Abstract
Enterohemorrhagic Escherichia coli O157:H7 (EHEC) is an enteric pathogen that causes potentially fatal symptoms after intimate adhesion, modulation of intestinal epithelial signal transduction, and alteration of epithelial function (eg, barrier disruption). Although the epithelial barrier is critical to gut homeostasis, only a few agents, such as transforming growth factor (TGF)-β, can enhance or protect epithelial barrier function. Our aims were to delineate the mechanism(s) behind TGF-β-induced barrier enhancement and to determine whether TGF-β could prevent EHEC-induced barrier disruption. Using monolayers of the human T84 colonic epithelial cell line, we found that TGF-β induced a significant increase in transepithelial electrical resistance (a measure of paracellular permeability) through activation of ERK MAPK and SMAD signaling pathways and up-regulation of the tight junction protein claudin-1. Additionally, TGF-β pretreatment of epithelia blocked the decrease in transepithelial electrical resistance and the increase in transepithelial passage of [3H]-mannitol caused by EHEC infection. EHEC infection was associated with reduced expression of zonula occludens-1, occludin, and claudin-2 (but not claudin-1 or claudin-4); TGF-β pretreatment prevented these changes. These studies provide insight into EHEC pathogenesis by illustrating the mechanisms underlying TGF-β-induced epithelial barrier enhancement and identifying TGF-β as an agent capable of blocking EHEC-induced increases in epithelial permeability via maintenance of claudin-2, occludin, and zonula occludens-1 levels.
Infection with the intestinal pathogen enterohemorrhagic Escherichia coli O157:H7 (EHEC) causes diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome in some individuals, the latter being a result of EHEC-derived Shiga toxins.1 Enteropathogenic E. coli (EPEC) is a related pathogen that causes much morbidity and mortality by eliciting watery diarrhea.2 Antibiotics, although useful in certain cases of EPEC infection, are primarily ineffective and may even be harmful during EHEC infection, leaving no effective medical therapy for infected individuals.3 Thus, understanding the interaction(s) between EHEC and the enteric epithelium may be important in defining novel therapeutic targets.
Under normal circumstances the epithelial lining of the gut forms a semipermeable barrier, the selectivity of which is in large part determined by the intercellular tight junctions. The tight junctions consist of interdigitating occludin molecules and one or more claudins that are linked to the actin cytoskeleton via a complex of proteins including the zonula occludens (ZO) 1, 2, and 3.4,5 Recent evidence suggests that members of the claudin family play a critical role in tight junction formation and determine the permeability characteristics in a variety of tissues including the gut.6 Claudin-1 in particular is believed to regulate epithelial permeability. Evidence includes the lethality of claudin-1-deficient mice,7 reduced claudin-1 expression after interferon (IFN)-γ-induced barrier defects in thyrocytes,8 increased claudin-1 expression leading to barrier enhancement of airways and kidney epithelia,9,10 and the significant redistribution of claudin-1 at the onset of rotavirus-induced paracellular permeability in intestinal epithelial monolayers.11
Investigations with model epithelial cell lines have shown that EHEC forms intimate attaching and effacing lesions on host cells by eliciting and modulating a variety of intracellular signaling pathways to reorganize the cytoskeleton while evoking a significant increase in epithelial permeability. This diminished epithelia barrier is accompanied by loss of ZO-1 from the tight junction region12 and may be of pathological significance by allowing pathogens and antigens unimpeded access to the mucosa. Indeed, the paracellular pathway may be one way for Shiga toxin to enter the circulation because the receptor for the toxin has not been identified on human gut epithelium.13 Thus, agents capable of enhancing or maintaining the epithelial barrier, through preservation of the tight junction or via modulating intracellular signaling pathways that compete with those activated by bacteria, may be of value in reversing the effects of EHEC infection.
Transforming growth factor (TGF)-β is a multifunctional cytokine with immunosuppressive properties produced by many cell types including intestinal epithelia and can enhance epithelial barrier function. For instance, monolayers of human colon-derived epithelia treated with TGF-β display an increase in transepithelial electrical resistance (TER).14 Moreover, TGF-β also prevents or reduces epithelial barrier disruption caused by infection with Cryptosporidium parvum, exposure to IFN-γ, or exposure to conditioned medium from activated immune cells.14–17 However, neither the ability of TGF-β to ameliorate EHEC-induced barrier disruption nor the mechanisms underlying preservation of epithelial barrier function have been reported. Thus, the aims of the present study were 1) to define the mechanism behind TGF-β enhancement of epithelial barrier function in terms of intracellular signaling pathways and tight junction protein expression, 2) to characterize the effects of EHEC on tight junction protein expression and distribution, and 3) to determine whether TGF-β could block the EHEC-induced increase in epithelial permeability.
Materials and Methods
Cell and Bacterial Culture
T84 cells were grown and maintained in a 1:1 (vol/vol) mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium, supplemented with 10% fetal bovine serum, 1.5% HEPES, and 2% penicillin-streptomycin (all from Life Technologies, Grand Island, NY) at 37°C, 5% CO2. Enterohemorrhagic E. coli (EHEC) O157:H7 (strain CL-56)12 and enteropathogenic E. coli (EPEC) O127:H6 (strain E2348/69)18 were maintained on 5% sheep blood agar plates (PML Microbiologicals, Mississauga, Canada) at 4°C, cultured overnight in Luria-Bertani broth at 37°C with continuous agitation, diluted in Luria-Bertani broth, and then grown for 4 to 6 hours (to mid-logarithmic growth phase) before T84 monolayer infection. The bacterial suspension was pelleted (2500 rpm, 20 minutes), resuspended in antibiotic-free T84 cell culture medium, and 108 CFU were added to the apical surface of epithelial monolayers at an MOI of 100 (multiplicity of infection; 100 infectious units/epithelial cell). A laboratory strain of nonpathogenic E. coli, HB101, was used as a control and was cultured as described above.19
Measurement of Epithelial Barrier
T84 cells were seeded onto filter supports (106 cells/well, surface area 1 cm2; Costar Inc., New York, NY) and grown to confluence (≥6 days), at which point TER was monitored by a voltmeter and chopstick electrodes (Millicell-ERS; Millipore, Bedford, MA). To assess the effects of TGF-β, the cytokine was added to the basolateral surface of T84 monolayers (human recombinant TGF-β1, 10 ng/ml; R&D Systems, Minneapolis, MN), and TER was recorded at 4, 8, 16, 24, 48, and 72 hours after treatment. Based on previous studies showing that TGF-β has barrier-enhancing effects at doses of 1 to 100 ng/ml,14,20 we selected a dose of TGF-β of 10 ng/ml for this study. The acute effects of TGF-β were assessed by wash out experiments, in which monolayers were exposed to TGF-β for 1 hour, rinsed twice with culture media, and maintained daily in fresh culture media with TER recorded at 24-hour intervals. For pharmacological inhibition of ERK MAPK, T84 monolayers were pretreated with the MEK1 inhibitor PD98059 (apical and basolateral, 1 hour, 25 μmol/L; Calbiochem, San Diego, CA20), exposed to TGF-β (10 ng/ml, 1 hour), rinsed, and maintained with fresh culture media for 72 hours, and TER was then recorded.
In bacterial co-culture experiments, T84 monolayers were treated with TGF-β (10 ng/ml) 45 minutes before infection, with TGF-β remaining for the duration of infection, and TER recorded 16 hours later (a time point at which EHEC infection reduces epithelial barrier function12). In additional studies, 2.5 μCi of [3H]-mannitol (∼180 d; Sigma Chemical Co., St. Louis, MO) was added to the apical side of filter-grown monolayers at the end of the experiment. As an inert small molecule, mannitol will cross the epithelium predominantly via the paracellular pathway. Four hours later 10-μl and 500-μl aliquots of culture medium were obtained from the apical and basal culture well, respectively, and radioactivity determined in a scintillation counter (Becton Dickinson, Mississauga, Canada). The transepithelial passage of the labeled mannitol was calculated.
Adenoviral Infection
T84 cells were seeded as described above and, at 4 hours after seeding, infected for 16 hours with replication-deficient adenovirus constructs encoding SMAD7 (Ad-SMAD7, MOI = 50; a generous gift from Dr. ten Dijke, The Netherlands Cancer Institute, Leiden, The Netherlands).21,22 Cells were rinsed, grown for 3 days, and then treated with TGF-β (10 ng/ml, 24 hours) and TER recorded 72 hours after cytokine exposure.
Immunoprecipitation for SMAD2/3
T84 monolayers were seeded onto filter supports (3 × 106 cells/well, surface area 4.7 cm2; Costar Inc.), grown to confluence, serum-starved for 24 hours, and treated with TGF-β (10 ng/ml, 5 to 60 minutes). Whole cell protein extracts were prepared, protein concentrations determined,23 and samples (1 mg/ml in phosphate-buffered saline) incubated with gentle agitation at room temperature for 2 hours with mouse anti-human SMAD2/3 antibody (2 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), followed by centrifugation and incubation with protein A-conjugated beads (Easy-View; Sigma-Aldrich, St. Louis, MO). Immunoprecipitated SMAD2/3 was recovered following the manufacturer’s protocol and loaded immediately onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel.
Immunoblotting
Samples were prepared from T84 monolayers as described above ± TGF-β (10 ng/ml) ± bacteria (MOI = 100). Proteins (40 μg/sample) were electroblotted onto a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA) and blocked in 5% bovine serum albumin in Tris-buffered saline (containing 0.1% Tween-20, TBST) or 5% milk diluted in TBST, according to the manufacturer’s directions.
Phospho-SMAD2/3, SMAD2/3, and SMAD7
Blots were probed with mouse anti-human phospho-serine antibody (phospho-SMAD2/3), rabbit anti-human SMAD2/3, or goat anti-human SMAD7 diluted in bovine serum albumin-TBST (1:1000, 1:1000, and 1:750, respectively; 1 hour at room temperature; Abcam, Cambridge, UK), washed in TBST, and exposed to the appropriate horseradish peroxidase-conjugated secondary antibodies (1:3000, Santa Cruz Biotechnology).
FLAG
Blots were probed with mouse anti-FLAG diluted in milk-TBST for 1 hour at room temperature (1:1000, Sigma-Aldrich), washed in TBST, and exposed to horse-radish peroxidase-conjugated rabbit anti-mouse IgG (1:3000, 1 hour room temperature; Santa Cruz Biotechnology).
Phospho-ERK1/2 and Tight Junction Proteins
Blots were either probed for 1 hour at room temperature (mouse anti-human phospho-ERK1/2, 1:800; rabbit anti-human ERK1/2, 1:3000; Santa Cruz Biotechnology) or overnight at 4°C (rabbit anti-human claudin-1, 0.5 μg/ml; rabbit anti-human claudin-2, 1.0 μg/ml; mouse anti-human claudin-4, 1.5 μg/ml; rabbit anti-human occludin, 0.25 μg/ml, rabbit anti-human ZO-1, 1.5 μg/ml; Zymed Laboratories, San Francisco, CA), washed in TBST (5×) and then exposed to the appropriate horseradish peroxidase-conjugated secondary antibodies (1:2000 to 1:15,000, 1 hour room temperature; Santa Cruz Biotechnology). Immunoreactive proteins were visualized using chemiluminescence (ECL; Amersham Pharmacia, Piscataway, NJ) and exposure to Kodak XBL film (Eastman Kodak Co., Rochester, NY). Initial tight junction protein immunoblots were stripped and reprobed for β-actin (goat anti-human actin, 1:200; bovine anti-goat 1:400; Santa Cruz Biotechnology) to assess total protein loading. Subsequently, we used claudin-4 expression as an internal control because this did not change with infection or TGF-β treatment and is a tight junction protein. The densitometry ratio between the tight junction protein of interest and the internal control from each extraction was compared between control (ie, naïve) and treated epithelia.
Confocal Microscopy
T84 cells were seeded onto filter supports (105 cells/well, surface area 0.33 cm2) and either treated with TGF-β for 72 hours (10 ng/ml) or infected with EHEC O157:H7 for 16 hours (MOI = 100) ± TGF-β pretreatment for 45 minutes (10 ng/ml) (as described above). Monolayers were rinsed in phosphate-buffered saline, fixed in 4% formalin, permeabilized with 0.1% Triton X-100 (Mallinckrodt Inc., Paris, KY), and blocked for 30 minutes in blocking solution [5% bovine serum albumin/5% goat serum (Sigma-Aldrich) in 0.1% Triton X-100]. Primary and secondary antibodies were diluted in blocking solution (rabbit anti-human claudin-1, 15 μg/ml, mouse anti-human claudin-2, 2 μg/ml, or rabbit anti-human ZO-1, 2 μg/ml, Zymed Laboratories; Alexa Fluor 488 goat anti-rabbit IgG or Alexa Fluor 488 goat anti-mouse IgG, 1.5 μg/ml, Molecular Probes, Eugene, OR) and exposed to monolayers for 1 hour at room temperature. After extensive washing, filters were excised from the polystyrene filter supports, and mounted on glass slides in Gel/Mount anti-fade reagent (Biomeda Corp., Foster City, CA). Images were acquired using an inverted laser-scanning microscope (LSM 510, Axiovert 100 mol/L; Zeiss, Oberkochen, Germany) equipped with argon (450 to 514 nm). Immunoreactive claudin and ZO-1 were excited using the 488-nm laser and collected using a standard fluorescein isothiocyanate filter set. For each experiment, image acquisition (ie, confocal microscope settings) and processing was identical between controls and TGF-β-, EHEC-, or TGF-β + EHEC-treated cells. Images are presented as either en face to illustrate the distribution of the tight junction protein immunoreactivity or as a composite Z-stack reconstruction, which shows the monolayer in transverse profile with the basally located nuclei identified by propidium iodide staining (red) and the tight junction proteins by fluorescein isothiocyanate-labeled secondary antibodies (green).
[3H]-Thymidine Incorporation
T84 cells were seeded onto 96-well plates (4 × 104 cells/well) and grown for 4 days. [3H]-Thymidine (1 μCi/well; DuPont-New England Nuclear, Wilmington, DE) was added for the duration of TGF-β treatment (10 ng/ml, 72 hours). Cells were harvested onto glass-fiber filter disks and assayed in 5 ml of aqueous counting scintillant for β-ray emission, and counts per minute were determined in a scintillation counter (Becton Dickinson). Results are expressed as mean cpm ± SEM.
Bacterial Growth
EHEC was cultured to mid-logarithmic growth phase (described in Cell and Bacterial Culture), resuspended in antibiotic-free T84 cell culture media ± TGF-β (10 ng/ml), and incubated at 37°C with continuous agitation for 16 hours. Bacterial growth was determined by spectrophotometric analysis of each sample (OD600: 0.1 = 109 CFU). In similar experiments, supernatants from naïve T84 monolayers or TGF-β-treated T84 cells were added to EHEC in mid-logarithmic growth phase, cultured as above, and OD600 measured.
Interleukin (IL)-8 Enzyme-Linked Immunosorbent Assay
T84 cells were seeded onto filter supports (106 cells/well, surface area 1 cm2), grown to confluence, and infected with EHEC ± TGF-β pretreatment (as described above) for 16 hours. Subsequently, culture medium samples were collected from the basolateral wells and frozen at −20°C until the concentration of IL-8 was determined by commercial enzyme-linked immunosorbent assay (R&D Systems). Detection limit of the assay was 16 pg/ml.
Statistical Analysis
Data are presented as means ± SEM. Data were analyzed using one-way analysis of variance followed by posthoc comparisons using Tukey’s test, or Student’s t-test, with P < 0.05 accepted as the level of statistical significance.
Results
TGF-β-Induced Increase in Epithelial Barrier Function Is Mediated by ERK MAPK and SMAD Signaling Pathways
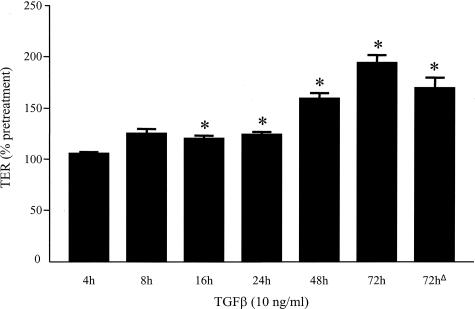
Figure 1 shows that TGF-β (10 ng/ml) evoked a time-dependent increase in TER that was significant at 16 hours after treatment and more pronounced at 72 hours when there was a twofold increase greater than pretreatment values. Epithelial TER was similarly increased 72 hours after a single 1-hour pulse with TGF-β, indicating that acute exposure to this cytokine has long-term effects (Figure 1, bar labeled 72hΔ). Indeed, our earlier publication showing that 1-hour TGF-β treatment causes alterations in epithelial ion transport 16 hours later supports this observation.20 Reflecting the high TER baseline of control monolayers, there was no significant difference in [3H]-mannitol flux across control and TGF-β-treated epithelial monolayers.
Figure 1.
Bar graph showing increased TER across T84 cell monolayers after continuous TGF-β treatment (10 ng/ml, 4 to 72 hours) or after a 1-hour pulse with cytokine (denoted as 72hΔ). TER values were recorded before cytokine treatment for comparison against values at the end of each time point (indicated as percent pretreatment value), and these were compared against naïve, time-matched controls (mean ± SEM, *P < 0.05, n = 3 to 10 monolayers). Pretreatment TER values were 609 ± 189 Ω·cm2 and 607 ± 158 Ω·cm2 for naïve controls and pre-TGF-β treatment, respectively.
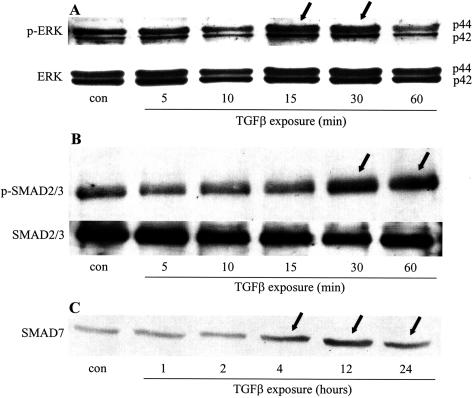
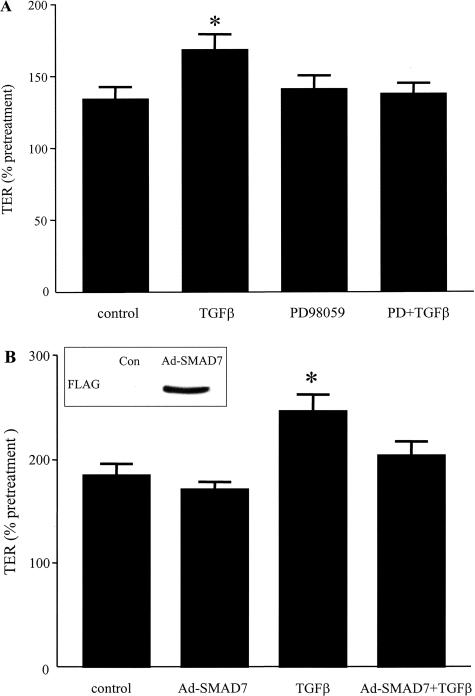
TGF-β is known to activate the ERK MAPK pathway and mobilize SMAD proteins in multiple cell types.24 Figure 2A is an immunoblot showing ERK 1/2 activation (ie, increased phosphorylation) in whole cell protein extracts of T84 cells after 15 and 30 minutes of TGF-β exposure. Figure 2B shows increased levels of phosphorylated SMAD2/3 30 to 60 minutes after cytokine treatment, indicating SMAD2/3 activation. SMAD7 is a naturally occurring inhibitor of the SMAD signaling pathway that is typically induced after TGF-β activation and provides a negative feedback mechanism for TGF-β signaling.24 In TGF-β-treated T84 epithelia, SMAD7 expression was increased 4 hours after cytokine exposure and remained elevated at 24 hours (end of experiment, Figure 2C). Inhibition of ERK MAPK and SMAD signaling via the pharmacological inhibitor PD98059 and via Ad-SMAD7, respectively, completely prevented the TGF-β-induced increase in epithelial TER (Figure 3).
Figure 2.
TGF-β activates ERK MAPK and SMAD signaling pathways in colonic epithelia. Immunoblot of protein extracts from serum-starved TGF-β-treated (10 ng/ml) T84 epithelial cells probed for phosphorylated ERK1/2 (p-ERK) followed by stripping and reprobing for total ERK1/2 (A), serine-phosphorylated SMAD2/3 (p-SDMA2/3) followed by stripping and reprobing for total SMAD2/3 (B), or SMAD7 protein expression (C). All panels depict representative images from three separate experiments (arrows highlight lanes where protein expression is visibly different from untreated controls).
Figure 3.
Inhibition of ERK MAPK and SMAD signaling pathways prevents the TGF-β-induced increases in TER. T84 cell monolayers were pretreated with the inhibitor of ERK MAPK signaling PD98059 (PD, 25 μmol/L) (A) or infected with an adenovirus encoding inhibitory SMAD7 (Ad-SMAD7, MOI = 50) ± TGF-β (10 ng/ml) (B) and TER values (percent change from pretreatment) were compared against naïve controls and TGF-β-only treated cells (means ± SEM, *P < 0.05, n = 9 to 12 monolayers; average control TER values were 784 ± 251 Ω/cm2). B, inset: FLAG-tagged adenoviral SMAD7 protein expression confirming adenoviral infection.
TGF-β Stimulates Increased Expression of the Tight Junction Protein Claudin-1
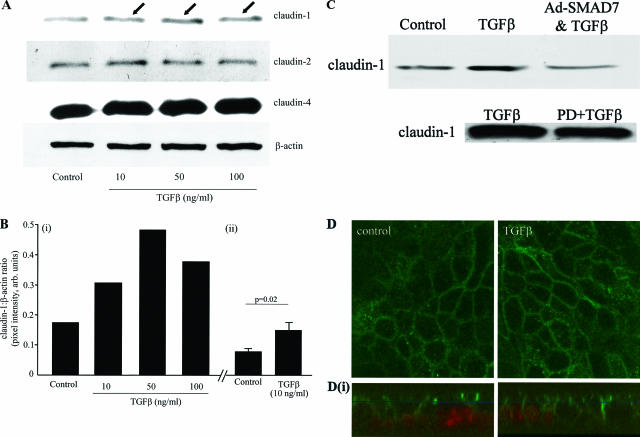
To determine the structural mechanism behind the epithelial barrier enhancement, we compared tight junction protein expression between TGF-β-treated T84 monolayers and untreated controls. TGF-β exposure (10, 50, or 100 ng/ml, 72 hours) resulted in twofold to threefold up-regulation of claudin-1, whereas neither claudin-2 nor claudin-4 were significantly affected (Figure 4). Figure 4B (i) shows densitometry analysis of the representative blot shown in Figure 4A, and Figure 4B (ii) shows quantification of the increased claudin expression in six epithelial preparations exposed to TGF-β (10 ng/ml, 72 hours) compared to whole cell protein extracts from time-matched controls. Supporting the TER data (Figure 3), inhibition of ERK MAPK and SMAD signaling reduced the TGF-β-induced increases in claudin-1 expression (Figure 4C). The increased claudin-1 could have been retained solely in a central cytosolic position. However, confocal microscopy revealed a chicken-wire pattern of claudin-1 immunoreactivity that is typical of tight junction protein expression at the margins of polarized epithelia (Figure 4D). Moreover, assessment of z axis reconstructions showed that the claudin-1 immunoreactivity was similar in control and TGF-β-treated monolayers and was mostly confined to the apical lateral membranes. However, there was a tendency for claudin-1 to extend further down the lateral membrane in epithelia exposed to TGF-β (immunostaining was noted in the cytosolic compartment in both treated and control epithelial preparations, consistent with normal protein production and turnover). Neither occludin nor ZO-1 expression were consistently or significantly affected by TGF-β treatment (data not shown). The increased claudin-1 expression was not simply a reflection of increased proliferation because [3H]-thymidine incorporation was actually reduced after 72 hours of TGF-β exposure (31,477 ± 4861 versus 5294 ± 612 cpm, control versus TGF-β respectively, n = 12; P < 0.001).
Figure 4.
TGF-β increases epithelial claudin-1 expression. A: A representative immunoblot of protein extracts from TGF-β-treated (72 hours) T84 cell monolayers shows increased claudin-1 expression (arrows) with exposure to all three TGF-β concentrations, whereas claudin-2 and claudin-4 were not consistently increased (blots reprobed for β-actin as a control; n = 3). The densitometry analysis (integrated pixel intensity of claudin-1 was compared to the β-actin) of this blot is shown on the left portion of B (i) whereas the right portion (ii) shows the statistically significant increase in claudin-1 induced by TGF-β (10 ng/ml, 72 hours exposure, n = 6 T84 cell protein extracts). C: Representative immunoblots (n = 2) showing that TGF-β-induced increases in claudin-1 are reduced by pretreatment with PD98059 (PD, 25 μmol/L) or infection with Ad.SMAD7 to block ERK MAPK and SMAD2/3 signaling, respectively. D: En face confocal scanning laser microscopy reveals a chicken wire pattern of claudin-1 immunoreactivity in control epithelia, which is slightly more prominent in TGF-β-treated T84 cell monolayers (images representative of n = 8 monolayers). The corresponding Z-stack series below each panel (i) shows that the majority of the claudin-1 immunoreactivity (green) is in the lateral cell membrane in the apical compartment of the cell above the nucleus (red) and that TGF-β treatment may result in more claudin-1 along the lateral cell membrane. Original magnifications, ×1260.
TGF-β Prevents Epithelial Barrier Dysfunction Caused by EHEC
TGF-β protects T84 monolayers against loss of barrier function caused by infection with C. parvum.15 Thus, we tested whether TGF-β (10 ng/ml) could prevent the barrier dysfunction caused by other pathogenic bacteria by challenging TGF-β-treated T84 monolayers with EHEC O157:H7 (MOI = 100, 16 hours). Figure 5 shows that a 45-minute TGF-β pretreatment prevented the decrease in TER caused by EHEC infection. EHEC infection caused an ∼30-fold increase in transepithelial flux of [3H]-mannitol: control = 0.49 ± 0.13% versus EHEC infection = 10.63 ± 2.36% of apical mannitol (based on cpm; n = 10 T84 monolayers from four experiments; P < 0.05). TGF-β pretreatment of the epithelial monolayer reduced the EHEC barrier disruption as gauged by [3H]-mannitol flux (control = 0.79 ± 0.09, EHEC = 21.34 ± 3.42, TGF-β + EHEC = 11.61 ± 2.51% of apical mannitol; n = 3 monolayers from one of two experiments).
Figure 5.
Bar graph showing that T84 cell monolayers pretreated with TGF-β (45 minutes, 10 ng/ml) exhibit decreases in TER caused by EHEC (16 hours after infection). Controls consisted of untreated monolayers or infection with the nonpathogenic E. coli HB101 (HB101). Data are presented as the percent change in TER compared to pretreatment values (means ± SEM, *P < 0.05 compared to untreated control and E. coli HB101-treated monolayers; n = 6 to 12 monolayers). Note that TGF-β treatment alone causes a small, but significant increase in TER after 16 hours (#P < 0.05 compared to control), consistent with the time-course data presented in Figure 1. Average pretreatment TER values ranged between 632 to 931 Ω·cm2.
Infection with the related enteric pathogen EPEC also causes epithelial barrier disruption.18 Similar to EHEC infection, TGF-β pretreatment protected T84 monolayers against barrier dysfunction induced by EPEC [103.1 ± 1.7% versus 57.2 ± 3.3%* pretreatment TER, respectively; *P < 0.01 compared to control pretreatment values (95.2 ± 3.3%); n = 3 monolayers].
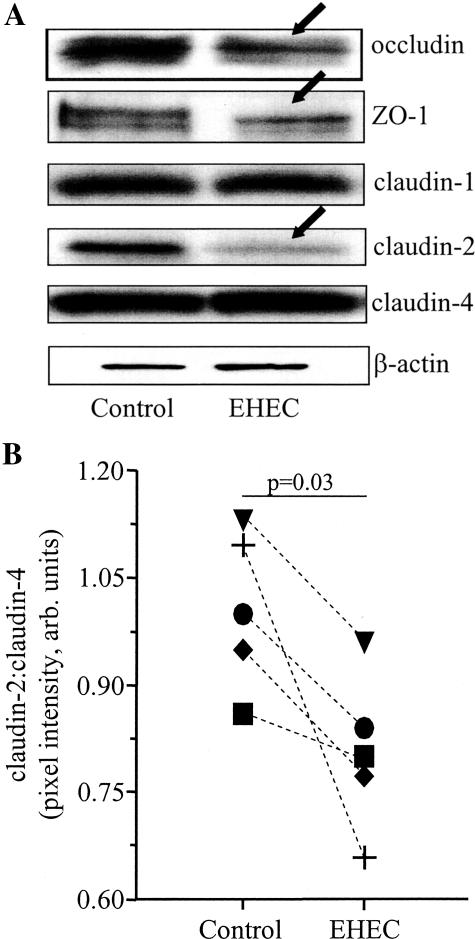
EHEC Infection Causes Reduced Tight Junction Protein Expression
Infection with pathogenic bacteria, such as EHEC, EPEC, and Shigella flexneri, decreases expression of tight junctions and associated proteins.12,25–28 However, little is known about their effects on claudin protein expression. Figure 6A shows representative immunoblots from an analysis of tight junction proteins in our model system. Corroborating our earlier immunolocalization studies,12 ZO-1 expression was reduced by 34 ± 3% 16 hours after EHEC infection (n = 9 T84 cell extracts from three experiments). Similarly, occludin expression was reduced by 27 ± 5% by EHEC infection compared to controls (n = 6 T84 cell extracts from two experiments). Of the three claudins examined, only claudin-2 was significantly reduced by EHEC: claudin-2 expression was significantly reduced by 16 ± 2%* compared to controls (n = 15 T84 cell extracts from five experiments, *P = 0.03; Figure 6B).
Figure 6.
EHEC reduces epithelial tight junction protein expression. A: Representative immunoblots for tight junction proteins in protein extracts from T84 cell monolayers 16 hours after EHEC infection (arrows indicate reduced expression; representative of six to nine monolayers from three experiments; doublets in occludin and ZO-1 immunoblots likely indicate phosphorylated and nonphosphorylated forms). B: Claudin-2 is significantly reduced by comparison of claudin-2:claudin-4 ratio in five experiments (each symbol indicates the average of three epithelial preparations/experiment).
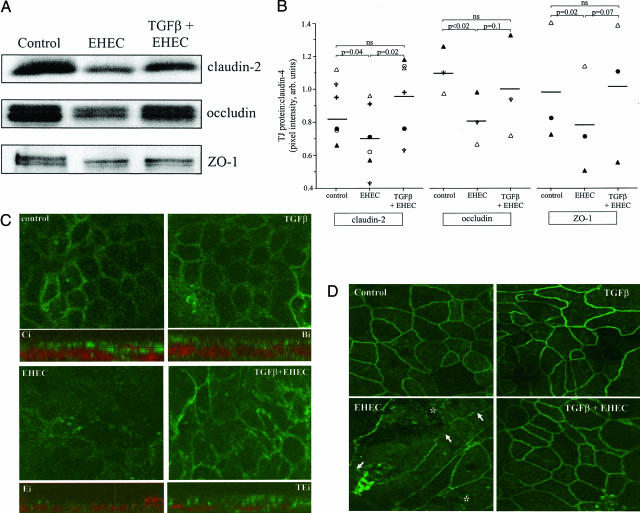
Complementing TGF-β prevention of EHEC-induced increased in epithelial paracellular permeability, immunoblotting revealed that claudin-2, occludin, and ZO-1 expression was preserved in EHEC-infected T84 monolayers pretreated with TGF-β (Figure 7A). Furthermore, confocal microscopy revealed an obvious disruption of the normal distribution of claudin-2-like immunoreactivity in EHEC-treated T84 cell monolayers, an effect that was virtually completely prevented if the cells were pretreated with TGF-β (10 ng/ml, 45 minutes) (Figure 7C). In addition, the accompanying Z-stack compilation images in Figure 7C (each one below the appropriately labeled en face image) not only confirm the lack of claudin-2 expression in EHEC-treated epithelia, they also demonstrate that although claudin-2 can be found throughout the cell, there is clear accumulation of the immunoreactivity at the cell’s apical pole and juxtaposed to a location compatible with the tight junction. The distribution of claudin-2 immunoreactivity in control cells (Ci) and in TGF-β + EHEC (TEi)-treated T84 cell monolayers was virtually indistinguishable (Figure 7C). Finally, confocal microscopic assessment revealed that epithelial monolayers treated with TGF-β displayed ZO-1 immunoreactivity patterns comparable to controls. In contrast, EHEC-infected T84 cells (MOI = 100, 16 hours) had strikingly reduced ZO-1 protein expression (matching the immunoblot analysis). TGF-β pretreatment (45 minutes, 10 ng/ml) prevented this EHEC-induced rearrangement of ZO-1 (Figure 7D).
Figure 7.
TGF-β pretreatment prevents EHEC-induced reductions in tight junction protein expression. A: Representative immunoblot showing that TGF-β (10 ng/ml) prevents EHEC (MOI = 100, 16 hours)-induced reduction in claudin-2, occludin, and ZO-1. The events are quantified in B using the integrated pixel intensity of the tight junction protein:claudin-4 bands (n = 3 to 6 separate experiments, each indicated by a different symbol). C: Shown is the epithelial distribution of claudin-2-like immunoreactivity both as en face images and in corresponding composite Z-stack images below each panel (control, Ci; TGF-β, Ti; EHEC, Ei; TGF-β+EHEC, TEi) (images taken in the x-y plane using confocal microscopy; n = 5 monolayers). The diffuse peripheral claudin-2 immunoreactivity in control preparations is lost in EHEC-infected cells and restored to a large extent by TGF-β pretreatment. The z axis images show that although claudin-2 immunoreactivity (green) can occur throughout the cell, the majority occurs in an apical location (nucleus shown as red based on propidium iodide staining) and is located at the tight junction (note intense punctate staining that indicates tight junction regions of adjacent cells). Similarly, and as shown in D, confocal microscopy confirms that TGF-β pretreatment prevents EHEC (MOI = 100, 16 hours)-induced disruption of the distribution of ZO-1. Representative images of T84 cell monolayers immunostained for ZO-1 taken in the x-y plane using confocal scanning laser microscopy (n = 5 to 7 monolayers; arrows denote loss of ZO-1 at the cell periphery and the increased punctate appearance of ZO-1 is highlighted by the asterisk). Original magnifications, ×1260.
TGF-β-Induced Barrier Protection Is Independent of an Effect on Bacterial Growth
Because cytokines can possess antimicrobial activity,25 we tested whether TGF-β itself, or epithelial factors induced by TGF-β (eg, antimicrobials), could impair EHEC growth. TGF-β did not directly affect EHEC viability after 16 hours of co-incubation (OD600: EHEC 1.3 ± 0.1 versus TGF-β + EHEC 1.3 ± 0.1; n = 2). Bacterial growth was similarly unaffected by supernatants from TGF-β-treated T84 monolayers (data not shown), suggesting that an epithelial-derived factor was also not involved in the preservation of epithelial barrier function via induction of bacterial cell death or inhibition of bacterial proliferation.
TGF-β Does Not Prevent EHEC-Induced IL-8 Production by Intestinal Epithelia
EHEC infection induces IL-8 production from intestinal epithelia.29 Given the protective effects of TGF-β on barrier function, we tested whether pretreatment with TGF-β would prevent EHEC-induced IL-8 production from T84 monolayers. In comparison to untreated control monolayers or TGF-β-treated epithelia (which produced 108 ± 18 pg/ml and 130 ± 20 pg/ml of IL-8, respectively), EHEC-infected T84 cells produced significant amounts of IL-8 that was not prevented by TGF-β pretreatment (193 ± 27* pg/ml and 186 ± 27* pg/ml, respectively; *P < 0.05 compared to control and TGF-β only; n = 5 to 6 monolayers).
Discussion
Intestinal epithelia serve multiple physiological roles, including the provision of a defensive barrier to protect against potentially harmful luminal contents. The importance of maintaining an intact barrier is underscored by recent reports stating increased intestinal permeability contributes to the pathogenesis of several enteric disorders, including inflammatory bowel disease.30 Thus, our aims were to define the mechanism behind TGF-β-induced barrier enhancement and to determine whether this cytokine could prevent epithelial barrier disruption caused by infection with pathogenic bacteria, principally EHEC. We show that TGF-β enhances the epithelial barrier via ERK MAPK and SMAD signaling pathways and up-regulates claudin-1 protein expression. Moreover, we determined that TGF-β preserves the epithelial barrier during EHEC infection and prevents EHEC-induced loss of specific tight junction and tight junction-associated proteins, namely claudin-2, occludin, and ZO-1.
This study corroborates our earlier findings20 and those of others,16 showing that TGF-β enhances the epithelial barrier (at least as gauged by TER). Subsequent molecular assessment of TGF-β-treated T84 epithelia revealed an expected increase in ERK MAPK and SMAD2/3 activation as judged by phosphorylation on immunoblots. Moreover, use of inhibitors of ERK and SMAD2/3 signaling blocked TGF-β-enhancement of barrier function. Whether these pathways regulate epithelial paracellular permeability independently or via intracellular cross-talk (Figure 8)31 remains to be determined. TER, although primarily reflective of tight junction permeability, can also be influenced by membrane ion channels and transporter activity. We found that TGF-β reduced expression of a Cl− channel (ie, the CFTR).32 However, unlike the present study the ion transport effect was insensitive to ERK blockade with PD98058,20 suggesting that the TGF-β-induced increased TER is due to modulation of the tight junction. Also noteworthy, is the fact that a 1-hour TGF-β pulse resulted in increased TER 72 hours later. This lasting effect of TGF-β on epithelial barrier enhancement is unlike the effects of other cytokines, for example IFN-γ, in which the cytokine-induced barrier defect is not observed with an exposure of less than 6 hours duration (D. M. McKay, unpublished observation).
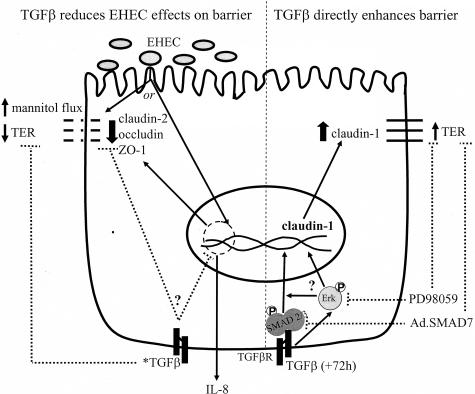
Figure 8.
Model showing putative mechanisms of TGF-β enhancement of epithelial transepithelial resistance and prevention of EHEC-induced barrier disruption but not IL-8 production (*TGF-β; added 45 minutes before EHEC and epithelium assessed 16 hours later).
Having observed physiological changes indicative of altered epithelial tight junction function, we assessed putative changes in the structural elements of the junction. TGF-β exposure led to increased protein expression of claudin-1, but not claudin-2, claudin-4, occludin, or ZO-1. The increased claudin-1 expression was reduced by use of inhibitors of ERK and SMAD2/3 signaling. Immunolocalization studies confirmed that the claudin-1 protein was being trafficked to the site of the tight junctions and that it was indeed possibly extending further down the lateral membrane compared to the pattern of immunoreactivity in control T84 cell monolayers. Our data are supported by the work of Kinugasa and colleagues,33 who found that IL-17 enhances development of the paracellular barrier in immature T84 epithelial monolayers by activation of ERK MAPK and up-regulation of claudin-1 and claudin-2 mRNA and protein expression. However, unlike IL-17, TGF-β did not increase claudin-2 protein expression. This difference indicates cytokine-specific effects on tight junction proteins and suggests distinct roles for claudins during epithelial monolayer development versus barrier enhancement of an established monolayer. Indeed, the protein kinase C agonist bryostatin-1 enhances the barrier function of confluent T84 monolayers by recruiting claudin-1, ZO-2, and occludin to the tight junction complex.34 Although the contribution of other tight junction proteins is less clear, up-regulation of claudin-1 appears to be a common mechanism by which colonic epithelial barrier function can be established and/or enhanced.
In addition to barrier enhancement, TGF-β preserves intestinal epithelial barrier function after exposure to agents known to cause barrier disruption, such as IFN-γ, supernatants from superantigen-stimulated immune cells, and infection with C. parvum.14,15,17 Here we show that TGF-β protects against intestinal epithelial barrier dysfunction caused by infection with EHEC (and EPEC), which is not due to effects on EHEC growth or viability. EHEC and EPEC infections are associated with tight junction changes. Specifically, EHEC infection disrupts the distribution of ZO-1,12 and EPEC causes dephosphorylation of occludin and its subsequent dissociation from the tight junction.26 We observed that pretreatment with TGF-β prevented the EHEC-induced reductions in occludin and ZO-1 and the disruption of ZO-1 distribution. Also, considering the important role of claudin proteins in tight junction formation35 and the claudin-4 epithelial barrier disruption caused by Clostridium perfringens,36 we examined the effects of EHEC infection on claudin expression. Our data show that in the colon-derived T84 cell line, EHEC specifically affects claudin-2 but not claudin-1 or claudin-4, as exhibited by the significant reduction in claudin-2 protein expression and dissociation from the tight junction (preliminary data suggest that EPEC similarly affects claudin-2 expression; unpublished observation). The EHEC-induced reduction in claudin-2 expression was significantly less pronounced in TGF-β-treated cells, correlating with the preservation of the other tight junction proteins (ie, occludin, ZO-1) and the maintenance of the epithelial barrier (ie, TER, [3H]-mannitol flux). At present, the exact role of claudin-2 in tight junction barrier function is unclear. Furuse and colleagues,37 showed that introduction of claudin-2 into high-resistance Madin-Darby canine kidney (MDCK) epithelia caused decreased TER and led to the concept that claudin-2 forms conductive pores within tight junction strands. Indeed, claudin-2 has since been shown to induce cation-selective channels in tight junctions in the MDCK cell line.38 In contrast, induction of claudin-2 expression in low-resistance MDCK cells led to a 20% increase in TER.39 Furthermore, using T84 cells monolayers Nishiyama and colleagues40 found that increased TER was associated with up-regulation of claudin-2 protein expression at the tight junction. Similarly, the loss of barrier integrity observed in IFN-γ + tumor necrosis factor-α-treated T84 cell monolayers was accompanied by reduced claudin-2 expression,41 and, conversely, the enhancement of the barrier property of Caco-2 epithelial cells (another human colon-derived cell line) by retinoic acid was associated with increased claudin-2 expression.42 Thus, the exact role of claudin-2 in epithelial barrier function is controversial, may be cell-type-specific, and may be influenced by the other constituents of the tight junction. Collectively our data suggest that the structural basis for barrier preservation by TGF-β lies in preventing EHEC-mediated down-regulation of claudin-2, occludin, and ZO-1 protein expression and in maintaining the normal distribution of these proteins. Because we found no evidence of direct TGF-β up-regulation of claudin-2 expression, we speculate that TGF-β antagonism of EHEC-induced disruption of epithelial barrier function could be due to interference with a bacteria-induced epithelial signaling pathway43 or stabilization of claudin-2 mRNA (Figure 8).
In terms of other effects on host cell function by EHEC, Dahan and colleagues44 determined that S. boulardii significantly decreased epithelial IL-8 secretion during EHEC infection by inhibiting the MAPK and nuclear factor (NF)-κB signaling pathways responsible for this proinflammatory response. Given the anti-inflammatory properties of TGF-β, we tested the ability of TGF-β to prevent epithelial IL-8 secretion during EHEC infection. TGF-β did not prevent EHEC-induced epithelial IL-8 secretion, indicating that the effect of TGF-β was specific for protection against EHEC-mediated disruption of the epithelial barrier. However, we cannot exclude the possibility that TGF-β may regulate other aspects of an EHEC-induced proinflammatory response. For example, TGF-β exerts anti-inflammatory properties against Bacteroides vulgatus- and lipopolysaccharide-mediated NF-κB activation and subsequent IL-6 secretion in intestinal epithelia.45 Because activation of NF-κB regulates not only IL-8 gene expression but also the expression of several other proinflammatory mediators such as tumor necrosis factor-α and IL-6,46,47 TGF-β may exert anti-inflammatory properties against EHEC-induced expression of such genes. Finally, EHEC-derived Shiga toxin and its translocation across the epithelium pose a significant biological threat to renal function that may culminate in hemolytic uremic syndrome. It is therefore noteworthy that elevated levels of TGF-β are associated with reduced incidence of hemolytic uremic syndrome in children infected with EHEC O157:H7,48 making it feasible that increased TGF-β enhances epithelial barrier function and limits toxin entry into the body.
TGF-β influences many aspects of intestinal epithelial homeostasis. We found that TGF-β enhancement of epithelial barrier function was associated with increased claudin-1 expression, an ERK MAPK- and SMAD2/3-dependent event. In addition, preservation of epithelial barrier integrity in the face of EHEC infection by TGF-β was accompanied by maintenance of the levels and distribution of claudin-2, occludin, and ZO-1 (Figure 8). Thus, awareness of TGF-β enhancement of epithelial barrier function is now complemented by data on the intracellular signaling pathways and structural modification of the tight junctions that mediate this physiological and protective effect of TGF-β in the intestine. Overall, the data presented offer compelling evidence that differential regulation of tight junction protein expression, particularly claudins, by TGF-β provides beneficial effects capable of enhancing the epithelial barrier and protecting it against severe insult by pathogenic bacteria.
Acknowledgments
We thank Jun Lu for technical assistance.
Footnotes
Address reprint requests to Derek M. McKay, Ph.D., Intestinal Disease Research Programme, McMaster University, HSC-3N5C, 1200 Main St. West, Hamilton, ON, Canada L8N 3Z5. E-mail: mckayd@mcmaster.ca.
Supported by the Canadian Institutes of Health Research (grant MT-13421 to D.M.M.).
K.L.H. has been a recipient of a Natural Sciences and Engineering Research Council studentship (2001 to 2003) and a Canadian Institutes of Health Research/Canadian Digestive Health Foundation partnership award (2003 to 2004).
References
- Karmali MA, Steele BT, Petric M, Lin C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;1:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- Vallance BA, Finlay BB. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2000;97:8799–8806. doi: 10.1073/pnas.97.16.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. Am J Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann NY Acad Sci. 2000;915:129–135. doi: 10.1111/j.1749-6632.2000.tb05235.x. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedelind S, Ericson LE, Karlsson JO, Nilsson M. Interferon-γ down-regulates claudin-1 and impairs the epithelial barrier function in primary cultured human thyrocytes. Eur J Endocrinol. 2003;149:215–221. doi: 10.1530/eje.0.1490215. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849–855. doi: 10.1016/S0171-9335(99)80086-7. [DOI] [PubMed] [Google Scholar]
- Dickman KG, Hempson SJ, Anderson J, Lippe S, Zhao L, Burakoff R, Shaw RD. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am J Physiol. 2000;279:G757–G766. doi: 10.1152/ajpgi.2000.279.4.G757. [DOI] [PubMed] [Google Scholar]
- Philpott DJ, McKay DM, Mak W, Perdue MH, Sherman PM. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect Immunity. 1998;66:1680–1687. doi: 10.1128/iai.66.4.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley BP, Thorpe CM, Acheson DW. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immunity. 2001;69:6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchon SM, Martins CAP, Guerrant RL, Roche JK. Regulation of intestinal barrier function by TGFβ1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–5739. [PubMed] [Google Scholar]
- Roche JK, Martins CA, Cosme R, Fayer R, Guerrant RL. Transforming growth factor beta1 ameliorates intestinal epithelial barrier disruption by Cryptosporidium parvum in vitro in the absence of T lymphocytes. Infect Immunity. 2000;68:5635–5644. doi: 10.1128/iai.68.10.5635-5644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchon SM, Fiocchi C, Takafuji V, Roche JK. Transforming growth factor-β1 preserves epithelial barrier function: identification of receptors, biochemical intermediates, and cytokine antagonists. J Cell Physiol. 1999;181:55–66. doi: 10.1002/(SICI)1097-4652(199910)181:1<55::AID-JCP6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- McKay DM, Singh PK. Superantigen-activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via interferon-γ and tumor necrosis factor-α. Inhibition of increased permeability, but not diminished secretory responses by transforming growth factor β2. J Immunol. 1997;159:2382–2390. [PubMed] [Google Scholar]
- Philpott DJ, McKay DM, Sherman PM, Perdue MH. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- Nazli A, Yang PC, Jury J, Howe KL, Watson JL, Soderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164:947–957. doi: 10.1016/S0002-9440(10)63182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KL, Gauldie J, McKay DM. TGF-β effects on epithelial ion transport and barrier: reduced Cl− secretion blocked by a p38 MAPK inhibitor. Am J Physiol. 2002;283:C1667–C1674. doi: 10.1152/ajpcell.00414.2001. [DOI] [PubMed] [Google Scholar]
- Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, ten Dijke P, Gressner AM. Smad 7 prevents activation of hepatic stellate cells and live fibrosis in rats. Gastroenterology. 2003;125:178–191. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- Kato S, Ueda S, Tamaki K, Fujii M, Miyazono K, ten Dijke P, Morimatsu M, Okuda S. Ectopic expression of Smad 7 inhibits transforming growth factor-β responses in vascular smooth muscle. Life Sci. 2001;69:2641–2652. doi: 10.1016/s0024-3205(01)01350-9. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NFκΒ-independent pathway. FASEB J. 2003;17:1319–1321. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- Mehra A, Wrana JL. TGFβ and the SMAD signal transduction pathway. Biochem Cell Biol. 2002;80:605–622. doi: 10.1139/o02-161. [DOI] [PubMed] [Google Scholar]
- Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect Immunity. 2001;69:5679–5688. doi: 10.1128/IAI.69.9.5679-5688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol. 2002;4:367–381. doi: 10.1046/j.1462-5822.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- Dahan S, Busuttil V, Imbert V, Peyron JF, Rampal P, Czerucka D. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NFκΒ and AP-1 in T84 cells. Infect Immunity. 2002;70:2304–2310. doi: 10.1128/IAI.70.5.2304-2310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- Howe KL, Wang A, Hunter MM, Stanton BA, McKay DM. TGFb down-regulation of the CFTR: a means to limit epithelial chloride secretion. Exp Cell Res. 2004;298:473–484. doi: 10.1016/j.yexcr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- Yoo J, Nichols A, Mammen J, Calvo I, Song JC, Worrell RT, Matlin K, Matthews JB. Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins. Am J Physiol. 2003;285:C300–C309. doi: 10.1152/ajpcell.00267.2002. [DOI] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Colegio OR, van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC. Interleukin-2 receptor-β subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem. 2001;276:35571–35580. doi: 10.1074/jbc.M106013200. [DOI] [PubMed] [Google Scholar]
- Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- Baltes S, Nau H, Lampen A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev Growth Differ. 2004;46:503–514. doi: 10.1111/j.1440-169x.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, Madara JL. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol. 1999;277:C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- Dahan S, Dalmasso G, Imbert G, Peyron JF, Rampal P, Czerucka D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect Immunity. 2003;71:766–773. doi: 10.1128/IAI.71.2.766-773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller D, Holt L, Kim SC, Schwabe RF, Sartor RB, Jobin C. Transforming growth factor-β1 inhibits non-pathogenic Gram negative bacteria-induced NFκΒ recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–23860. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-α and IL-6 production through NF-κΒ in peritoneal mast cells. Biochim Biophys Acta. 2003;1643:75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Proulx F, Litalien C, Turgeon JP, Mariscalco MM, Seidman E. Circulating levels of transforming growth factor-β1 and lymphokines among children with hemolytic uremic syndrome. Am J Kidney Dis. 2000;35:29–34. doi: 10.1016/s0272-6386(00)70297-6. [DOI] [PubMed] [Google Scholar]