. doi: 10.1007/s00280-002-0537-0. [DOI] [PubMed] [Google Scholar]
- Pan BF, Sweet DH, Pritchard JB, Chen R, Nelson JA. A transfected cell model for the renal toxin transporter, rOCT2. Toxicol Sci. 1999;47:181–186. doi: 10.1093/toxsci/47.2.181. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Rev Physiol Biochem Pharmacol. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui KK. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13:866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- Najjar TA, Saad SY. Cisplatin pharmacokinetics and its nephrotoxicity in diabetic rabbits. Chemotherapy. 2001;47:128–135. doi: 10.1159/000048512. [DOI] [PubMed] [Google Scholar]
- Grover B, Buckley D, Buckley AR, Cacini W. Reduced expression of organic cation transporters rOCT1 and rOCT2 in experimental diabetes. J Pharmacol Exp Ther. 2004;308:949–956. doi: 10.1124/jpet.103.058388. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Tikellis C, Burns WC, Thallas V, Forbes JM, Cao Z, Osicka TM, Russo LM, Jerums G, Ghabrial H, Cooper ME, Kantharidis P. Reduced tubular cation transport in diabetes: prevented by ACE inhibition. Kidney Int. 2003;63:2152–2161. doi: 10.1046/j.1523-1755.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Tikellis C, Kantharidis P, Burns WC, Cooper ME, Forbes JM. The role of advanced glycation in reduced organic cation transport associated with experimental diabetes. J Pharmacol Exp Ther. 2004;311:456–466. doi: 10.1124/jpet.104.070672. [DOI] [PubMed] [Google Scholar]
- Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett. 1996;395:153–156. doi: 10.1016/0014-5793(96)01030-7. [DOI] [PubMed] [Google Scholar]
- Cetinkaya I, Ciarimboli G, Yalcinkaya G, Mehrens T, Velic A, Hirsch JR, Gorboulev V, Koepsell H, Schlatter E. Regulation of human organic cation transporter hOCT2 by PKA, PI3K, and calmodulin-dependent kinases. Am J Physiol. 2003;284:F293–F302. doi: 10.1152/ajprenal.00251.2002. [DOI] [PubMed] [Google Scholar]
- Pietig G, Mehrens T, Hirsch JR, Cetinkaya I, Piechota H, Schlatter E. Properties and regulation of organic cation transport in freshly isolated human proximal tubules. J Biol Chem. 2001;276:33741–33746. doi: 10.1074/jbc.M104617200. [DOI] [PubMed] [Google Scholar]
- Gautam A, Ng OC, Boyer JL. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987;7:216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Struwe K, Arndt P, Gorboulev V, Koepsell H, Schlatter E, Hirsch JR. Regulation of the human organic cation transporter hOCT1. J Cell Physiol. 2004;201:420–428. doi: 10.1002/jcp.20081. [DOI] [PubMed] [Google Scholar]
- Mehrens T, Lelleck S, Cetinkaya I, Knollmann M, Hohage H, Gorboulev V, Boknik P, Koepsell H, Schlatter E. The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J Am Soc Nephrol. 2000;11:1216–1224. doi: 10.1681/ASN.V1171216. [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Karbach U, Gorboulev V, Arndt P, Koepsell H. Membrane localization of the electrogenic cation transporter rOCT1 in rat liver. Biochem Biophys Res Commun. 1998;248:673–678. doi: 10.1006/bbrc.1998.9034. [DOI] [PubMed] [Google Scholar]
- Kloft C, Appelius H, Siegert W, Schunack W, Jaehde U. Determination of platinum complexes in clinical samples by a rapid flameless atomic absorption spectrometry assay. Ther Drug Monit. 1999;21:631–637. doi: 10.1097/00007691-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Zisowsky J, Becker A, Weykam S, Kassack M, Jaehde U. Assessment of platinum sensitivity in human tumor cells. Int J Clin Pharmacol Ther. 2003;41:612–613. doi: 10.5414/cpp41612. [DOI] [PubMed] [Google Scholar]
- Lang D, Dohle F, Terstesse M, Bangen P, August C, Pauels HG, Heidenreich S. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol. 2002;168:6152–6158. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]
- Caglar K, Kinalp C, Arpaci F, Turan M, Saglam K, Ozturk B, Komurcu S, Yavuz I, Yenicesu M, Ozet A, Vural A. Cumulative prior dose of cisplatin as a cause of the nephrotoxicity of high-dose chemotherapy followed by autologous stem-cell transplantation. Nephrol Dial Transplant. 2002;17:1931–1935. doi: 10.1093/ndt/17.11.1931. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Borch RF. Quiescent LLC-PK1 cells as a model for cis-diamminedichloroplatinum(II) nephrotoxicity and modulation by thiol rescue agents. Cancer Res. 1988;48:6017–6024. [PubMed] [Google Scholar]
- Gorboulev V, Shatskaya N, Volk C, Koepsell H. Subtype-specific affinity for corticosterone of rat organic cation transporters rOCT1 and rOCT2 depends on three amino acids within the substrate binding region. Mol Pharmacol. 2005;67:1612–1619. doi: 10.1124/mol.104.008821. [DOI] [PubMed] [Google Scholar]
- Popp C, Gorboulev V, Muller TD, Gorbunov D, Shatskaya N, Koepsell H. Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Mol Pharmacol. 2005;67:1600–1611. doi: 10.1124/mol.104.008839. [DOI] [PubMed] [Google Scholar]
- Pietruck F, Spleiter S, Daul A, Philipp T, Derwahl M, Schatz H, Siffert W. Enhanced G protein activation in IDDM patients with diabetic nephropathy. Diabetologia. 1998;41:94–100. doi: 10.1007/s001250050872. [DOI] [PubMed] [Google Scholar]
- Somogyi A, Gugler R. Clinical pharmacokinetics of cimetidine. Clin Pharmacokinet. 1983;8:463–495. doi: 10.2165/00003088-198308060-00001. [DOI] [PubMed] [Google Scholar]
- Hempenius J, Wieling J, Brakenhoff JP, Maris FA, Jonkman JH. High-throughput solid-phase extraction for the determination of cimetidine in human plasma. J Chromatogr B Biomed Sci Appl. 1998;714:361–368. doi: 10.1016/s0378-4347(98)00232-1. [DOI] [PubMed] [Google Scholar]
- Bird JE, Walser MM, Quebbemann AJ. Protective effect of organic cation transport inhibitors on cis-diamminedichloroplatinum-induced nephrotoxicity. J Pharmacol Exp Ther. 1984;231:752–758. [PubMed] [Google Scholar]
- Fenoglio C, Boicelli CA, Ottone M, Addario C, Chiari P, Viale M. Protective effect of procaine hydrochloride on cisplatin-induced alterations in rat kidney. Anticancer Drugs. 2002;13:1043–1054. doi: 10.1097/00001813-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Hanada K, Odaka K, Kudo A, Ogata H. Effects of disopyramide and verapamil on renal disposition and nephrotoxicity of cisplatin in rats. Pharm Res. 1999;16:1589–1595. doi: 10.1023/a:1018912806355. [DOI] [PubMed] [Google Scholar]
- Sleijfer DT, Offerman JJ, Mulder NH, Verweij M, van der Hem GK, Schraffordt Koops HS, Meijer S. The protective potential of the combination of verapamil and cimetidine on cisplatin-induced nephrotoxicity in man. Cancer. 1987;60:2823–2828. doi: 10.1002/1097-0142(19871201)60:11<2823::aid-cncr2820601138>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ito S, Weitzman S, Klein J, Greenberg M, Lau R, Atanakovic G, Koren G. Lack of cisplatin-ranitidine kinetic interactions: in vivo study in children, and in vitro study using dog renal brush border membrane vesicles. Life Sci. 1998;62:L387–L392. doi: 10.1016/s0024-3205(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Huang Q, Dunn RT, Jayadev S, DiSorbo O, Pack FD, Farr SB, Stoll RE, Blanchard KT. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci. 2001;63:196–207. doi: 10.1093/toxsci/63.2.196. [DOI] [PubMed] [Google Scholar]