Abstract
The acquisition of homing receptors that redirect lymphocyte trafficking to nonlymphoid tissues after antigen encounter is a fundamental aspect of effector T-cell development. Although a role for selectins and their ligands has been well characterized for trafficking of Th1 cells to nonlymphoid sites, mechanisms responsible for Th2 trafficking are not well understood. Using a flow chamber system in which the endothelial interactions of two distinct T-cell populations could be examined simultaneously, we directly compared the requirements for Th1 and Th2 cell tethering and rolling. We found that although Th2 cells expressed significantly lower levels of selectin ligands than Th1 cells, activation of the endothelium by Th2-derived factors induced rolling interactions that were comparable for both Th1 and Th2 populations. Further, in the absence of PSGL-1, no other adhesion molecule could effectively compensate for lack of PSGL-1 to mediate rolling of either Th1 or Th2 cells. Thus, both Th1 and Th2 populations express functional PSGL-1-based selectin ligands for tethering and rolling on activated endothelium, and both effector populations can use PSGL-1 as the dominant scaffold for functional selectin ligand expression.
The recognition of antigen by naïve T-cell precursors circulating through secondary lymphoid tissues initiates the development of effector T cells, which have enhanced functional properties adapted for eradication of pathogens. An important feature of effector T-cell development is the altered expression of surface molecules that redirects T cell trafficking to nonlymphoid tissues where pathogens may reside. Naïve T cells express L-selectin (CD62L) and CC chemokine receptor 7 (CCR7), which direct their homing to specialized high endothelial venules in secondary lymphoid tissues.1–6 Some developing effector T cells shed CD62L and express E- and P-selectin ligands, as well as distinct chemokine receptors and integrins, which direct their recruitment to nonlymphoid vascular beds.7–9 The major subsets of CD4+ effector T cells, Th1 and Th2 cells, differ in their profile of adhesion molecule and chemokine receptor expression as a possible mechanism for differential recruitment.10–16
The process of leukocyte recruitment is a multistep cascade that involves adhesion molecules and chemotactic factors and results in the emigration of a leukocyte from the vasculature into an adjacent tissue bed.7 For granulocytes and monocytes, this process is typically initiated through adhesive interactions between one of three selectin molecules (L-, E-, or P-selectin; CD62L, P, or E), and their respective ligands, or in some instances, via α4-integrin, which allows the leukocyte to tether to and roll on activated vascular endothelium.17–24 Through the process of rolling, the leukocyte surveys the endothelium for specific chemokines for which it expresses counterreceptors. On appropriate chemokine recognition, the leukocyte is signaled to activate integrins to mediate firm adhesion and ultimately transmigration across the endothelium.25–29 Lymphocytes use similar strategies for endothelial transmigration, whether at high endothelial venules of secondary lymphoid tissues or at activated endothelium in nonlymphoid tissues, although details of effector T cell trafficking are incompletely characterized.30,31
Naïve and effector T cells express differences in functional P-selectin glycoprotein ligand-1 (PSGL-1; CD162), a homodimeric sialoglycoprotein that is the major ligand for tethering and rolling on P-selectin, and an important ligand for E-selectin.10,11,32,33 In contrast to myeloid lineage cells (eg, neutrophils and monocytes), which constitutively express glycosyltransferases necessary for biosynthesis of selectin ligands, T cells express these enzymes only after effector T cell development. Thus, although PSGL-1 is highly expressed by both naïve and effector T cells, it is appropriately modified for selectin binding in effector T cells only.34–36 Importantly, Th1 and Th2 populations express different levels of α1,3-fucosyl transferase VII (FucT-VII), a key enzyme required for both P- and E-selectin ligand generation.37,38 Th1 cells express high levels of FucT-VII and therefore generate ligands for both P- and E-selectin molecules.10,34–36 In contrast, Th2 cells express low levels of FucT-VII, resulting in a reduction in fucosylated PSGL-1 and, consequently, P- and E-selectin ligand synthesis. Decreased expression of P- and E-selectin ligands on Th2 cells is thought to contribute, at least in part, to the deficiency of Th2 cells to traffic to nonlymphoid tissue sites where Th1 cells are readily recruited.10,11,39
Nevertheless, recent studies have demonstrated that both Th1 and Th2 populations can be recruited to the same tissue sites. In our own studies, it was shown that Th1 and Th2 cells localized to the intestinal mucosa in an antigen-specific model of colitis wherein adoptive transfers of both Th1- and Th2-polarized populations induced disease in recipient hosts.40 Similarly, in mouse models of asthma, Th1 and Th2 cells are recruited to airways of the lung after co-transfer and antigenic challenge.41,42 Based on the prevailing belief that Th2 cells are deficient in their interactions with P- and E-selectin molecules, and the absence of L-selectin expression by Th2 cells, it has been postulated that Th2 cells use selectin-independent mechanisms for rolling and recruitment to nonlymphoid tissues.41,43 Indeed, specific integrin molecules such as the α4-integrins, which have been shown to mediate rolling in vitro, are expressed by Th2 cells and could mediate Th2 recruitment, although this has not been directly shown.24,43
With a view toward identifying selectin-independent mechanisms for Th2 cell recruitment to nonlymphoid vascular beds, we have directly compared endothelial interactions of Th1 and Th2 cells in an in vitro flow chamber system and, in a parallel study, via intravital imaging of the intestinal lamina propria after adoptive transfers of Th1 and Th2 populations (see companion paper by Bonder et al44 in this issue). Effector CD4+ T cells were derived from wild-type and PSGL-1-deficient TCR transgenic mice to define the requirements of PSGL-1-dependent and -independent interactions. We find that although Th2 cells express lower levels of selectin ligands than Th1 cells, they nevertheless require PSGL-1 for tethering and rolling on inflamed endothelium. Further, under conditions of endothelial activation by Th2-derived factors, Th1 and Th2 populations demonstrate comparable rolling interactions. Thus, Th1 and Th2 cells require PSGL-1-based ligands for efficient tethering and rolling on activated endothelium and, in their absence, no other adhesion molecule can compensate.
Materials and Methods
Mice
DO11.10 TCR transgenic mice on a BALB/c background were bred in our specific pathogen-free facility and were screened at age 3 to 4 weeks for transgene expression by two-color flow cytometric analysis after staining of peripheral blood with anti-CD4 and the anti-clonotype mAb, KJ1-26. BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or bred in our facility. PSGL-1−/− mice on a C57BL/6 background (the kind gift of Dr. Barbara Furie, Harvard Medical School, Boston, MA) were backcrossed onto the BALB/c background then intercrossed with BALB/c DO11.10 TCR transgenic mice to generate PSGL-1-deficient DO11.10 mice (DO11.PSGL-1−/−). All mice were housed and treated according to National Institutes of Health guidelines under the auspices of the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Antibodies and Reagents
Purified blocking and biotin-conjugated anti-P-selectin (RB40.34) and anti-E-selectin (10E9.6), PerCP-labeled anti-CD4 (L3T4), phycoerythrin (PE)-labeled, anti-PSGL-1 (2PH1), anti-LFA-1 (2D7), anti-L-selectin (MEL-14), anti-VLA-4 (R1-2), and anti-ICAM-1 (3E2), and fluorescein isothiocyanate-labeled anti-interferon (IFN)-γ (XMG1.2) and anti-CD44 (Pgp-1) monoclonal antibodies (mAbs), allophycocyanin-conjugated streptavidin, and biotin-conjugated anti-Mac-1 (M1/70), anti-VCAM-1 (MVCAM.A), and anti-MAdCAM-1 (MECA-89) mAbs were purchased from PharMingen (San Diego, CA). Anti-CD3 (145-2C11), neutralizing anti-interleukin (IL)-12 (C17.8), and neutralizing and allophycocyanin-labeled anti-IL-4 (11B11) mAbs were purified from ascites by Dr. Roger Lallone (Brookwood Biotech, Birmingham, AL). P- and E-selectin human IgM fusion protein was kindly provided by Dr. John Lowe (University of Michigan, Ann Arbor, MI). PE-labeled anti-human IgM and recombinant mouse GM-CSF was purchased from Sigma (St. Louis, MO). Recombinant mouse IL-12, IL-4, IL-2, IL-5, IL-13, and tumor necrosis factor (TNF)-α were purchased from R&D Systems Inc. (Minneapolis, MN). Calcein, AM, and Cell Trace calcein red-orange, AM were obtained from Molecular Probes (Eugene, OR). The Cytofix/Cytoperm Plus kit with Golgi Stop (monensin) was purchased from PharMingen.
Endothelial Cell Lines
Primary murine aortic endothelial cell (MAEC) lines were generated as previously described,45 and were passaged into flasks or plates coated with 1% gelatin (Sigma). The MAEC line was maintained in MCDB 131 media supplemented with 10% fetal calf serum, 10 mmol/L glutamine, 100 μg/ml endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA), 0.225% sodium bicarbonate, 1 μg/ml hydrocortisone (Sigma), 10 U/ml heparin sodium, 100 μg/ml penicillin, and 100 μg/ml streptomycin. The bEnd.3 cell line46 was purchased from American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin.
Generation of Th1 and Th2 Cells
Th1 and Th2 populations were polarized in vitro from antigen-naïve CD4+ T cells isolated from DO11.10 or DO11.10.PSGL-1−/− mice. CD4+ T cells were purified from pooled spleen and lymph nodes by magnetic sorting using mouse anti-CD4 beads (Dynal-ASA, Oslo, Norway), with routine purities greater than 95% CD4+. Cells were plated at a ratio of 1:6 with irradiated (3000 rads) BALB/c splenocytes and 5 μg/ml OVA peptide 323-339 (OVAp). The addition of 50 U/ml rmIL-12 and 10 μg/ml anti-IL-4 (11B11) was used to generate Th1 populations, whereas Th2 cells were produced by adding 2000 U/ml rmIL-4, and 10 μg/ml of both anti-IL-12 (C17.8) and anti-IFN-γ (XMG1.2). Three days after initiation, Th1 and Th2 cultures were split 1:2 and 1:4, respectively, and supplemented with 50 U/ml rmIL-2. Th1 cells were harvested for experiments after 7 days. To ensure adequate polarization of Th2 populations, cells cultured for 7 days in Th2-polarizing conditions were polarized a second time, as before, with fresh irradiated BALB/c splenocytes, 5 μg/ml OVAp, 2000 U/ml rmIL-4, and 10 μg/ml of both anti-IL-12 and anti-IFN-γ. Conditioned medium was prepared from Th2 cells that were stimulated with plate-bound anti-CD3 for 72 hours. T cells were pelleted and the cleared supernatant was used as an endothelial cell stimulant (Th2 supernatant, or Th2 sup).
Flow Cytometric Analysis
Th1 and Th2 cells were stimulated for 6 hours with 50 ng/ml of PMA and 750 ng/ml of ionomycin or not at all. After 2 hours monensin was added to block cytokine secretion. Th1 and Th2 cells were stained with P- and E-selectin human IgM (hIgM) chimeras and PerCP-labeled anti-CD4 mAb. Staining was performed in Dulbecco’s modified Eagle’s medium supplemented with 0.1% bovine serum albumin, 0.1% sodium azide, with or without 5 mmol/L ethylenediamine tetraacetic acid (EDTA). Detection of the human IgM chimeras was achieved through secondary antibody staining with PE-conjugated anti-human IgM (Sigma). T cells were fixed and permeabilized with Cytofix Cytoperm and stained intracellularly with allophycocyanin-conjugated anti-IL-4 (11B11) and fluorescein isothiocyanate-conjugated IFN-γ. A separate aliquot of Th1 and Th2 cells was surface stained in phosphate-buffered saline supplemented with 1% bovine serum albumin and 0.2% sodium azide for 30 minutes on ice with PE-labeled anti-LFA-1, anti-L-selectin, anti-VLA-4, anti-PSGL-1, fluorescein isothiocyanate-conjugated anti-CD44 or biotin-conjugated anti-Mac-1. PE-conjugated streptavidin (PharMingen) was used to detect biotin-conjugated antibodies.
MAECs or bEnd.3 endothelial cells were activated with indicated stimuli (eg, TNF-α) or not at all before staining for flow cytometry. Endothelial cells were trypsinized for <5 minutes in 0.25% trypsin, 0.1% EDTA. Recovered endothelial cells were stained with biotin-conjugated anti-P-selectin, anti-E-selectin, anti-VCAM-1, anti-MAdCAM-1 and PE-labeled anti-ICAM-1. Allophycocyanin-labeled streptavidin was used to detect biotin-conjugated antibodies. All flow cytometric acquisition was performed on a FACSCalibur system and data analysis was conducted using CellQuest computer software (BD Biosciences, San Jose, CA).
In Vitro Flow Experiments and Analysis
Polarized Th1 and Th2 cells were harvested, and labeled with 2 μmol/L calcein, AM or Cell Trace calcein red-orange, AM, in serum-free Hanks’ balanced salt solution for 30 minutes at 37°C. This dye loading procedure was optimized to label cells with sufficient signal without interfering with the quality of rolling interactions (data not shown). T cells were washed and resuspended at 1 × 106 cells/ml in serum and phenol red-free Dulbecco’s modified Eagle’s medium supplemented with 25 mmol/L HEPES. The two labeled cell populations were combined at a 1:1 ratio. For flow experiments, a GlycoTech (Rockville, MD) flow chamber and gasket were inserted into a culture dish coated with MAECs or bEnd.3 cells to form a laminar flow chamber that could be viewed on a microscope. T cells were injected into the flow chamber at a shear stress of 0.5 to 1.5 dynes/cm2 using a programmable syringe pump (KD Scientific, New Hope, PA). A heated stage (designed and constructed by D.F.K.) was used to maintain the chamber temperature at 37°C. Flow chambers were viewed and images recorded with an Axiovert 100 microscope (Zeiss, Thornwood, NY) equipped with a color camera (JAI Corporation, Yokohama, Japan). Both bright-field and fluorescent images were collected.
A total of 3 minutes of video footage from four different fields of view was recorded. Images were digitized at high resolution (uncompressed, full screen, 720 × 486 pixel images captured at 30 images per second) using the Leitch Reality Digital Disk Recorder System (Leitch Technology Corp., Florence, KY). Color images were separated into RGB components using Metamorph software (Universal Imaging, Downingtown, PA) to produce a separate video for red- and green-labeled cells. Cell position measurements in each frame were determined using Videolab software (Ed Marcus Labs, Newton, MA).
Instantaneous velocities for each cell were determined using RS-1 programs designed by D.F.K. Cells that had instantaneous velocities of ≤50 μm/second were automatically selected and the motion of each cell was validated manually. To identify rolling cells, the critical velocity (Vcrit) was calculated as previously described.47 To calculate average rolling velocity, the total distance that a cell rolled was measured and divided by the total time during which the cell demonstrated rolling. Because the rolling behavior of cells was often discontinuous (representative high temporal resolution velocity plots generated from the motion of individual Th1 and Th2 cells are included in supplemental data (supplemental Figure S1 at http://www.amjpathol.org), a total rolling time, defined as the cumulative time during which cells had an instantaneous velocity of ≤50 μm/second, was automatically calculated for each rolling cell by the RS-1 program. Cells that did not roll more than one cell diameter in ≥5 seconds were considered firmly adherent and excluded from rolling time data. Less than 20% of cells that interacted on stimulated endothelium were firmly adherent.
Statistical Analyses
All statistical calculations were performed using Prism software (GraphPad, San Diego, CA). The number of interacting cells per 10 minutes and SD for at least four randomly selected fields of view were calculated for each experimental condition. From the same four fields of view in each experiment the average rolling velocity and SD of rolling cells was calculated. Statistical significance between experimental conditions was determined using an unpaired Student’s t-test. For rolling time comparisons, the median rolling time for all interacting cells in at least four randomly selected fields of view that were not firmly adherent was calculated, and statistical significance determined using a Mann-Whitney test. P values <0.05 were considered significant.
Results
Characterization of Wild-Type and PSGL-1−/− Th1 and Th2 Populations
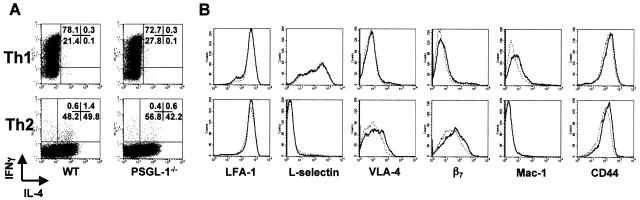
Effector T cells were generated from antigen-naïve CD4+ T cells derived from wild-type or PSGL-1−/− DO11.10 transgenic mice by stimulation with OVAp in the presence of IL-12 and anti-IL-4 antibody (Th1), or IL-4, anti-IL-12, and anti-IFN-γ antibodies (Th2).40 After 7 days in culture, Th1 cells were collected for experiments. To ensure a well-polarized population, Th2 cells were generated by two rounds of identical stimulation, and collected for experiments on day 14. Th1 and Th2 populations derived from naïve DO11.10 or DO11 PSGL-1−/− precursors demonstrated comparable cytokine phenotypes (Figure 1A). The frequencies of IFN-γ-expressing CD4+ T cells in wild-type and PSGL-1-deficient Th1 cultures were typically between 70 to 90% of total, with no detectable IL-4-expressing cells. The frequencies of IL-4-expressing cells in wild-type and PSGL-1−/− Th2-polarized populations were typically between 40% and 60% of total (Figure 1A); modest numbers of IFN-γ-expressing cells (less than 2%) were present in both wild-type and PSGL-1-deficient Th2 populations.
Figure 1.
Characterization of Th1 and Th2 cells from wild-type and PSGL-1-deficient DO11.10 TCR transgenic mice. Naïve CD4+ T cells from wild-type or PSGL-1-deficient DO11.10 mice were cultured with irradiated BALB/c splenocytes and OVAp for 7 days (Th1) or 14 days (Th2) under Th1 (rmIL-12 and anti-IL-4) or Th2 (rmIL-4, anti-IL-12, and anti-IFN-γ) polarizing conditions. A: For detection of intracellular cytokine expression 1 × 106 cells per condition were restimulated with PMA and ionomycin for 6 hours. Monensin was added for the final 4 hours to block cytokine secretion. T cells were fixed, permeabilized, and stained intracellularly for IFN-γ and IL-4. The frequency of IFN-γ+ and IL-4+ cells was assessed by flow cytometry on the lymphoid gate. Quadrant percentiles of lymphocyte-gated cells are indicated. B: For detection of surface phenotype, polarized Th1 and Th2 cells derived from wild-type (solid line) and PSGL-1-deficient (dotted line) DO11.10 mice were stained with monoclonal antibodies for the indicated adhesion molecules and analyzed by flow cytometry. Plots represent populations in the lymphocyte gate (30,000 events).
The surface expression profiles of wild-type and PSGL-1-deficient Th1 and Th2 populations were analyzed by flow cytometry to compare adhesion molecule expression between genotypes and T-effector lineages (Figure 1B). Wild-type and PSGL-1-deficient T cells polarized to a Th1 phenotype expressed comparable levels of LFA-1, L-selectin, VLA-4, β7, Mac-1, and CD44. Similarly, Th2 populations derived from either wild-type or PSGL-1-deficient DO11.10 cells had virtually identical expression patterns of the adhesion molecules tested. Notably, Th1 cells of either genotype included a sizeable fraction that retained or re-expressed L-selectin after polarization, and expressed slightly higher levels of Mac-1 than Th2 cells. Wild-type and PSGL-1-deficient Th2 cells expressed higher levels of both VLA-4 (α4β1 integrin) and β7 integrin compared to Th1 populations. Thus, although there were clear differences in the surface expression profiles between Th1 and Th2 populations, deficiency of PSGL-1 had no detectable effect on the cytokine or surface phenotype of either Th1 or Th2 populations.
Th1 and Th2 Cells Use PSGL-1 for Rolling on TNF-α-Stimulated Endothelium
To assess the importance of differentially expressed molecules for Th1 and Th2 rolling, the interactions of polarized T-cell populations with primary murine endothelial cells (MAECs) were observed under laminar flow. To permit direct comparisons, Th1 and Th2 cells were labeled with distinct fluorescent dyes, mixed at a 1:1 ratio and flowed together over nonstimulated MAECs or MAECs stimulated for 6 hours with TNF-α (Figure 2A, and supplemental videos SV-1 and SV-2 at http://www.amjpathol.org). Video footage was analyzed and the data outputted as position and instantaneous velocity plots (supplemental Figure S1 at http://www.amjpathol.org), from which the number of rolling cells and average rolling velocity were determined. Owing to the high temporal resolution of the analytical system used for these studies, we observed that a significant number of Th1 and Th2 cells rolled only transiently before being released, or were captured and rolled repetitively. Because of the intermittent nature of this type of rolling behavior, we found it less accurate to calculate rolling velocities as compared to a total rolling time that reflected the cumulative time that cells rolled on the endothelium, exclusive of periods when they were released before recapture (see Materials and Methods). This level of analysis provided an additional parameter by which the rolling interactions of polarized T cells could be compared.
Figure 2.
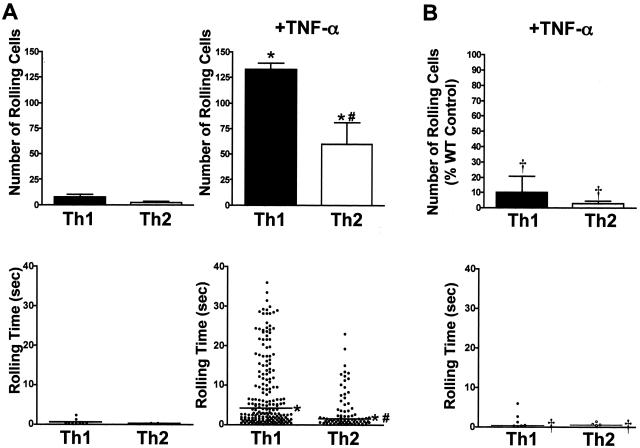
TNF-α-stimulated MAECs support rolling of Th1 and Th2 cells via a PSGL-1-dependent mechanism. A: Th1- and Th2-polarized cells derived from DO11.10 mice were labeled with 2 μmol/L Cell Trace calcein red-orange, AM (Th1), or calcein, AM (Th2), and resuspended at a concentration of 1 × 106 cells/ml. T cells were combined at a 1:1 ratio and flowed over MAECs with a shear stress of 0.5 dynes/cm2. MAECs were stimulated for 6 hours with nothing (left) or 10 ng/ml of TNF-α (right). VideoLab software was used to digitize and track 3-minute sequences of videotape and an RS-1 program was used to determine the rolling time of each cell (see Materials and Methods). The top panels indicate the mean number of rolling cells from 10 minutes of footage taken from four randomly selected fields of view in one of four representative experiments. Error bars indicate SEM. The bottom panel shows pooled rolling times of interacting cells from four fields of view for each condition. #Indicates statistical significance between T-cell subtypes. *Indicates statistical significance between methods of endothelial cell stimulation. P < 0.05. B: Th1- and Th2-polarized cells were derived from DO11.PSGL-1−/− mice as in A, and rolled on TNF-α-stimulated MAECs as above. Data in the top panel are expressed as the percentage of rolling wild-type (DO11.PSGL-1+/+) control cells. Error bars indicate SEM. †Indicates statistical significance between wild-type and PSGL-1-deficient cells (P < 0.05).
On average, less than three Th1 cells and less than one Th2 cell per field of view rolled on nonstimulated MAECs. The median rolling times of Th1 or Th2 cells on nonstimulated MAECs were transient and did not exceed 0.2 seconds. As expected, TNF-α stimulation of the endothelium significantly increased the number of interacting Th1 cells (on average, 47 cells per field of view), but it also induced significant Th2 interactions (on average, 18 cells per field of view). The median rolling time was increased to 4.2 seconds for Th1 and 1.5 seconds for Th2 cells. In supplemental Figure S2 (at http://www.amjpathol.org) and data not shown, naïve CD4+ T cells demonstrated no significant interactions with either nonstimulated or TNF-α-stimulated endothelium compared with polarized Th1 or Th2 cells. Thus, there were significant interactions of both Th1 and Th2 effector populations with TNF-α-induced endothelium, although the number of rolling cells and their median rolling time were significantly less for Th2 than Th1 cells.
To determine whether Th2 cells use selectin-independent interactions for rolling, and to define the contribution of PSGL-1 in Th1 interactions, the behavior of Th1 and Th2 cells derived from DO11.PSGL-1 mice was compared to wild-type DO11.10-derived populations on TNF-α-stimulated endothelium. To allow direct comparisons between PSGL-1−/− and wild-type cells, differentially labeled populations of the same effector phenotype were compared simultaneously. Surprisingly, both Th1 and Th2 cells demonstrated a striking dependence on PSGL-1 expression for interactions with TNF-α-stimulated MAECs (Figure 2B, and supplemental video SV-2 at http://www.amjpathol.org). PSGL-1-deficient Th1 and Th2 populations showed >80% reduction in the number of rolling cells compared to wild type, and the duration of total rolling times was similarly reduced. Notably, although both wild-type and PSGL-1-deficient Th2 cells expressed comparable levels of β7 integrin (Figure 1B), there was a strict dependence on PSGL-1 expression for endothelial interactions. Also, although wild-type and PSGL-1-deficient Th1 cells expressed significant levels of L-selectin, this did not appear to play a significant role in their interactions, from which it can be inferred that expression of L-selectin ligands by MAECs was absent or noncontributory.
Stimulation of Primary Endothelium by Th2-Derived Factors Induces PSGL-1-Dependent Rolling of Th1 and Th2 Cells
Although the marked diminution of both Th1 and Th2 rolling seen in the absence of PSGL-1 established an important role for selectin-/PSGL-1-dependent recruitment of both effector populations, the diminished interaction of Th2 cells relative to Th1 cells raised the possibility that TNF-α stimulation of the endothelium was not optimal for Th2 recruitment. Further, because TNF-α is produced at higher levels by activated Th1 cells, and its local production by infiltrating Th1 cells might augment Th1 recruitment, we speculated that Th2 cells might also produce factors that could mediate their own recruitment. We therefore examined the effects of Th2-derived factors on activation of MAECs for Th2 recruitment.
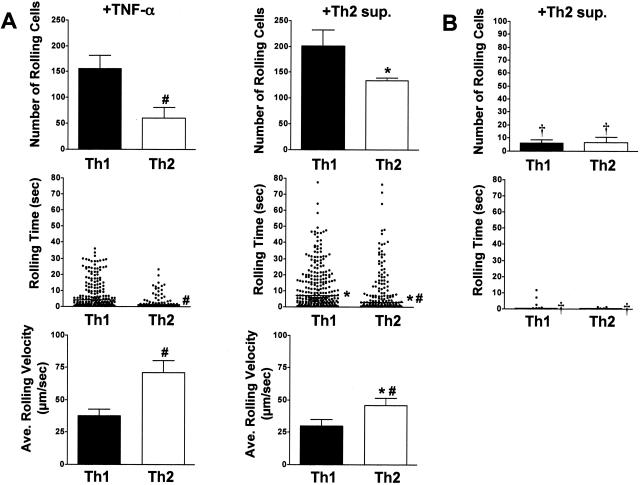
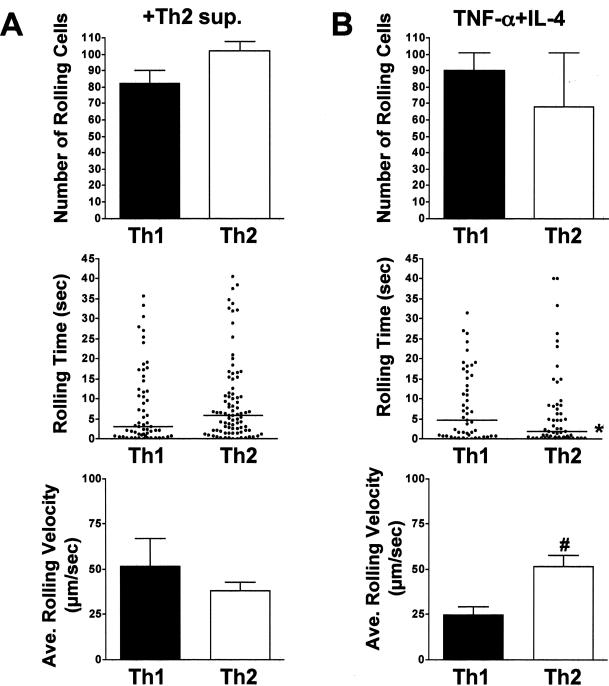
Supernatant from cultures of Th2 cells activated for 72 hours by plate-bound anti-CD3 antibody (Th2 sup) was used to stimulate MAECs, and effector T cell interactions were compared between TNF-α- and Th2-sup-induced endothelium. Wild-type and PSGL-1-deficient Th1 and Th2 populations were again compared to explore a possible role for selectin-independent recruitment. As shown in Figure 3A (and supplemental video SV-3 at http://www.amjpathol.org), the number of rolling wild-type Th2 cells was increased approximately twofold on MAECs stimulated with Th2 supernatant as compared with TNF-α stimulation. Notably, the number of wild-type Th1 cells that rolled were comparable to that induced by TNF-α. Moreover, the number of rolling Th2 cells increased to a level that was not statistically different from that of Th1 cells. In supplemental Figure S3A (at http://www.amjpathol.org) and data not shown, neither the number of rolling Th2 cells nor the rolling times was affected whether the cells were kept in polarizing conditions for 7 or 14 days. Interestingly, the median rolling time of both Th1 and Th2 populations on Th2 sup-activated endothelium was significantly prolonged compared to TNF-α-stimulated MAECs. The median rolling time for Th1 cells was increased from 4.2 seconds to 7.1 seconds and for Th2 cells from 1.5 seconds to 2.9 seconds. Despite the enhanced number of rolling cells and rolling times of Th2 cells on Th2-sup-induced MAECs, the median rolling time for Th2 cells remained less than that of Th1 cells on both TNF-α- and Th2-supernatant-stimulated MAECs. The average rolling velocity of Th2 cells was reduced more than 1.5-fold from 70.9 μm/second to 46.1 μm/second on Th2 sup-stimulated MAECs compared to TNF-α-stimulated endothelial cells. The reduction of Th2 rolling resulted in velocities that approached those of Th1 cells (30.0 μm/second) rolling under the same conditions. Importantly, the enhanced Th1 and Th2 interactions induced on endothelium stimulated with Th2-derived factors remained PSGL-1-dependent (Figure 3B). Thus, the enhanced recruitment of Th2 interactions elicited by endothelial stimulation by Th2-derived factors was inhibited to 3% of the wild-type control in the absence of PSGL-1. Th1 cells showed a comparable rolling deficit in the absence of PSGL-1 (10% of wild-type control). This suggested that enhanced Th2 recruitment stimulated by Th2-derived factors must act through a selectin-dependent mechanism.
Figure 3.
Activated Th2 cells produce soluble factors that stimulate enhanced recruitment of Th1 and Th2 cell rolling on MAECs. A: Th1- and Th2-polarized cells were derived from wild-type DO11.10 mice as in Figure 2, labeled with 2 μmol/L Cell Trace calcein red-orange, AM (Th1), or calcein, AM (Th2), mixed at a 1:1 ratio and flowed over MAECs with a shear stress of 0.5 dynes/cm2. MAECs were stimulated for 6 hours with Th2 supernatant. Data collection and analyses were performed as in Figure 2, and are from one of two representative experiments. Error bars indicate SEM. In the middle panel, rolling time data are pooled from four fields of view for each condition. Data in the bottom panel represent the average rolling velocity and SEM from four fields of view. #Indicates statistical significance between T-cell subtypes. *Indicates statistical significance between methods of endothelial cell stimulation. P < 0.05. B: Th1- and Th2-polarized cells were derived from DO11.PSGL-1−/− mice as in A, and rolled on Th2 supernatant-stimulated MAECs as above. Data in the top panel are expressed as the percentage of rolling wild-type (DO11.PSGL-1+/+) control cells. Error bars indicate SEM. †Indicates statistical significance between genotypes (P < 0.05).
Both Th1 and Th2 Populations Express Selectin Ligands
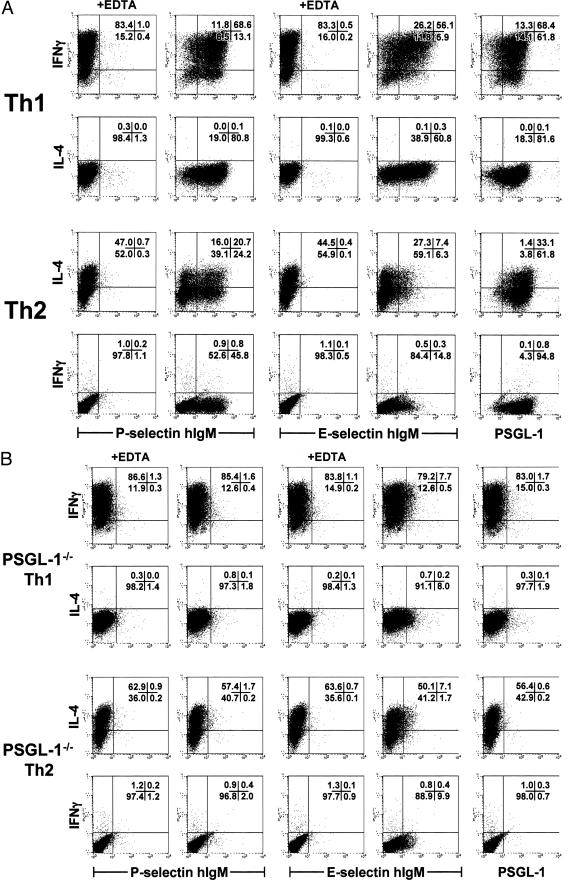
Because the PSGL-1 dependence of Th2 interactions implicated functional selectin ligand expression by Th2 cells, we directly examined Th1 and Th2 populations for the expression of P- and E-selectin ligands by flow cytometry (Figure 4). Simultaneous analysis for binding of P- and E-selectin-hIgM fusion proteins and intracellular cytokines permitted correlation of selectin ligand expression with effector cytokine production at the single-cell level. Nearly all wild-type Th1 and Th2 cells expressed PSGL-1 (Figure 4A). Of the IFN-γ-positive Th1 cells, 85.3% bound P-selectin and 68.2% bound E-selectin-hIgM compared to the EDTA-treated control. The fraction of IFN-γ-negative Th1 cells that expressed P-selectin ligand was slightly less (66.8%), but even fewer of the IFN-γ-negative Th1 cells expressed E-selectin ligand (33.3%). Strikingly, more than 56% of IL-4-producing Th2 cells bound P-selectin, whereas E-selectin binding by Th2 cells was ∼21%. Interestingly, although successive rounds of Th2 polarization increased the frequency of IL-4-producing cells from 30 to 60%, the percentage of P- and E-selectin-binding cells did not change under extended polarization conditions (supplemental data in Figure S3B at http://www.amjpathol.org). Through a similar evaluation of polarized Th1 and Th2 populations from DO11.PSGL-1−/− mice it was shown that binding of effector cytokine-secreting Th1 and Th2 cells to P- and E-selectin fusion protein was primarily PSGL-1-dependent (Figure 4B). In comparison to the EDTA control, less than 2% of IFN-γ-producing Th1 cells and IL-4-producing Th2 cells bound P-selectin IgM. In contrast, 9 to 12% of both effector cell populations expressed E-selectin ligand in the absence of PSGL-1. These data confirm that both Th1 and Th2 cells express P-selectin ligand, which is linked to the PSGL-1 scaffold. Moreover, whereas expression of E-selectin ligand by Th1 cells is primarily dependent on the PSGL-1 backbone, E-selectin ligand expression by Th2 cells is for the most part PSGL-1-independent.
Figure 4.
Analysis of P- and E-selectin ligand expression on wild-type and PSGL-1−/− Th1 and Th2 effector populations. Th1 or Th2 cells were derived from DO11.10 (A) or DO11.PSGL-1−/− (B) mice as in Figure 1 and restimulated with PMA and ionomycin for 6 hours. Monensin was added for the final 4 hours to stop cytokine secretion. T cells were surface-stained with PSGL-1 monoclonal antibody or P- or E-selectin human IgM chimeras. Five mmol/L EDTA was included in the staining media where indicated as a negative control for selectin binding. After surface staining, the cells were fixed, permeabilized, and stained intracellularly for IFN-γ and IL-4. The frequency of IFN-γ+ and IL-4+ cells that were PSGL-1+ and bound P- and E-selectin hIgM was assessed by flow cytometry on the lymphoid gate. Quadrant percentiles of lymphocyte-gated cells are indicated.
Th2-Derived Cytokines Enhance P-Selectin Expression by Endothelium as a Possible Mechanism for Enhanced Th2 Recruitment
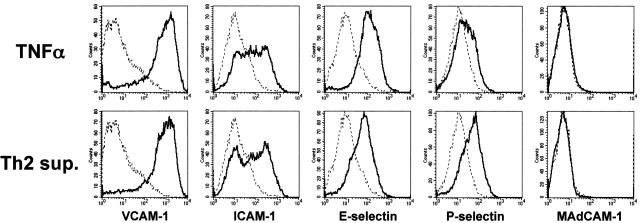
The foregoing experiments indicated that both Th1 and Th2 cells required PSGL-1-linked selectin ligands for rolling on activated endothelium. Because Th2-derived factors significantly enhanced Th2 rolling through a PSGL-1-dependent mechanism, it was inferred that Th2-derived factors acted either to enhance expression of P- or E-selectin by the endothelium, and/or induced expression of another adhesion component that could cooperate with selectin ligands to enhance Th2 rolling. In initial studies to directly evaluate the expression of selectins on MAECs, we found that expression levels were difficult to quantify by flow cytometry, whether stimulated with TNF-α or Th2 supernatants or not, despite functional expression of these molecules (data not shown).46 We therefore evaluated an independent murine endothelial cell line, bEnd.3, for which inducible selectin expression was more readily quantifiable. As shown in Figure 5, 6 hours of stimulation with TNF-α increased expression of VCAM-1, ICAM-1, P-selectin, and E-selectin compared to unstimulated bEnd.3 cells. Although no detectable levels of MAdCAM-1 were expressed after 6 hours, low levels of MAdCAM-1 were evident after 18 hours of TNF-α stimulation, as previously reported (data not shown).46 Activation of bEnd.3 cells with Th2 supernatant induced levels of VCAM-1, ICAM-1, E-selectin, and MAdCAM-1 comparable to that of TNF-α. However, P-selectin expression levels were increased approximately twofold more than those induced by TNF-α stimulation (mean fluorescent intensities: 33.5, TNF-α alone; 62.4, Th2 supernatant).
Figure 5.
TNF-α and Th2-derived factors induce adhesion molecule expression on bEnd.3 cells. Confluent bEnd.3 cells were either left unstimulated (dotted line) or stimulated (solid line) for 6 hours with TNF-α (10 ng/ml) or Th2 supernatant prepared as described in Materials and Methods. Endothelial cells were briefly trypsinized (<5minutes), stained with monoclonal antibodies for the indicated adhesion molecules, and analyzed by flow cytometry. Histograms represent 30,000 events.
Figure 6.
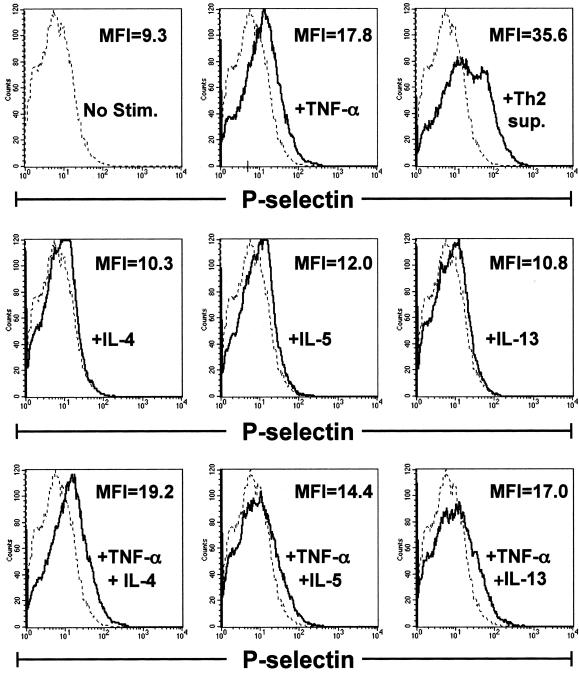
Th2-derived cytokines do not induce enhanced endothelial cell expression of P-selectin above that induced by TNF-α alone. Confluent bEnd.3 cells were stimulated for 6 hours with nothing (dotted line), Th2 sup, TNF-α (10 ng/ml), IL-4 (20 ng/ml), IL-5 (10 ng/ml), IL-13 (20 ng/ml), or the indicated combinations of cytokines (solid lines). Endothelial cells were recovered, stained with monoclonal antibodies for P-selectin, and analyzed by flow cytometry. In overlay histograms, the mean fluorescence intensity (MFI) for the stimulated condition is given. The MFI of unstimulated bEnd.3 cells = 9.3. Histograms represent 30,000 events.
In agreement with previous studies using MAECs, Th2 rolling on bEnd.3 cells was significantly enhanced by pretreatment with Th2-derived factors, and was comparable to rolling of Th1 cells (see supplemental data, Figure S4A at http://www.amjpathol.org). In accord with a requirement for P-selectin by Th2 cells, PSGL-1 deficiency on Th2 cells reversed their enhanced rolling of Th2 on endothelium activated by Th2 supernatant (supplemental data, Figure S4B at http://www.amjpathol.org).
In an effort to define the Th2-derived factor(s) responsible for enhanced Th2 rolling, we initially examined the induction of expression of P-selectin by bEnd.3 cells incubated for 6 hours with individual cytokines or combinations of cytokines present in the Th2 supernatants. As shown in Figure 6, flow cytometric analyses showed that IL-4, IL-5, and IL-13 alone and in combination with TNF-α did not induce increased P-selectin expression above that found for TNF-α alone. Similarly, GM-CSF or GM-CSF plus TNF-α did not enhance selectin expression levels (data not shown). Nevertheless, when we examined the functional effects of different cytokines on Th2 rolling, we found that the combined treatment of endothelium with TNF-α plus IL-4 significantly increased Th2 rolling (Figure 7 and data not shown). Thus, the number of rolling Th1 and Th2 cells was equivalent on endothelium treated with either Th2 supernatant or TNF-α/IL-4, and there was no significant difference in the numbers of Th2 cells rolling under the two conditions. Further, the median rolling times for Th1 and Th2 cells on endothelium stimulated with Th2 supernatant were comparable (3.0 versus 5.9 seconds, respectively), as were the rolling times of Th1 cells on TNF-α/IL-4-stimulated endothelium (4.7 each). Notably, however, although the number of rolling Th2 cells was similar for both conditions, the median rolling time for Th2 cells on bEnd.3 cells stimulated with Th2 supernatant was significantly longer than that for TNF-α/IL-4-stimulated endothelium (5.9 versus 1.9 seconds), reflected in the slower average rolling velocity of the former. This was likely due to the increased induction of P-selectin induced by Th2 supernatant, and was not attributable to differential induction of VCAM-1, ICAM-1, E-selectin, or MAdCAM-1, because flow cytometric analysis of expression levels for these adhesion molecules were not detectably altered after bEnd.3 stimulation with either Th2 supernatant or TNF-α/IL-4 (Figure 5 and data not shown). Thus, although enhanced expression of P-selectin certainly contributed to enhanced Th2 rolling via interactions with PSGL-1, undefined interactions induced by IL-4 acted independently of increased P-selectin induction to enhance Th2 cell rolling.
Figure 7.
IL-4 plus TNF-α stimulation of endothelium induces features of Th2 rolling comparable to Th2-derived cytokine cocktail. DO11.10 Th1- and Th2-polarized cells were labeled with calcein, AM, or Cell Trace calcein red-orange, AM, mixed 1:1 and flowed with a shear stress of 0.5 dynes/cm2 more than bEnd.3 cells stimulated for 6 hours with Th2 sup (A) or TNF-α + IL-4 (B) (10 ng/ml and 20 ng/ml, respectively). Data collection and analyses were performed as described in Materials and Methods. The top panels indicate the mean number of rolling cells in 10 minutes of footage taken from four randomly selected fields of view. Error bars indicate SEM. The middle panels show pooled rolling times of interacting cells from four fields of view. In the bottom panels, the average rolling velocity and SEM from four fields of view is shown. Error bars indicate SEM. #Indicates statistical significance between T-cell subtypes. *Indicates statistical significance between methods of endothelial cell stimulation (P < 0.05).
Discussion
In this study, we have explored selectin-dependent and -independent pathways that might explain previously reported differences observed for Th1 versus Th2 effector cell recruitment to nonlymphoid sites. The premise at the outset was that Th2 cells likely used selectin-independent interactions for tethering and rolling on endothelia, since it is well established that in contrast to Th1 development, Th2 development is associated with relatively limited induction of glycosyltransferases that mediate posttranslational modification of scaffolds that display P- and E-selectin ligands, such as PSGL-1. Here and in a related in vivo study (see companion paper by Bonder et al44) we show that, in fact, Th2 cells, like Th1 cells, require selectin-dependent interactions for efficient interaction with at least some activated endothelium. Further, both Th1 and Th2 cells can use PSGL-1 as the dominant, if not exclusive, rolling adhesion molecule. Finally, in contrast to Th1 cells, which express higher levels of both P- and E-selectin ligands after in vitro derivation, Th2 cells require additional, undefined interactions, such as those induced by IL-4, for optimal tethering and rolling. This supports a model wherein both Th1 and Th2 cells require selectin/PSGL-1 interactions to survey nonlymphoid endothelial beds, and implicates a positive feedback mechanism whereby local activation of tissue-localized Th2 cells or IL-4-producing innate immune cells might foster enhanced Th2 recruitment, perhaps through cooperative selectin-dependent and -independent mechanisms.
Although the in vitro system developed for the current studies may not perfectly replicate in vivo physiology, it does offer advantages for direct comparisons of Th1 and Th2 interactions with the same endothelial monolayer under identical conditions across a range of shear stresses (see supplemental videos, and supplemental Figure S5 at http://www.amjpathol.org). It also permits examination of T cell-endothelial interactions in the absence of other leukocyte populations (eg, platelets and neutrophils) that can express selectin ligands that might modify these interactions and complicate interpretation. The development of PSGL-1-deficient, DO11.10 TCR transgenic mice further enabled us to address the requirements for PSGL-1 in interactions between well-defined effector T-cell populations and endothelium. It also allowed us to define possible selectin-independent mechanisms for effector T-cell recruitment. Importantly, PSGL-1 deficiency had no untoward effects on either Th1 or Th2 development, as evidenced by the nearly identical effector cytokine profiles of wild-type and PSGL-1-deficient effector T-cell populations of each subset. Similarly, the expression of non-PSGL-1 adhesion molecules was unaffected by PSGL-1 deficiency; distinct adhesion molecule expression profiles observed for wild-type Th1 and Th2 cells were retained in polarized PSGL-1−/− effectors. Thus, in agreement with other studies,43,48 Th1 cells in this study expressed higher levels of Mac-1 and L-selectin, whereas Th2 cells expressed higher levels of VLA-4 and β7 integrin, irrespective of PSGL-1 genotype.
In previous reports, the trafficking of Th1 cells to nonlymphoid tissues was found to be selectin-dependent, although the particular selectin used varied, contingent on the tissue site and mode of local activation of the endothelium.10,39,49,50 In our own earlier report, Th1 recruitment to a subcutaneous depot of antigen and adjuvant was E-selectin-dependent, and was unaffected by P-selectin deficiency.50 This was despite the expression of functional PSGL-1 by Th1 cells, and implied that the local, inflamed subcutaneous endothelium preferentially expressed E-selectin. In contrast, Haddad and colleagues39 found that Th1 cell recruitment to the noninflamed intestine was P-selectin-dependent, and unaffected by blockade of E-selectin, in agreement with our own in vivo studies.44 Importantly, in each of these studies, blockade of P- and/or E-selectin, whether by antibodies or genetic deficiency, ablated Th1 cell trafficking to nonlymphoid tissues, establishing a requirement for selectin-mediated recruitment to these sites. These data are consistent with the expression of both E- and P-selectin ligands by Th1 cells and highlight the requirement for selectin-mediated Th1 recruitment, as well as a level of potential redundancy and selectivity for Th1 recruitment that likely reflects local dominance of P- or E-selectin expression, which is dependent on the tissue site and the inflammatory factors elicited.15
Although previous studies have implicated selectin-dependent recruitment by Th1 cells, in none of these studies has a specific selectin ligand been identified. Thus, although Smithson and colleagues49 mapped a requirement for the fucosyltransferase, FucT-VII, and to a lesser extent, FucT-IV, for Th1 and cytotoxic T lymphocyte (CTL) trafficking to an inflamed cutaneous site, it was not determined which surface glycoprotein was a target for modification. Herein, we identify PSGL-1 as the principal Th1 ligand for both P- and E-selectin. Whereas P-selectin interacts with a single ligand generated by appropriate sialation and fucosylation of PSGL-1, non-PSGL-1 ligands have been described for E-selectin.51–54 Our data strongly implicate PSGL-1 as the dominant scaffold for Th1 expression of both P- and E-selectin ligands, but do not rule out a less significant role for non-PSGL-1, E-selectin ligands, which were detectable at low levels on both Th1 and Th2 cells but apparently could not compensate to mediate rolling in the absence of PSGL-1.
Detailed studies of the molecular requirements for Th2 cell trafficking have been limited.30 In at least one murine airway hypersensitivity model, a role for both Th1 and Th2 cells in eosinophilic airway inflammation has been described,41 with optimal Th2 recruitment only when antigen-activated Th1 cells participate in the response. In this model, an important role for VCAM-1 in Th2, but not Th1, recruitment has been defined. Randolph and colleagues41 found in co-transfer studies of Th1 and Th2 cells derived from DO11.10 mice that neutralization of TNF-α or blocking antibodies to VCAM-1 decreased Th2 (and eosinophil) recruitment stimulated by airway delivery of OVA, with neutralization of TNF-α itself leading to decreased VCAM-1 expression. In separate studies, Cohn and colleagues55 found that IL-4 production by Th2 cells was required for their recruitment to airways, but this could be overcome by exogenous administration of TNF-α. Taken together, these data suggested that TNF-α and IL-4 might act synergistically to recruit Th2 cells through a mechanism that is VCAM-1-dependent. In other studies, data supporting a role for P-selectin in Th2 recruitment to the lung were obtained. Lukacs and colleagues56 found that P-selectin-deficient mice, but not E-selectin-deficient or wild-type mice, demonstrated a marked decrease in Th2-type cytokines after antigenic challenge, although infiltration of Th2 cells was not directly characterized. More direct evidence for the role of the selectins and PSGL-1 in mediating Th2 rolling in vivo comes from studies of E-/P-selectin double-mutant mice in the ovalbumin-mediated airway hypersensitivity model.56 In these investigations, it was observed that Th2 recruitment to the airways was significantly inhibited in mice lacking both of these selectins. Moreover, recent studies have shown that Th2 cells generated in vivo can express selectin ligands, although none have directly addressed the importance of PSGL-1 for the generation of these ligands.57 In a Th2 model of lung inflammation, IL-4-producing cells expressing P- but not E-selectin ligand could be detected in the lungs of infected mice. Also, in human systems, Th2 cells cultured under defined media conditions could be induced to express the CLA epitope, a ligand for E-selectin.58,59 Our data support a requirement for PSGL-1 in Th2 recruitment, but also show that TNF-α and IL-4 cooperate to enhance Th2 interactions with the endothelium through distinct mechanisms. This is consistent with the possibility that cooperative effects of TNF-α and IL-4 reported previously in airway hypersensitivity models reflect a requirement for both P-selectin and VCAM-1 for Th2 recruitment, and is in accord with data herein, which document that in addition to P-selectin ligand, Th2 cells, but not Th1 cells, express elevated levels of the VCAM-1 counterreceptor VLA-4 (α4β1-integrin; CD49d/CD29). In this regard, it is noteworthy that expression by the endothelium of high levels of VCAM-1 alone was insufficient to mediate significant Th2 rolling in the absence of PSGL-1 (Figures 1B and 6), suggesting that VLA-4/VCAM-1 interactions do not mediate Th2 recruitment in the absence of PSGL-1/selectin interactions. Future studies using P- and E-selectin-deficient recipients, or PSGL-1-deficient Th2 cells, should definitively address this possibility.
Notably, a significant fraction of Th1 cells in the current study retained or re-expressed significant L-selectin (CD62L) after antigen activation and effector cell commitment. This was in striking contrast to Th2 cells, which did not express detectable L-selectin after effector differentiation. This could have important implications for preferential recruitment of Th1 cells under certain conditions in vivo, because chronically inflamed endothelium can express L-selectin ligands and granulocytes adherent to inflamed endothelium may also display L-selectin ligands.30 Indeed, appropriately glycosylated PSGL-1 is itself an important ligand for L-selectin,60 raising the possibility that adherent T cells might themselves serve as a substrate for augmented naïve or Th1 recruitment via L-selectin binding. Nevertheless, we detected no role for L-selectin-mediated interactions for Th1 rolling in our in vitro system, reflecting perhaps either limited or absent expression of L-selectin ligands by endothelial lines activated under the conditions used in this study and/or the absence of granulocytes and platelets in the experimental system. However, there was also no apparent role for L-selectin in the recruitment of Th1-type cells to subcutaneous tissues in our previous in vivo study, in which E-selectin or combined E-/P-selectin deficiency abrogated Th1 recruitment.50 Similarly, in our related in vivo study (see companion paper44), expression of L-selectin by Th1 cells was not compensatory for trafficking to the intestine in the face of P-selectin antibody blockade. Whether this was due to an absence of L-selectin ligand expression by the endothelium or the lack of granulocyte recruitment under the in vivo conditions examined in these studies, or rather a reflection of a lack of a significant role for granulocyte adhesion in effector T-cell recruitment, is unclear and will require further investigation.
In summary, the current study supports a model of effector T-cell recruitment wherein both Th1 and Th2 cells are primarily dependent on PSGL-1-linked selectin ligands for interactions with the endothelium that initiate their recruitment to nonlymphoid tissues. Because E-selectin ligand biosynthesis requires higher FucT-VII activity than P-selectin ligand biosynthesis,37,38 and lower levels of glycosyltransferase activity are induced during Th2 development, Th2 cells are particularly deficient in E-selectin binding activity, and thus appear to be dependent on other cooperative adhesive interactions for efficient rolling on inflamed endothelia. Previous studies that have demonstrated preferential recruitment of Th1 cells to nonlymphoid tissues,39,50,61,62 would appear to reflect a strict requirement for high levels of P-selectin and/or cooperative adhesion interactions for Th2 cells, rather than a general dependence on nonselectin interactions for Th2 recruitment.
Supplementary Material
Acknowledgments
We thank the members of the Weaver and Bullard laboratories for helpful comments and manuscript review, Drs. John Lowe and Peter L. Smith for provision of plasmids that express P- and E-selectin-hIgM fusion proteins, Dr. Barbara Furie for PSGL-1-deficient mice, and Noelle LeLievre for manuscript preparation and editorial critique.
Footnotes
Address reprint requests to Casey T. Weaver, BBRB 870, 845 19th St. S., Birmingham, AL 35294-2170. E-mail: weaver@path.uab.edu.
Supported by grants from the National Institutes of Health (grants RO1 AI35783 and DK64400 to C.T.W. and training grant AI007051 to P.R.M.), the National Multiple Sclerosis Society (to L.H.) the Crohn’s and Colitis Foundation of America (to D.C.B.), and a training fellowship from the Crohn’s and Colitis Foundation of Canada (to C.S.B.).
D.C.B. and C.T.W. contributed equally to this study.
Supplemental material for this article can be found on http://www.amjpathol.org.
References
- Bradley LM, Watson SR, Swain SL. Entry of naive CD4 T cells into peripheral lymph nodes requires L-selectin. J Exp Med. 1994;180:2401–2406. doi: 10.1084/jem.180.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeber DA, Green NE, Sato S, Tedder TF. Lymphocyte migration in L-selectin-deficient mice. Altered subset migration and aging of the immune system. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, Haskard DO, Tedder TF, Ager A. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio D, Sinigaglia F. Chemokines and their receptors: trafficking cues for Th1 and Th2 cells. Eur Cytokine Netw. 2000;11:495–496. [PubMed] [Google Scholar]
- Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol. 1999;21:263–285. doi: 10.1007/BF00812257. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenkamp A, Nagata K, Murphy K, Wu L, Lanzavecchia A, Sallusto F. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003;33:474–482. doi: 10.1002/immu.200310023. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Abbassi O, Kishimoto TK, McIntire LV, Smith CW. Neutrophil adhesion to endothelial cells. Blood Cells. 1993;19:245–260. [PubMed] [Google Scholar]
- Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, Kontgen F, Stewart CL, McIntyre KW, Will PC, Burns DK, Wolitzky BA. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B, Issekutz TB, Kubes P. The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med. 1996;183:1995–2006. doi: 10.1084/jem.183.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Ramos CL, Steeber DA, Muller W, Wagner N, Tedder TF, Ley K. The roles of L-selectin, beta 7 integrins, and P-selectin in leukocyte rolling and adhesion in high endothelial venules of Peyer’s patches. J Immunol. 1998;161:2449–2456. [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, Baleux F, Arenzana-Seisdedos F, Lapidot T, van Kooyk Y, Lobb RR, Alon R. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinamon G, Grabovsky V, Winter E, Franitza S, Feigelson S, Shamri R, Dwir O, Alon R. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001;69:860–866. [PubMed] [Google Scholar]
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Semin Immunol. 2002;14:83–92. doi: 10.1006/smim.2001.0345. [DOI] [PubMed] [Google Scholar]
- Alon R, Grabovsky V, Feigelson S. Chemokine induction of integrin adhesiveness on rolling and arrested leukocytes local signaling events or global stepwise activation? Microcirculation. 2003;10:297–311. doi: 10.1038/sj.mn.7800195. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to nonlymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–336. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–370. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AE, Nolan SL, Ridger VC, Hellewell PG, Norman KE. Recombinant P-selectin glycoprotein ligand-1 directly inhibits leukocyte rolling by all 3 selectins in vivo: complete inhibition of rolling is not required for anti-inflammatory effect. Blood. 2003;101:3249–3256. doi: 10.1182/blood-2002-07-2329. [DOI] [PubMed] [Google Scholar]
- van Wely CA, Blanchard AD, Britten CJ. Differential expression of alpha3 fucosyltransferases in Th1 and Th2 cells correlates with their ability to bind P-selectin. Biochem Biophys Res Commun. 1998;247:307–311. doi: 10.1006/bbrc.1998.8786. [DOI] [PubMed] [Google Scholar]
- Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on alpha1,3-fucosyltransferase VII gene expression. J Exp Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- Knibbs RN, Craig RA, Natsuka S, Chang A, Cameron M, Lowe JB, Stoolman LM. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad W, Cooper CJ, Zhang Z, Brown JB, Zhu Y, Issekutz A, Fuss I, Lee HO, Kansas GS, Barrett TA. P-selectin and P-selectin glyco-protein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med. 2003;198:369–377. doi: 10.1084/jem.20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- Lim YC, Wakelin MW, Henault L, Goetz DJ, Yednock T, Cabanas C, Sanchez-Madrid F, Lichtman AH, Luscinskas FW. Alpha4beta1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation. 2000;7:201–214. [PubMed] [Google Scholar]
- Bonder CS, Norman MU, MacRae T, Mangan PR, Weaver CT, Bullard DC, McCafferty D-M, Kubes P. P-selectin can support both Th1 and Th2 lymphocyte rolling in the intestinal microvasculature. Am J Pathol. 2005;167:1647–1660. doi: 10.1016/S0002-9440(10)61248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil CG, Bullard DC. In vitro culture and characterization of gene targeted mouse endothelium. Acta Physiol Scand. 2001;173:151–157. doi: 10.1046/j.1365-201X.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- Kevil CG, Chidlow JH, Bullard DC, Kucik DF. High-temporal-resolution analysis demonstrates that ICAM-1 stabilizes WEHI 274.1 monocytic cell rolling on endothelium. Am J Physiol. 2003;285:C112–C118. doi: 10.1152/ajpcell.00334.2002. [DOI] [PubMed] [Google Scholar]
- van Wely CA, Beverley PC, Brett SJ, Britten CJ, Tite JP. Expression of L-selectin on Th1 cells is regulated by IL-12. J Immunol. 1999;163:1214–1221. [PubMed] [Google Scholar]
- Smithson G, Rogers CE, Smith PL, Scheidegger EP, Petryniak B, Myers JT, Kim DS, Homeister JW, Lowe JB. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Zollner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes HG, Vestweber D. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol. 1997;136:707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies LG, Sperandio M, Underhill GH, Yousif J, Smith M, Priatel JJ, Kansas GS, Ley K, Marth JD. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100:3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, John A, Berlin A, Bullard DC, Knibbs R, Stoolman LM. E- and P-selectins are essential for the development of cockroach allergen-induced airway responses. J Immunol. 2002;169:2120–2125. doi: 10.4049/jimmunol.169.4.2120. [DOI] [PubMed] [Google Scholar]
- Kretschmer U, Bonhagen K, Debes GF, Mittrucker HW, Erb KJ, Liesenfeld O, Zaiss D, Kamradt T, Syrbe U, Hamann A. Expression of selectin ligands on murine effector and IL-10-producing CD4+ T cells from non-infected and infected tissues. Eur J Immunol. 2004;34:3070–3081. doi: 10.1002/eji.200424972. [DOI] [PubMed] [Google Scholar]
- Akdis M, Klunker S, Schliz M, Blaser K, Akdis CA. Expression of cutaneous lymphocyte-associated antigen on human CD4(+) and CD8(+) Th2 cells. Eur J Immunol. 2000;30:3533–3541. doi: 10.1002/1521-4141(2000012)30:12<3533::AID-IMMU3533>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Colantonio L, Rossi B, Constantin G, D’Ambrosio D. Integration and independent acquisition of specialized skin- versus gut-homing and Th1 versus Th2 cytokine synthesis phenotypes in human CD4(+) T cells. Eur J Immunol. 2004;34:2419–2429. doi: 10.1002/eji.200425159. [DOI] [PubMed] [Google Scholar]
- Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz W, Allemand Y, Borges E, von Laer D, Hallmann R, Vestweber D, Hamann A. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J Exp Med. 1999;189:1765–1776. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.