Abstract
P-selectin expression has been reported in platelets, endothelial cells, and vascular smooth muscle cells in response to vascular injury. Here, we report P-selectin expression on macrophages in the arterial wall after carotid denudation injury and spontaneous atherosclerosis in atherosclerosis-prone apoE-deficient (apoE−/−) mice. Double-immunofluorescence staining revealed robust P-selectin expression in macrophage-rich regions of both denudation-induced carotid neointimal lesions and innominate atherosclerotic plaques. Co-localization of P-selectin with macrophages was verified at the single cell level using double immunostaining plus 4,6-diamidino-2-phenylindole (for nuclei) counterstaining. No platelet staining was seen in association with the macrophage staining, excluding platelet contamination. Furthermore, P-selectin mRNA expression was readily detectable in macrophage-rich plaques of atherosclerotic innominate arteries and blood monocyte-derived macrophages from apoE−/− mice. Strong P-selectin expression was also seen in the areas of regenerated endothelium after arterial injury. In addition, co-localization of P-selectin with vascular smooth muscle cells was readily observed in denudation-injured carotid arteries at 7 and 14 days. We conclude that macrophages in carotid injury-induced neointimal lesions and spontaneous atherosclerotic plaques of the innominate artery acquire the ability to express P-selectin, as does regenerating endothelium. These findings provide a potential new paradigm in macrophage-mediated vascular inflammation, atherosclerosis, and neointimal hyperplasia after arterial injury.
P-selectin (CD62P) is constitutively expressed and stored in the α-granules of platelets1 and the Weibel-Palade bodies of endothelial cells2 and translocates rapidly to the cell surface in response to several inflammatory stimuli. P-selectin participates in the early steps of leukocyte recruitment and mediates interactions of platelets and leukocytes with the damaged vessel wall through multiple mechanisms.3–6 Recent studies from both our laboratory and others demonstrate that P-selectin plays a pivotal role in inflammation,3 thrombosis,5 atherosclerosis,7 and neointima formation after arterial injury.8–12 Platelet P-selectin plays a critical role in the development of atherosclerosis in atherosclerosis-prone apoE-deficient (apoE−/−) mice. We found that intravenous injection of activated wild-type but not P-selectin-null platelets promote leukocyte recruitment (particularly monocyte) on the atherosclerosis-prone endothelium and exacerbate atherosclerosis in apoE−/− mice.7 After vascular injury, P-selectin-deficient mice have significantly reduced neointima formation,11,12 demonstrating a critical role of P-selectin in the response to arterial injury. We found a 94% reduction in neointima area after carotid wire denudation injury in apoE and P-selectin double-knockout (apoE−/−P-sel−/−) mice compared with wild-type apoE−/− mice.8 Furthermore, using bone marrow transplantation to create chimeric mice showed that lack of platelet P-selectin resulted in an intermediate degree (62% reduction) in neointima area.10 Based on these findings, we concluded that platelet P-selectin played a predominantly protective role but that P-selectin on other cell types [eg, vascular smooth muscle cells (SMCs), macrophages, and regenerated endothelium] may also influence the response to vascular injury.
Macrophages are abundantly present in spontaneous atherosclerotic lesions and neointimal lesions after arterial injury. Although it is well known that macrophages play a pivotal role in the development of atherosclerosis and neointima formation after balloon angioplasty, the underlying molecular mechanisms are not completely understood. Macrophages contribute to the local inflammatory response in part through the production of a variety of proinflammatory mediators, including adhesion molecules, chemokines, cytokines, free oxygen radicals, and matrix metalloproteinases.13–16 The present study tested the hypothesis that macrophages within vascular injury-induced neointimal lesions and spontaneous atherosclerotic plaques in atherosclerosis-prone apoE−/− mice express P-selectin, an important mediator of inflammation. Our results for the first time demonstrate the expression of P-selectin in macrophages both within neointimal lesions of denudation-injured carotid arteries and spontaneous atherosclerotic plaques of innominate arteries in apoE−/− mice.
Materials and Methods
Experimental Animals
ApoE−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee at the University of Virginia Health System.
Mouse Carotid Denudation Injury Model
ApoE−/− mice at the age of 10 to 12 weeks were fed a Western atherogenic diet containing 21% fat by weight (TD 88137 Harlan-Teklad, Madison, WI; 0.15% cholesterol and 19.5% casein without sodium cholate) for 1 week before and 4 weeks after carotid injury. Wire denudation injury of the left common carotid artery of the mouse was performed under ketamine/xylazine anesthesia as previously described.8,9 Endothelial denudation was confirmed by scanning electron microscopy as previously described.17 Animals were sacrificed at defined time points after wire denudation injury. Arteries were perfusion-fixed with 4% paraformaldehyde and embedded in paraffin.
Immunofluorescence Microscopy
Paraffin-embedded arterial sections (5 μm thick) were incubated with primary antibody overnight at 4°C. Antibody binding was detected with a biotinylated secondary antibody and visualized by streptavidin conjugated-Alexa Fluor 555 (red) or Alexa Fluor 488 (green) (both from Molecular Probes, Eugene, OR). P-selectin and platelets were stained with a polyclonal P-selectin antibody (provided by Dr. Samuel A. Green at the University of Virginia) and a polyclonal integrin β3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), respectively, and visualized by Alexa Fluor 488 (green); macrophages and vascular SMCs were stained with Mac-2 (M3/38; Accurate) and smooth muscle α-actin monoclonal antibody (1A4; Sigma, St. Louis, MO), respectively, and visualized by Alexa Fluor 555 (red). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI, blue). For double-immunofluorescence staining, sections were stained first for P-selectin followed by detection of Mac-2 or α-smooth muscle actin (SMA). Overlapping images (yellow) of P-selectin with Mac-2 or α-SMA indicates co-localization of P-selectin with macrophages or vascular SMCs. Irrelevant class- and species-matched immunoglobulins as well as incubations without the primary antibody were used as controls. Sections were analyzed by fluorescence microscopy (Olympus BX51 microscope) and Image Pro Plus 3.0 software (Media Cybernetics, Silver Spring, MD).
Cell Isolation and Culture
Mouse peritoneal macrophages (MPMs) were prepared from the peritoneal fluid of apoE−/− mice (10 weeks old) 4 days after intraperitoneal injection of 3 ml of 4% thioglycolate in saline.18 The MPMs obtained (10 to 20 × 106 per mouse) were plated at a density of 3 × 106 cells/35-mm dish and incubated in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) containing 10% fetal bovine serum until the day of the experiment.
Mouse peripheral blood monocyte-derived macrophages (PBMDMs) were isolated from 3 ml of the blood of three apoE−/− mice (10 weeks old) by density gradient centrifugation using Histopaque-1077 kit (Sigma).19 The monocytes obtained were suspended in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 8 μg/ml of insulin and incubated for 2 hours at 37°C in 35-mm cell culture dishes. Nonadherent cells were removed by washing twice with phosphate-buffered saline. The remaining adherent cells were cultured in the same medium. The medium was replaced every 3 days. After 10 days of incubation with recombinant mouse macrophage colony-stimulating factor (M-CSF, 50 ng/ml; Calbiochem, La Jolla, CA), the cells were used as mouse monocyte-derived macrophages. Cell viability was typically >95% by the trypan blue exclusion method. In addition, mouse bone marrow-derived macrophages were prepared as described as a control.20 After 7 days of incubation with 50 ng/ml of M-CSF, the cells were used as bone marrow-derived macrophages.20
Flow Cytometry
Flow cytometry was performed using a FACSCalibur (Becton-Dickinson, Mountain View, CA). MPMs were identified using fluorescein isothiocyanate-conjugated rat anti-mouse Mac-3 (M3/84; BD Pharmingen, San Diego, CA). P-selectin expression was analyzed using fluorescein isothiocyanate-conjugated rat anti-mouse CD62P monoclonal antibody (RB40.34, BD Pharmingen). Appropriate isotype-matched control antibodies were used as controls.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was extracted from a pool of five innominate arteries with severe atherosclerotic plaques under the dissecting microscope and from primary cultures of the MPMs or PBMDMs with TRIzol (Invitrogen, Carlsbad, CA) treated with DNAase I to remove genomic DNA and then purified with the use of a RNA purification kit (Invitrogen), and reverse-transcribed using the Superscript II (Life Technologies Inc., Rockville, MD) and oligo-dT primers. The following mouse PCR primers were used: P-selectin forward, 5′-GTGCAGAGCGGTCAAATGC-3′, and reverse, 5′-CTGAGAGCTTTCTTAGCAGAGC-3′ (303 bp); β-actin forward, 5′-GTGGGCCGCTCTAGGCACCAA-3′, and reverse, 5′-CTCTTTGATGTCACGCACGATTTC-3′ (540 bp). PCR reaction was performed by an initial cycle at 95°C for 4 minutes followed by 30 cycles for P-selectin or 18 cycles for β-actin of 94°C for 45 seconds, 58°C for 45 seconds, 72°C for 1 minute, and ending with a 5-minute extension at 72°C. The amplified products were separated on 1.5% agarose gels. Total cellular RNA extracted from normal innominate arteries of wild-type C57BL6J mice and from mouse SVEC4–10 endothelial cells (CRL-218; American Type Culture Collection, Rockville, MD) was used as negative and positive controls for P-selectin mRNA expression, respectively.
Statistical Analysis
Data are reported as the number of arteries in each group, and are expressed as the mean ± SD. Statistical analysis was performed with one-way analysis of variance or Student’s t-test as appropriate. The correlation study between P-selectin expression and Mac-2-positive macrophage area within lesions was evaluated by linear correlation analysis, followed by analysis of variance.
Results
P-Selectin Expression in Neointimal Macrophages after Carotid Artery Denudation Injury in Atherogenic ApoE−/− Mice
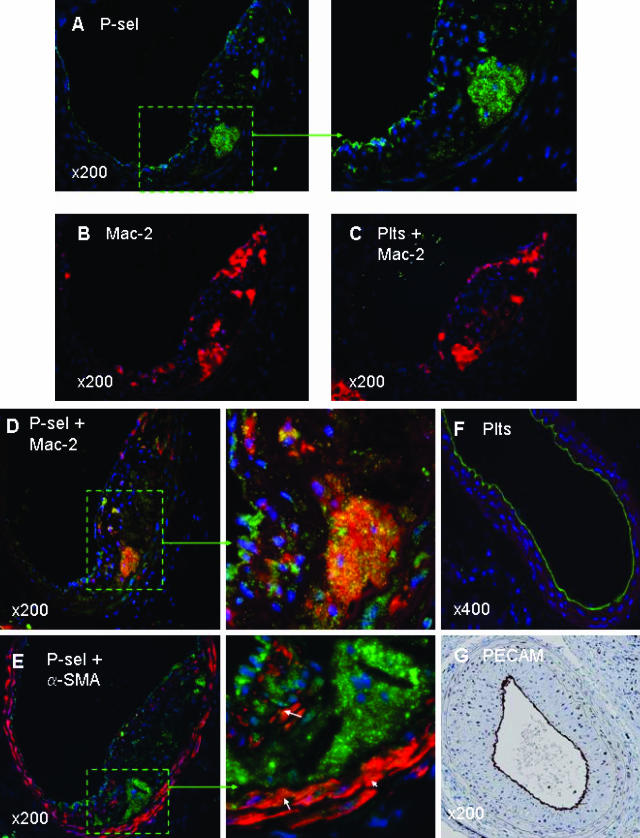
Paraffin-embedded sections of wire-injured carotid arteries were processed for double-immunofluorescence staining. The neointimal lesions used for analysis included injured carotid arteries of apoE−/− mice (n = 11) 28 days after wire denudation. Similar to our previous reports,8,9 quantitative immunohistochemistry using Image Pro Plus software revealed that in paraffin sections of the injured carotid arteries, the Mac-2-positive macrophage area within the neointimal lesions was ∼20%. Robust P-selectin expression was seen in macrophage-rich regions within neointimal lesions (Figure 1; A, D, and E). Co-localization of P-selectin with Mac-2-positive macrophages was verified by double immunostaining plus DAPI (for nuclei) counterstaining (Figure 1D and magnified inset).
Figure 1.
Immunofluorescence staining illustrating expression of P-selectin in neointimal macrophages. Shown are representative images of wire-injured carotid arteries from an apoE−/− mouse at 28 days after wire denudation. Five adjacent arterial sections (A–E) per mouse were used to identify 1) P-selectin (green, A, and the magnified inset); 2) macrophages (red, B); 3) platelets plus macrophages double stained (yellow, C); 4) P-selectin plus macrophages double stained (yellow, D, and the magnified inset); 5) P-selectin plus vascular SMCs double stained (yellow, E, and the magnified inset). Nuclei were counterstained with DAPI (blue). Note the robust P-selectin expression in macrophage-rich regions (A, D, and E), and co-localization of P-selectin with macrophages as observed with double staining plus DAPI counterstaining (D and the magnified inset). C: No platelet-positive staining was seen within the neointimal macrophages. A and E: Robust P-selectin expression was also seen along the luminal lining. In addition, scanty positive staining of P-selectin was seen in α-SMA-positive vascular SMCs both in the neointima or media (E and the magnified inset). F: Platelet staining along the luminal lining at 4 hours after carotid wire denudation injury. G: Complete re-endothelialization at 28 days after carotid wire injury using anti-CD31 (PECAM) staining. Original magnifications: ×200 (A–E, G); ×400 (F).
Because positive macrophage P-selectin staining may arise from contamination with platelets or platelet-derived microparticles, we performed double staining of platelets and macrophages in the adjacent carotid sections. Results showed that neointimal macrophages were stained positive for P-selectin (Figure 1D and magnified inset), but not for platelets (Figure 1C). As a positive control, we performed platelet staining using integrin β3 polyclonal antibody on a 4-hour wire-injured carotid artery, when scanning electron microscopy reveals large numbers of platelets deposited on the injured surface (data not shown). Results showed robust platelet staining along the luminal border, reflecting platelet deposition (Figure 1F). Taken together, these data indicate that macrophage P-selectin-positive staining was not secondary to platelet contamination. Furthermore, a direct correlation exists between the P-selectin-positive area and the Mac-2-positive macrophage area within neointimal lesions. Linear correlation analysis revealed that the correlation between P-selectin-positive staining and Mac-2-positive staining was much higher for macrophage-rich regions versus macrophage-poor regions (R2 = 0.90 versus R2 = 0.02, P < 0.001).
Robust P-selectin expression was also seen along the luminal border at 28 days (Figure 1; A, D, and E, and the respective magnified insets), indicating that P-selectin is expressed by regenerated endothelium that was determined by use of anti-CD31 (PECAM-1) antibody staining (Figure 1G). In addition, weak but clearly positive staining of P-selectin co-localized with α-SMA-positive vascular SMCs within either the neointimal lesions or the media of carotid arteries 28 days after denudation injury (Figure 1E and magnified inset).
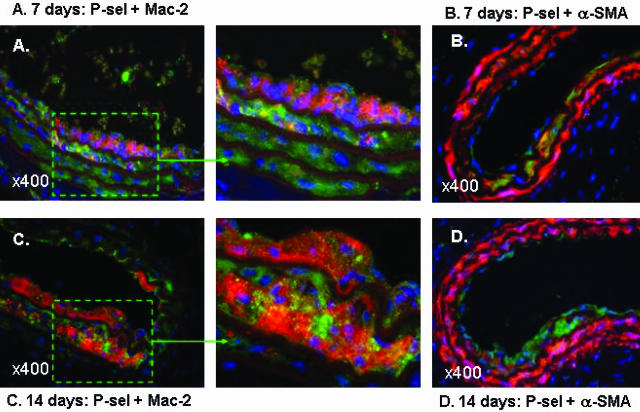
Furthermore, we investigated the co-localization of expression of P-selectin with macrophages and vascular SMCs at earlier time points (including 7 and 14 days) after carotid denudation injury. Co-localization of P-selectin with Mac-2-positive macrophages was observed in the developing intimal lesions and media at 7 days (Figure 2A and the magnified inset) and 14 days (Figure 2C and the magnified inset) after wire injury. Moreover, consistent with a previous report21 that showed expression of P-selectin by α-SMA-positive SMCs, the present study confirmed co-localization of P-selectin with α-SMA-positive SMCs in the wire-injured carotid arteries of apoE−/− mice at 7 days (Figure 2B) and 14 days (Figure 2D). In contrast, P-selectin staining was not seen in the media of noninjured carotid arteries of apoE−/− mice (data not shown). Taken together, these observations suggest that vascular SMCs, when in a proliferating or activated state, acquire the ability to express P-selectin in response to acute vascular injury.
Figure 2.
Immunofluorescence staining illustrating co-expression of P-selectin with macrophages and vascular SMCs of wire-injured carotid arteries from apoE−/− mice at 7 days and 14 days after wire denudation. Four arterial sections were used to identify 1) P-selectin plus macrophage double staining at 7 days (yellow, A, and the magnified inset); 2) P-selectin plus vascular SMC double staining at 7 days (yellow, B); 3) P-selectin plus macrophage double staining at 14 days (yellow, C, and the magnified inset); 4) P-selectin plus vascular SMC double staining at 14 days (yellow, D). Nuclei were counterstained with DAPI (blue). A and C: Note that co-localization of P-selectin plus macrophages were readily visible in intimal lesions. B and D: Co-localization of P-selectin plus vascular SMCs was noted in media. Original magnifications, ×400.
P-Selectin Expression in Plaque Macrophages of Spontaneous Atherosclerotic Lesions in the Innominate Arteries of ApoE−/− Mice
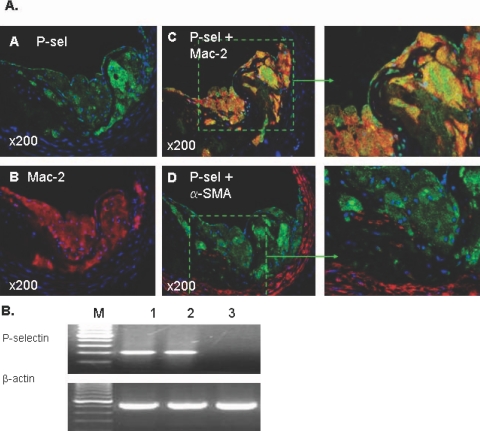
Paraffin-embedded sections of atherosclerotic innominate arteries were processed for double-immunofluorescence staining. The spontaneous atherosclerotic lesions used for analysis included innominate arteries of older (>25 weeks old) apoE−/− mice (n = 6). The pattern of expression of P-selectin (Figure 3A) was similar to that seen in the injured carotid arteries described above (Figure 1). The atherosclerotic lesions were rich in macrophages (Figure 3A, B) but poor in vascular SMCs (Figure 3A, D). Robust P-selectin expression was seen in macrophage-rich (Mac-2-positive) plaques (Figure 3A; A, C, and D). Co-localization of P-selectin with macrophages was observed with double immunostaining plus DAPI (for nuclei) counterstaining (Figure 3A, C and the magnified inset). In contrast to the earlier time points after carotid wire injury, there was a weak co-localization of P-selectin with α-SMA-positive vascular SMCs within either atherosclerotic plaques or the medial layer (Figure 3A, D and the magnified inset).
Figure 3.
A:Immunofluorescence staining illustrating expression of P-selectin in plaque macrophages of spontaneous atherosclerosis of the innominate artery in a 25-week-old apoE−/− mouse. Four adjacent arterial sections were used to identify 1) P-selectin (green, A); 2) macrophages (red, B); 3) P-selectin plus macrophages double stained (yellow, C, and the magnified inset); 4) P-selectin plus vascular SMCs double stained (yellow, D, and the magnified inset). Nuclei were counterstained with DAPI (blue). B and D: The atherosclerotic lesions were rich in macrophages but poor in vascular SMCs. Note that robust P-selectin expression was seen in macrophage-rich regions (A, C, and D), and co-localization of P-selectin with macrophages was observed with double staining plus DAPI counterstaining (C and the magnified inset). A and D: Robust P-selectin expression was also seen along the luminal surface. In contrast, co-localization of P-selectin with α-SMA-positive vascular SMCs was scanty both in the atherosclerotic plaque and the media (D and the magnified inset). B: RT-PCR analysis of P-selectin mRNA expression. Total RNA was extracted from a pool of five innominate arteries with severe atherosclerosis under the dissecting microscope. P-selectin mRNA was readily detectable in the atherosclerotic arteries (lane 2) but not in normal innominate arteries of wild-type C57BL6J mice (lane 3). Mouse SVEC4–10 endothelial cells used as a positive control (lane 1). Original magnifications, ×200.
To address whether P-selectin was transcribed in macrophages, we extracted total RNA from a pool of macrophage-rich areas of five innominate arteries with severe atherosclerotic lesions. RT-PCR analysis demonstrated that P-selectin mRNA was readily detectable in the innominate atherosclerotic plaques but not in normal innominate arteries from wild-type C57BL6J mice (Figure 3B).
P-Selectin Expression in MPMs and Blood Monocyte-Derived Macrophages
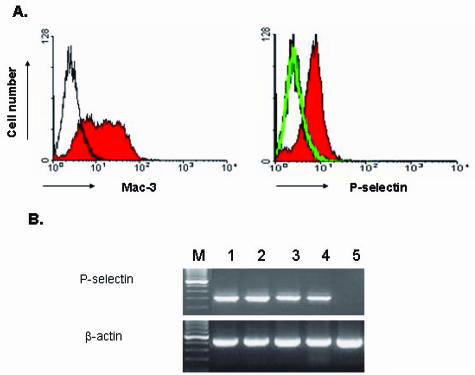
After intraperitoneal injection of thioglycolate, circulating monocytes migrate out of the bloodstream into the peritoneal cavity and develop into inflammatory peritoneal macrophages. P-selectin expression has been reported in MPMs at the mRNA and protein levels in normal C57BL/6J mice.22 In this study, we performed flow cytometry to assess P-selectin expression in thioglycolate-elicited MPMs from hyperlipidemic apoE−/− mice after 3 days in culture. The macrophage population was identified using a rat anti-mouse monoclonal antibody (Mac-3) specific to macrophages but not to monocytes (Figure 4A, left). A rat anti-mouse P-selectin monoclonal antibody (RB40.34) was used to assess P-selectin expression. Results showed P-selectin expression in the MPMs derived from apoE−/− mice (Figure 4A, right). In control experiments with MPMs from apoE and P-selectin double-knockout (apoE−/−P-sel−/−) mice, no positive P-selectin labeling was observed (Figure 4A, right). RT-PCR analysis confirmed P-selectin mRNA expression in the MPMs from apoE−/− mice (Figure 4B, lane 2). Because macrophages accumulated in arterial wall are derived from circulating monocytes, we have further determined whether PBMDMs and bone marrow-derived macrophages (BMDMs) from apoE−/− mice can synthesize P-selectin. RT-PCR analysis showed that P-selectin mRNA was easily detectable in the BMDMs (Figure 4B, lane 3) and PBMDMs (Figure 4B, lane 4) after 7 and 10 days in culture, respectively, but not detectable in the peripheral blood monocytes (Figure 4B, lane 5). These results provide strong evidence that macrophages during differentiation, possibly in the wall of atherosclerotic arteries, acquire the ability to express P-selectin.
Figure 4.
A: Flow cytometry analysis of P-selectin expression in MPMs. MPMs were prepared from P-selectin wild-type apoE−/− mice and P-selectin-null apoE/P-selectin double-knockout mice. After 3 days in culture, the MPM population (left) from apoE−/− mice was identified with a rat anti-mouse Mac-3 monoclonal antibody (shaded area) or with an isotype control rat IgG1 (thin line). P-selectin expression (right) in MPMs was assessed with a rat anti-mouse P-selectin antibody (RB40.34) or with an isotype control rat IgG1. Macrophages from apoE−/− mice showed positive P-selectin labeling (right, shaded area) but cells from P-selectin-null mice showed negative labeling (right, thin line overlapped with the isotype control labeling). B: RT-PCR analysis of P-selectin expression in MPMs, BMDMs, and PBMDMs. P-selectin mRNA was readily detectable in primary cultures of MPMs (lane 2), BMDM (lane 3), and PBMDMs (lane 4) derived from apoE−/− mice after 3, 7, and 10 days in culture, respectively, but was not detectable in monocytes prepared from peripheral blood (lane 5). Sample of mouse SVEC4–10 endothelial cells was used as a positive control (lane 1).
Discussion
P-selectin expression has been reported in platelets, endothelial cells, and vascular SMCs in response to vascular injury, but not in macrophages within injured or diseased arterial walls. Macrophages are abundantly present in spontaneous atherosclerotic plaques and neointimal lesions after arterial injury. Using the apoE−/− mouse carotid denudation injury model, both the present study and our previous reports8,9 have shown that the percentage of Mac-2-positive macrophages in neointimal lesions was appropriately 20% at 28 days after injury. The present study tested the hypothesis that arterial macrophages within vascular injury-induced neointimal lesions of carotid arteries and spontaneous atherosclerotic plaques in the innominate arteries of apoE−/− mice can express P-selectin. We report that P-selectin is expressed by regenerating endothelium and macrophages that are recruited after arterial injury and to sites of spontaneous atherosclerosis. These novel findings may help to extend our understanding of the role of the adhesion molecule P-selectin in atherosclerotic vascular disease, extending beyond its role in the early steps of leukocyte recruitment.
P-selectin expressed by activated platelets and endothelial cells plays a pivotal role in mediating the early steps of leukocyte (particularly monocyte) recruitment in inflammation and vascular diseases.3 Platelet P-selectin has been shown to play a critical role in the development of atherosclerosis and neointima formation after vascular injury.7–12 We and others have demonstrated that circulating platelet-derived P-selectin facilitates spontaneous atherosclerosis development in apoE−/− mice.7,23 In the response to vascular injury, circulating platelets rapidly adhere to sites of endothelial denudation and may trigger a cascade of early inflammatory events and subsequent neointimal growth after arterial injury. For example, Smyth and colleagues12 demonstrated that in P-selectin knockout mice platelets were able to adhere to the wire-injured femoral artery wall but were severely impaired in their ability to recruit leukocytes 1 hour after injury. These findings support a critical role of platelet P-selectin in leukocyte recruitment particularly in the early phase after vascular injury.
Previous studies have demonstrated expression of P-selectin by vascular SMCs in the response to vascular injury. Kumar and colleagues11 reported that P-selectin staining was absent in the P-selectin knockout mouse carotid section but intense in the media and developing neointima of normal mice 7 days after carotid ligation injury. Recently, using double immunostaining and PCR analysis of mRNA expression, Zeiffer and colleagues21 verified expression of P-selectin in vascular SMCs in the wire-injured carotid arteries of apoE−/− mice 14 days after injury. Consistent with those findings, the present study demonstrated co-localization of P-selectin with vascular SMCs in the wire-injured carotid arteries of apoE−/− mice at 7 days and 14 days, with much weaker staining at 28 days after carotid injury. Furthermore, the present study demonstrated sparse co-localization of P-selectin with vascular SMCs within either advanced atherosclerotic plaques or the media of innominate arteries. Together with the fact that P-selectin staining was almost absent in the media of noninjured carotid arteries, we speculate that vascular SMCs, when in a proliferating or activated state, acquire a proinflammatory phenotype and the ability to transiently express P-selectin in the response to acute vascular injury. To date, the exact role of vascular SMC-derived P-selectin in the response to vascular injury has not been elucidated. In vitro laminar flow assays suggest a proadhesive role of vascular SMC-derived P-selectin in adhesion/arrest of monocytes after endothelial denudation injury.21
Intriguingly, the present study provides novel findings indicating that P-selectin is also expressed by regenerating endothelium and macrophages that are recruited after arterial injury. We speculate that P-selectin expressed on regenerating endothelium, via interaction with its ligand PSGL-1 expressed on leukocytes and platelets, may mediate the chronic interactions of leukocytes and platelets with the diseased/injured arterial wall in the development of atherosclerosis and the response to vascular injury. The potential importance and molecular mechanisms involved in the ability of arterial macrophages to express P-selectin in atherosclerosis and vascular injury remains to be investigated. Macrophage expression of P-selectin may modulate, in either an autocrine or paracrine manner, a number of cellular functions including 1) macrophage production of proinflammatory mediators; 2) macrophage production of reactive oxygen species/NADPH oxidases; 3) macrophage differentiation into foam cells; and 4) growth, apoptosis, and inflammatory activation of vascular SMCs and endothelial cells. In future studies, we propose to define the functional importance of macrophage P-selectin signaling in inflammation and vascular disease, by generating macrophage-specific P-selectin knockout mice using the Cre-loxP recombination system, together with other approaches, such as small RNA interference and bone marrow transplantation techniques.
In conclusion, the present study for the first time demonstrates that P-selectin is expressed by macrophages and regenerating endothelium in neointimal lesions of carotid arteries after denudation injury and in spontaneous atherosclerotic plaques of innominate arteries in atherosclerosis-prone, hyperlipidemic apoE−/− mice. These novel findings suggest that expression of P-selectin by these cell types may play an important role in the response to vascular injury, particularly in the late phase after injury, and the chronic proinflammatory state of atherosclerosis.
Footnotes
Address reprint requests to Ian J. Sarembock, M.D., Cardiovascular Division, University of Virginia Health System, Box 800158, Charlottesville, VA 22908-0158. E-mail: ijs4s@virginia.edu.
Supported by the National Institutes of Health (grants HL-66264 to I.J.S. and HL-58108 to K.L.) and by the American Heart Association (grant 0530166N to G.L.).
References
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Andre P. P-selectin in haemostasis. Br J Haematol. 2004;126:298–306. doi: 10.1111/j.1365-2141.2004.05032.x. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- Manka D, Collins RG, Ley K, Beaudet AL, Sarembock IJ. Absence of P-selectin, but not intercellular adhesion molecule-1, attenuates neointimal growth after arterial injury in apolipoprotein E-deficient mice. Circulation. 2001;103:1000–1005. doi: 10.1161/01.cir.103.7.1000. [DOI] [PubMed] [Google Scholar]
- Phillips JW, Barringhaus KG, Sanders JM, Hesselbacher SE, Czarnik AC, Manka D, Vestweber D, Ley K, Sarembock IJ. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107:2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- Manka D, Forlow SB, Sanders JM, Hurwitz D, Bennett DK, Green SA, Ley K, Sarembock IJ. Critical role of platelet P-selectin in the response to arterial injury in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1124–1129. doi: 10.1161/01.ATV.0000127619.04687.f4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Hoover JL, Simmons CA, Lindner V, Shebuski RJ. Remodeling and neointimal formation in the carotid artery of normal and P-selectin-deficient mice. Circulation. 1997;96:4333–4342. doi: 10.1161/01.cir.96.12.4333. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Reis ED, Zhang W, Fallon JT, Gordon RE, Coller BS. Beta(3)-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–2507. doi: 10.1161/01.cir.103.20.2501. [DOI] [PubMed] [Google Scholar]
- Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S35–S40. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- Vainio S, Ikonen E. Macrophage cholesterol transport: a critical player in foam cell formation. Ann Med. 2003;35:146–155. doi: 10.1080/07853890310008198. [DOI] [PubMed] [Google Scholar]
- Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadler JL, Funk CD, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- Hutter R, Valdiviezo C, Sauter BV, Savontaus M, Chereshnev I, Carrick FE, Bauriedel G, Luderitz B, Fallon JT, Fuster V, Badimon JJ. Caspase-3 and tissue factor expression in lipid-rich plaque macrophages: evidence for apoptosis as link between inflammation and atherothrombosis. Circulation. 2004;109:2001–2008. doi: 10.1161/01.CIR.0000125526.91945.AE. [DOI] [PubMed] [Google Scholar]
- Manka DR, Wiegman P, Din S, Sanders JM, Green SA, Gimple LW, Ragosta M, Powers ER, Ley K, Sarembock IJ. Arterial injury increases expression of inflammatory adhesion molecules in the carotid arteries of apolipoprotein-E-deficient mice. J Vasc Res. 1999;36:372–378. doi: 10.1159/000025676. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra SH, de Souza W, DaMatta RA. Toxoplasma gondii partially inhibits nitric oxide production of activated murine macrophages. Exp Parasitol. 2002;100:62–70. doi: 10.1006/expr.2001.4675. [DOI] [PubMed] [Google Scholar]
- Comalada M, Xaus J, Sanchez E, Valledor AF, Celada A. Macrophage colony-stimulating factor-, granulocyte-macrophage colony-stimulating factor-, or IL-3-dependent survival of macrophages, but not proliferation, requires the expression of p21(Waf1) through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2257–2267. doi: 10.1002/eji.200425110. [DOI] [PubMed] [Google Scholar]
- Zeiffer U, Schober A, Lietz M, Liehn EA, Erl W, Emans N, Yan ZQ, Weber C. Neointimal smooth muscle cells display a pro-inflammatory phenotype resulting in increased leukocyte recruitment mediated by P-selectin and chemokines. Circ Res. 2004;94:776–784. doi: 10.1161/01.RES.0000121105.72718.5C. [DOI] [PubMed] [Google Scholar]
- Tchernychev B, Furie B, Furie BC. Peritoneal macrophages express both P-selectin and PSGL-1. J Cell Biol. 2003;163:1145–1155. doi: 10.1083/jcb.200310079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]