Abstract
Tumor necrosis factor (TNF) plays a critical role in the host response to the intracellular pathogen Listeria monocytogenes (LM). TNF exists in soluble and membrane-bound forms and exhibits both unique and overlapping activities. We examined the role of membrane TNF in the absence of secreted TNF for host resistance in knockin mice in which the endogenous TNF was replaced by a regulated, noncleavable allele (mem-TNF). Macrophages expressing mem-TNF produced nitric oxide and displayed normal bactericidal activity. Although mice completely deficient in TNF (TNF−/−) succumbed to LM infection within 4 days, mem-TNF mice controlled LM infection at a low dose (104 CFU) but succumbed at a higher dose of infection (105 CFU). In contrast to complete TNF deficiency, mem-TNF mice developed confined microabscesses that expressed inducible nitric oxide synthase. The transfer of lymphocytes from immunized mem-TNF, but not TNF−/−, mice protected TNF−/− mice from fatal infection. Taken together the data suggest that in the absence of soluble TNF, the presence of membrane-expressed TNF on phagocytes and lymphocytes partially restores host defense to LM infection.
Protective immunity to Listeria monocytogenes (LM) infection, both in humans and experimental animals, is based on orchestrated action of T cells, macrophages, and cytokines, including interferon (IFN)-γ, interleukin (IL)-12, and tumor necrosis factor (TNF).1 A critical role for TNF in anti-LM defense is inferred from neutralization and gene deletion experiments in mice.2,3 In addition, the TNF-related cytokines lymphotoxin (LT)-α and LT-β are also required to control LM infection.4 Both secreted TNF and LT-α signal through p55 and p75 TNF receptors (TNFR1 and TNFR2, respectively). The cell-bound LT-αβ heterotrimers recognize the LT-βR. TNF-R1 signaling appears to be critical for the control of LM infection2,3 and LT-βR also plays a distinct role, while the contribution of TNFR2 is less well defined.
TNF is expressed by a variety of cells, including macrophages and T cells, and is a major regulator of inflammation and leukocyte trafficking.5 TNF is first produced as an integral membrane protein and is subsequently cleaved by the metalloproteinase-disintegrin TACE (TNF-α converting enzyme)6,7 into the secreted trimeric TNF. Although the role of TNF in controlling intracellular bacterial infections is uncontested, the function of membrane TNF in host resistance is less understood.
Several biological functions of membrane TNF have been described, such as cytotoxicity, polyclonal activation of B cells, induction of IL-10 by monocytes, induction of chemokines, and ICAM-1 expression on endothelial cells.8–10 The transgenic overexpression of membrane TNF has demonstrated an in vivo role in the control of LM and mycobacterial infection.11–13 However, these models were potentially nonphysiological as transgenic expression of a membrane-only form of TNF results in artificially high and nonselective expression of membrane TNF. The recent generation of mice with functional, normally regulated and expressed membrane-bound TNF, obtained by knocking-in an uncleavable Δ1-9, K11E TNF allele (mem-TNF mice), represents a major advance and allows interesting insights in the role of membrane TNF in lymphoid structure development and inflammation.14
The question whether membrane TNF expression may be sufficient to control Listeria infection is becoming very relevant. Indeed, TNF-neutralizing therapies using antibodies or soluble receptors have been very successful in severe inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, or psoriasis, with more than a million patients treated. However, opportunistic infections including Listeria or tuberculosis represent a major complication of these therapies.15–18 Therefore, the identification of the respective roles of membrane-bound versus soluble TNF in host response to infection may elucidate second generation anti-TNF therapies targeted to spare the host defense to infections.
In the present investigation we asked whether membrane TNF is sufficient to contain LM infection. We used the mem-TNF knockin mice and compared the host resistance to LM infection in mem-TNF mice and TNF-deficient mice. Our data demonstrate that membrane TNF is biologically active and may act as a substitute for soluble TNF at low-dose infection because mem-TNF mice survive an LM infection that is fatal for complete TNF−/− mice. Furthermore, cell transfer experiments from immunized mem-TNF mice to TNF-deficient mice suggest that LM-antigen-specific T cells that express membrane-only TNF are sufficient to confer protection against LM infection.
Materials and Methods
Mice
Mem-TNF,14 TNF−/−,19 and C57BL/6 control mice were bred in our specific pathogen-free animal facility at the Centre National de la Recherche Scientifique. Mem-TNF mice were generated on a C57BL/6 background while TNF−/− mice were backcrossed for 10 generations onto the C57BL/6 background. For all experiments adult (8 to 12 weeks old) animals were kept in sterile confinement in a P2 animal unit. The infected mice were monitored regularly for clinical status and weighed daily. Mice were bled before and at 1, 2, 3, and 4 days after infection to assess the hematological parameters in the blood using a Technikon H1E analyzer (Bayer, Paris, France). All animal experiments complied with the French Government’s ethical and animal experiment regulations.
Culture of Bacteria
LM wild-type L028 and ActA deficient strains were provided by Prof. P. Cossart (Pasteur Institute, Paris, France) and cultured in trypticase soy broth (soybean casein digest medium; Biovalley, Marve la Vallée, France). Bacteria were aliquoted and stored in 30% glycerol at −80°C at a concentration of 5 × 109 CFU/ml. Heat-killed LM (HKLM) was prepared by incubation at 60°C for 1 hour followed by two washes with sterile phosphate-buffered saline (PBS).
Primary Macrophage and Dendritic Cell Culture
Murine bone marrow cells were isolated from femurs and differentiated into macrophages after culturing at 106 cells/ml for 7 days in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 20% horse serum and 30% L929 cell-conditioned medium as described before.20,21 Seven days after washing and reculturing in fresh medium, the cell preparation consisted of a homogenous population of macrophages (>97% CD11b+ cells). Alternatively, murine bone marrow cells were differentiated into myeloid dendritic cells (>98% CD11c+ cells) after culturing at 2 × 105 cells/ml for 10 days in RPMI supplemented with 10% fetal calf serum, glutamine, antibiotics, and 4% J558L cell-conditioned medium as a source of GM-CSF, as described.22
Bone marrow-derived macrophages (BMDMs) and dendritic cells were plated in 96-well microculture plates (at 105 cells/well) and stimulated with lipopolysaccharide (LPS) (Escherichia coli, serotype O111:B4 at 100 ng/ml; Sigma), HKLM (at a bacteria:cell ratio of 200:1), and live LM (at a bacteria:cell ratio of 2:1). After 24 hours of stimulation, the supernatants were harvested for cytokine determination.
Macrophage Killing Assay
To test LM killing, macrophages from the different strains were incubated for 20 minutes at 37°C with LM at a multiplicity of infection (MOI) of 1 in Dulbecco’s modified Eagle’s medium complemented with l-glutamine (5 mmol/L) and 10% fetal calf serum. After addition of gentamicin (10 μg/ml), macrophages were extensively washed to remove extracellular bacilli, and incubated in the same medium with and without recombinant mouse TNF (rmTNF, 10 ng/ml; PreproTech, Rocky Hill, NJ). After 3 and 6 hours of incubation, the number of viable intracellular bacteria in each well was determined by culturing on tryptic soy broth agar plates. Plating was performed in duplicate serial dilutions and macrophage killing was assessed by the determination of CFU in infected macrophages from two individual mice per group.
Cytokine Determination
IL-12p40 or IFN-γ were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits following the instructions of the manufacturer (Duoset; R&D Systems, Abingdon, UK). As described previously, bioactive TNF was assessed using the WEHI 164 cell-based bioassay, which has a higher sensitivity than ELISA, detecting 0.5 pg/ml of TNF.23
Flow Cytometry
After stimulation, macrophages were harvested, washed once in PBS containing 0.5% bovine serum albumin (PBS/BSA), and incubated on ice at 105 cells/50 μl with 2% mouse serum for 20 minutes. After centrifugation (10 minutes at 200 × g, 4°C), macrophages were incubated in PBS/BSA with primary antibodies (anti-CD40-PE clone 2G9, anti-CD86-PE clone GL1, or anti-CD11b-PerCP Cy5.5 clone M1/70) for 20 minutes in the dark. All antibodies were from BD Pharmingen (San Diego, Ca). After washing with PBS/BSA, cells were analyzed on a Becton Dickinson LSR analyzer.
Mouse Infection, Vaccination, and Transfer of Splenic T Cells
Infection
Mice were injected intravenously in the caudal vein with 104 or 105 CFU of LM per mouse as described before.21 On day 3, liver and spleen were harvested. The number of viable bacteria in organ homogenates was determined by plating serial dilutions on trypticase soy broth agar plates (Biovalley) and incubating for 24 hours at 37°C followed by counting CFU.
Restimulation and Transfer of Splenic Cells
Splenocyte suspensions were obtained from C57BL/6, TNF−/−, and mem-TNF mice 7 days after injection of 106 ACTA-deficient LM or saline. Nonadherent cells were restimulated in vitro with LM, HKLM, listeriolysin (LLO) peptide (fragment 189-201; Neosystem, Strasbourg, France), or Mycobacterium bovis-bacillus Calmette-Guerin (BCG), and the supernatants were assessed for IFN-γ levels by ELISA. For the cell transfer, 2 × 107 nonadherent cells (>95% lymphocytes with 65% CD3+ T cells) in 200 μl were injected intravenously into TNF−/− recipient mice, followed 1 hour later by intravenous injection of LM at 104 CFU/mice. Body weight was recorded daily, and the spleen and liver were taken 3 days after infection to enumerate viable bacteria.
Histology and Immunohistochemistry
Samples of liver and spleen were fixed in 10% buffered formalin (Shandon, Pittsburgh, PA). Tissues were dehydrated in ethanol and embedded in paraffin. Sections (4 μm) were cut and stained with hematoxylin and eosin (H&E) for evaluation of pathological changes. The number of microabscesses was quantified by counting 20 microscopic fields at ×100 magnification. The size of microabscesses was assessed by measuring the diameter of 20 randomly selected micro-abscesses per mouse at ×400 magnification.
For immunohistochemical analysis, liver and spleen were embedded with Tissue-Tek (Sakura, Zoeterwoude, The Netherlands) in cryomolds, immediately frozen on dry ice, and stored at −80°C as described before.24 The frozen tissues were cut at 5-μm thickness on a cryostat (Leica, Nussloch, Germany), air-dried, and stored at −80°C. Before use the sections were fixed in acetone (10 minutes at 4°C), and endogenous peroxidase activity was blocked using methanol with 1% H2O2 (30 minutes). Endogenous biotin in the liver was blocked using PBS containing 0.1% avidin (20 minutes) and PBS containing 0.01% biotin (20 minutes). The tissue sections were incubated with appropriate normal serum (30 minutes) before incubation for 2 hours at 37°C with the primary antibody. Antibodies to GR1, F4/80, and inducible nitric oxide synthase (iNOS) were from BD Pharmingen. The sections were then incubated for 30 minutes at 37°C with the appropriate biotinylated secondary antibody. Avidin-biotin peroxidase complexes were added to the sections for 30 minutes (ABC Vector kit; Vector Laboratories, Burlingame, CA), washed, and developed with diaminobenzidine substrate (DAKO, Glostrup, Denmark). After rinsing in PBS, the sections were mounted in Eukitt (Kindler and Co., Freiburg, Germany).
Nitrite Measurements
Nitrite concentrations in supernatants from macrophages were determined using the Griess reaction (1% sulfanilamide in 2.5% phosphoric acid and 0.1% n-1-napthylethylenediamide dichloride in 2.5% phosphoric acid).25 After a 30-minute incubation at room temperature under agitation, the absorbance at 540 nm was measured (NO2− was quantified using NaNO2 as a standard).
Statistical Analysis
Statistical evaluation of differences between the experimental groups was determined by Kaplan-Meier test for survival curves, Mann-Whitney U-test for ex vivo experiments, and Student’s t-test for in vitro data, using Prism software.
Results
Absence of Secreted TNF, but Normal Nitric Oxide Production and Killing of LM by Mem-TNF Macrophages
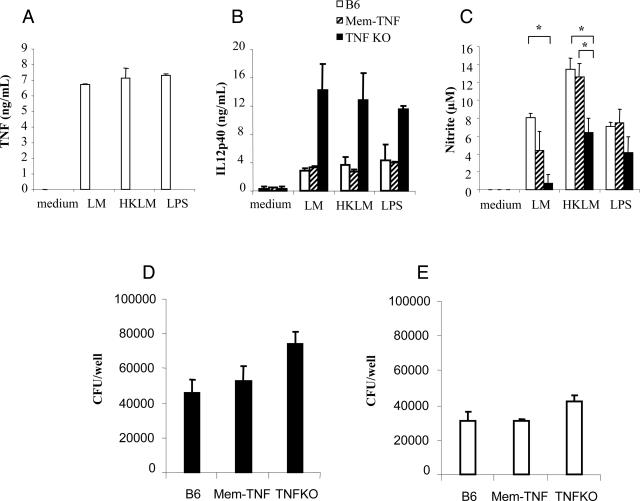
We first investigated whether cultured BMDM secrete TNF in response to LPS, LM, or heat-killed LM (HKLM). Soluble TNF was secreted by BMDM from wild-type mice but was essentially undetectable in culture supernatants of BMDM from mem-TNF and TNF−/− mice using the sensitive WEHI bioassay (Figure 1A), in agreement with the original report.14 To confirm that mem-TNF knockin mice do not secrete TNF in vivo, mice were injected intraperitoneally with 100 μg of LPS, blood was collected 90 minutes later, and serum was analyzed for TNF content. In contrast to wild-type mice, which displayed significant serum TNF levels, TNF was undetectable in the sera of both TNF−/− and mem-TNF mice (data not shown). We next determined whether IL-12p40 and nitric oxide production by LPS-, LM-, or HKLM-stimulated BMDMS was dependent on TNF expression. Macrophages secreted IL-12p40 on stimulation with LPS, LM, or HKLM, and we observed unexpectedly high IL-12p40 levels in TNF−/− as compared to wild-type macrophages (Figure 1B). An overproduction of Th1 cytokines has been demonstrated previously in TNF-deficient mice on Mycobacterium bovis-bacillus Calmette-Guerin (BCG) infection,26,27 suggesting that TNF has a regulatory role for Th1 cytokine response. Such a deregulation of Th1 response has not been reported for LM infection, so we then asked whether mem-TNF macrophages retained an augmented IL-12p40 response comparable to TNF−/− macrophages. Interestingly, mem-TNF macrophages expressed IL-12p40 levels comparable to those of wild-type macrophages. The data therefore suggest that membrane TNF prevents the exaggerated IL-12p40 responses seen in the complete absence of TNF, but the mechanism of deregulated Th1 response is still unclear.26,27
Figure 1.
Impaired soluble TNF but normal IL-12p40, nitrite production, and killing of LM by macrophages from mem-TNF mice. A–C: TNF (A), IL-12p40 (B), and nitrite (C) production measured in the supernatant of macrophages from C57BL/6, mem-TNF, and TNF−/− mice 24 hours after infection by LM (MOI of 2) or stimulation by heat-killed LM (HKLM; 200 bacteria per cell) or LPS (100 ng/ml). Results are means ± SD of two mice per genotype and are representative of three independent experiments (*P < 0.05). D and E: Killing of viable LM by macrophages expressed as CFU per 105 macrophages at 3 hours. D: Macrophages were infected with LM (MOI of 1) for 20 minutes, washed extensively to remove extracellular bacilli, and further incubated for 3 hours, after which CFU were determined. E: Addition of recombinant mouse TNF (10 ng/ml) corrected the killing of LM by TNF−/− macrophages. Results expressed as CFU per 2 × 105 macrophages at 3 hours after infection showed no significant differences. Data are given as the mean ± SD (n = 2 mice with duplicate CFU analysis per group) and are from one representative experiment of two independent experiments.
Killing of bacilli critically depends on activation of NOS2.28 We therefore tested the production of nitrite after LM macrophage activation. Nitrite production in mem-TNF BMDMs stimulated with LM, HKLM, or LPS was similar to the levels seen in wild-type cells but partially reduced in TNF−/− macrophages (Figure 1C). To assess the biological relevance of the measured nitrite levels, the bactericidal activity of mem-TNF macrophages was tested. Macrophages from mem-TNF mice had capacity to kill LM comparable to that of wild-type cells, and CFU levels were higher in TNF-deficient macrophages at 3 hours (Figure 1D) and 6 hours (data not shown), but this did not reach statistical significance. Addition of recombinant mouse TNF to the macrophages in culture increased the bactericidal effect in all groups, but importantly, it corrected the defect of TNF-deficient macrophages (Figure 1E). Therefore, the data suggest that membrane TNF expressed by macrophages is sufficient to induce early nitrite production by macrophages and killing of LM, both of which are impaired in the complete absence of TNF. Lastly, LM-induced up-regulation of co-stimulatory molecules in macrophages and dendritic cells was TNF-independent because CD40 and CD86 expression was comparable in wild-type, mem-TNF, and TNF−/− macrophages (data not shown).
Taken together, these observations confirm the absence of functional soluble TNF in mem-TNF mice14 and imply that membrane-bound TNF can substitute soluble TNF in some of its functions: macrophages from mem-TNF mice were able to produce nitrite and control infection in vitro. Therefore, it was reasonable to assume that membrane TNF may confer resistance to LM infection in vivo.
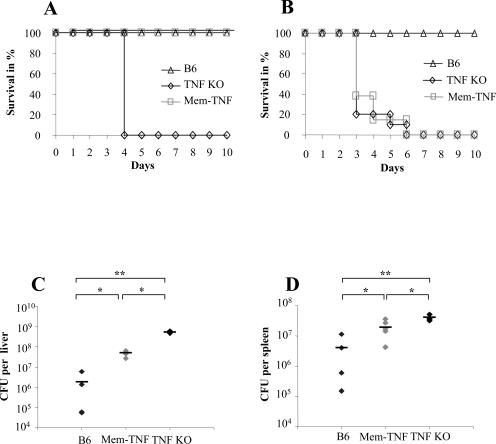
Mem-TNF Mice Are More Resistant to LM Infection Than TNF−/− Mice
A critical role for TNF has been shown in the control of LM infection.2,3 We confirmed the role of TNF and further ascribed it to both soluble and membrane TNF (Figure 2, A and B). Soluble TNF was dispensable at low infection dose of LM (104 CFU) because mem-TNF mice survived a dose at which TNF−/− mice succumbed (P < 0.05, Figure 2A). However, at the higher infectious dose of 105 CFU, both TNF−/− and mem-TNF mice, but not wild-type mice, succumbed to infection (P < 0.05, Figure 2B). The increased resistance of mem-TNF mice correlated with reduced bacterial burden in the liver of these mice as compared to TNF−/− mice after infection with 104 CFU of LM (P < 0.05, Figure 2C). In mem-TNF mice the CFU values were slightly higher than in wild-type mice in liver and spleen (P < 0.05; Figure 2, C and D). Thus, the bacterial burden in mem-TNF was intermediate from the values found in wild-type and TNF−/− mice. The results indicate that in the absence of soluble TNF, membrane-bound TNF confers substantial protection to LM infection, sufficient to control a low-dose but not a high-dose infection.
Figure 2.
Enhanced control of LM infection in mem-TNF mice compared to TNF-deficient mice. A and B: Survival on intravenous infection with doses of LM at 104 CFU (A, n = 4 mice; Kaplan-Meier B6 versus TNF KO, P < 0.01; mem-TNF versus TNF KO, P < 0.01) or at 105 CFU (B, n = 9 to 13; B6 versus mem-TNF, P < 0.01; B6 versus TNF KO, P < 0.01). C and D: Bacterial load in the liver (C) and spleen (D) of mem-TNF, TNF−/− mice (KO), and wild-type (B6) mice 3 days after infection with 104 CFU. Each group comprised four to six mice; mean values ± SD are given. *P < 0.05, **P < 0.01. The results are from one experiment representative of two independent experiments.
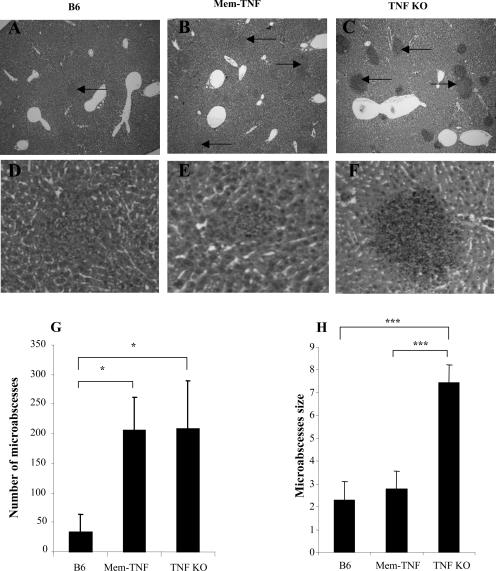
Smaller and Confined Hepatic Microabscesses in Mem-TNF Mice
To gain more insight in the cellular mechanisms of mem-TNF sustained resistance we next investigated the morphology of liver tissue 3 days after infection. The typical hepatic microabscesses rich in neutrophils induced by LM infection were more abundant in mem-TNF mice as compared to wild-type controls while the microabscesses appeared larger with diffuse infiltration and necrotic areas in the liver parenchyma in TNF−/− mice (Figure 3, A–F). The number of microabscesses was increased in mem-TNF and TNF−/− livers as compared to the wild-type controls (Figure 3G). In contrast, the size of the micro-abscesses increased in TNF−/− mice but appeared essentially normal in mem-TNF mice (Figure 3H).
Figure 3.
Numerous but confined, smaller hepatic microabscesses in mem-TNF mice compared to TNF-deficient mice. A–F: Histological sections of livers showing small confined microabscesses in mem-TNF and wild-type mice and spreading infection in TNF-deficient mice (TNF KO) (H&E staining). G: Increased number of microabscesses in mem-TNF and TNF-deficient mice as compared to wild-type controls. *P < 0.05. H: Normal-sized microabscesses in mem-TNF mice as compared to TNF-deficient mice. Mean diameters ± SD (arbitrary units) of abscesses are given. ***P < 0.001. Wild-type, mem-TNF, and TNF-deficient mice were infected with 105 CFU of LM and examined for histology 2 days after infection (n = 4 mice per group). Original magnifications: ×40 (A–C); ×100 (D–F).
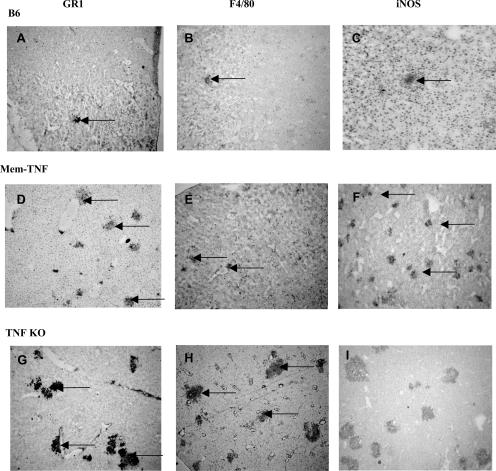
Concomitant with the increased size, semiquantitative immunohistochemistry analysis of liver sections revealed increased neutrophil (GR1+) and macrophage (F4/80+) levels in TNF−/− (Figure 4, G and H) but not in mem-TNF-infected mice (Figure 4, D and E) compared to wild-type mice (Figure 4, A and B). The hepatic expression of iNOS, as assessed by immunostaining,24 was similar in microabscesses of mem-TNF mice (Figure 4F) as compared to wild-type controls (Figure 4C) but distinctly more pronounced than in TNF−/− mice (Figure 4I). Therefore, the morphological assessment revealed increased leukocyte recruitment in TNF−/− mice. Membrane TNF expression allowed a controlled recruitment of neutrophils. The activation of inflammatory cells seemed more effective in the liver of membrane TNF-expressing mice as compared to complete TNF deficiency, as illustrated by the iNOS expression levels.
Figure 4.
Increased neutrophil recruitment and iNOS expression in hepatic microabscesses from mem-TNF mice. Wild-type, mem-TNF, and TNF-deficient mice were infected with 104 CFU of LM, and frozen liver sections were immunostained with GR1, F4/80, or NOS2 antibodies, as described in Materials and Methods. Black arrows point to positive staining in microabscesses. Representative micrographs are shown (n = 4 mice per group). Original magnifications, ×40.
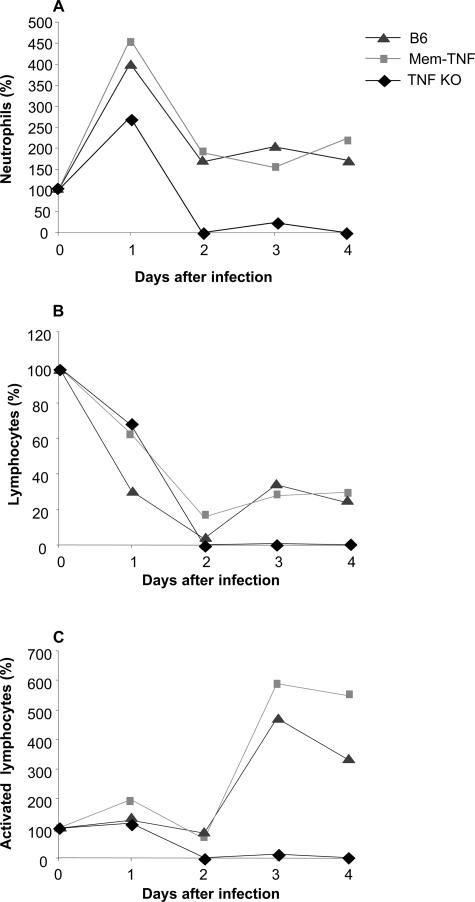
To follow more closely the systemic effects of LM infection, blood differential leukocyte counts were determined. An augmented base-line leukocyte count in TNF−/− and LT-α−/− mice has been reported before and may be due to a homing defect,29,30 which is not seen in mem-TNF mice (data not shown). To normalize the data we expressed the changes in blood cell counts as percentage of the cell counts before infection. LM infection induced an initial increase (day 1) followed by a decrease of neutrophil counts on day 2 after infection (Figure 5A). This neutropenia was transient in wild-type and mem-TNF mice and recovered thereafter (data not shown). Neutropenia was pronounced and sustained in TNF−/− mice. Lymphocyte counts were reduced in all groups at 2 days, in line with published data on massive apoptosis of lymphocytes in the spleen. Lymphocyte numbers partially recovered thereafter in mem-TNF and wild-type mice but remained very low in TNF−/− mice (Figure 5B). Activated lymphocytes, as defined by their morphology, were found in controls and mem-TNF mice but absent in TNF−/− mice (Figure 5C). Therefore, membrane TNF provides protective signals that allow a controlled systemic and hepatic inflammation with a rapid recovery of blood counts after infection.
Figure 5.
Sustained reduction of circulating neutrophils and lymphocytes in TNF-deficient, but not mem-TNF, mice after LM infection. Changes of neutrophil (A), lymphocyte (B), and activated lymphocyte (C) blood counts after LM infection are shown as percentage of the preinfection cell counts. Mice were infected with 104 CFU of LM, and hematological parameters were determined at days 0 and 1 to 4 (n = 4) using a Technikon H1E analyzer.
T-Cell Response to LM and Protection of TNF-Deficient Mice by Transfer of Immune T Cells from Mem-TNF Mice
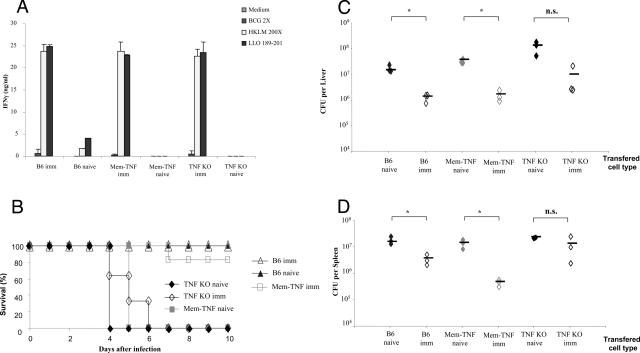
Because the expression of co-stimulatory molecules was normal in the complete absence of TNF, we asked whether TNF-deficient mice infected with the attenuated, ActA-deficient LM strain were able to mount protective immunity. First, the T-cell response induced by ActA-deficient LM infection was tested, on ex vivo restimulation of splenic T lymphocytes with HKLM and LLO peptide 189-201. T cells from mem-TNF and TNF−/− mice injected with 106 ActA-deficient LM attenuated strain 7 days before responded with a comparable IFN-γ production as wild-type mice to restimulation by HKLM or LLO 189-201 (Figure 6A). This response was specific to LM because no restimulation was obtained with the unrelated mycobacterium BCG. Therefore, TNF appears not to be required for an antigen-specific immune response, consistent with previous reports.31,32
Figure 6.
Enhanced resistance of TNF-deficient mice to virulent LM infection after lymphocyte transfer from immune mem-TNF mice. A: Production of IFN-γ on antigen restimulation of T cells from LM-infected mice, independent of TNF. Splenic T cells from wild-type, mem-TNF, and TNF−/− mice, either naïve or infected with 106 CFU of ActA-deficient LM strain 7 days before (imm), were restimulated ex vivo with HKLM (200 bacteria per cell) and LLO 189-201 (1 μg/ml) or an irrelevant antigen (BCG at an MOI of 2): IFN-γ levels were measured in the supernatant at 24 hours by ELISA. Data are expressed as the mean ± SD (n = 3 mice). B: Enhanced survival of TNF-deficient mice receiving lymphocytes from immune mem-TNF mice. Splenic nonadherent cells (2 × 107) from naïve or ActA-deficient LM-vaccinated C57BL/6, mem-TNF, and TNF-deficient mice (as above) were transferred into TNF−/− mice 1 hour before intravenous infection with 104 CFU of LM. Survival was recorded daily (n = 3 naïve and n = 6 immune mice; Kaplan-Meier mem-TNF naïve versus mem-TNF imm, P < 0.05). C and D: Control of bacterial growth in TNF-deficient mice by adoptive transfer from immune mem-TNF, but not TNF-deficient, mice. Bacterial load in liver (C) and spleen (D) of TNF-deficient mice reconstituted with immunized splenic cells as in (B), 3 days after infection with 104 CFU of LM. Bacterial load expressed as CFU per organ, individual counts from one representative of two experiments are shown (*P < 0.05).
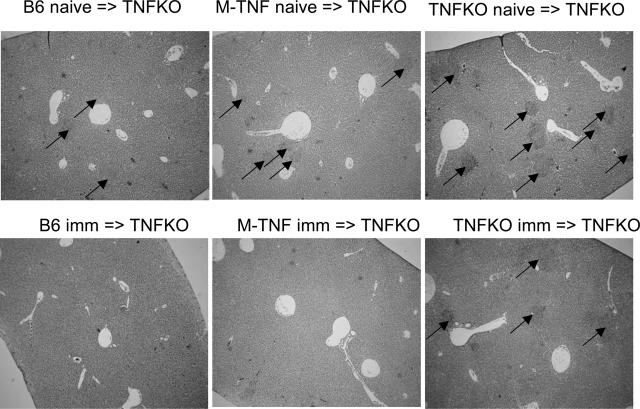
We next asked whether membrane TNF expressed on antigen-specific lymphocytes was sufficient to confer effective protection in vivo. To this end, TNF−/− mice received splenic lymphocytes (2 × 107 cells, 95% lymphocytes) from wild-type, mem-TNF mice, or TNF−/− mice, either naïve or preinfected for 7 days with ActA-deficient LM strain (106 CFU). The recipient mice were then challenged with 104 CFU of virulent LM. Lymphocytes from ActA-deficient LM-immunized wild-type and mem-TNF mice, but not TNF−/− mice (Figure 6A), prevented body weight loss (data not shown), and the reconstituted TNF−/− mice survived the virulent LM infection (P < 0.05, Figure 6B). The bacterial load in liver and spleen was tested on transfer of immune lymphocytes in TNF−/− mice. Three days after infection, CFU levels in liver and spleen were significantly lower in TNF−/− mice receiving an adoptive transfer of lymphocytes from immunized C57BL/6 or mem-TNF mice (Figure 6, C and D) but not from immune TNF−/− mice. Finally, TNF−/− mice transferred with immune lymphocytes from wild-type or mem-TNF mice, but not TNF−/− mice, had very small hepatic granulomas compared to TNF−/− control mice (Figure 7), indicating that the inflammation and infection were controlled by the adoptive transfer of mem-TNF-expressing lymphocytes.
Figure 7.
Controlled microabscesses in TNF−/− mice after adoptive transfer of lymphocytes from immune mem-TNF mice, but not TNF−/− mice. Splenic nonadherent cells (2 × 107) from naïve or vaccinated C57BL/6, mem-TNF, and TNF-deficient mice were injected intravenously into TNF−/− mice as in Figure 6. Representative sections of liver microabscesses were examined 3 days after infection with 104 CFU of LM (n = 4 mice per group, H&E staining). Black arrows point to microabscesses. Original magnifications, ×40.
The data demonstrate that membrane TNF is able to control a low-dose LM infection and that adoptive transfer of immune lymphocytes expressing membrane TNF confers protection in TNF−/− mice. Despite a normal T-cell response on vaccination, TNF−/− mice were not resistant to LM infection after transfer of immune TNF−/− lymphocytes. Therefore, the transfer experiments indicate that membrane-expressed TNF is essential to mount protective immunity in TNF-deficient mice.
Discussion
We report here that membrane TNF plays an important role in the control of LM infection using a knockin mouse model in which the endogenous TNF allele was replaced by a noncleavable membrane TNF.14 The sole expression of membrane TNF endows macrophages to kill LM in vitro and confers substantial protection to infection with LM in vivo. Further, the transfer of immune lymphocytes from membrane TNF mice confers resistance to LM infection in the absence of secreted TNF.
A critical role of TNF for the effective control and resolution of LM infection has been demonstrated previously.2,3 Furthermore, TNF derived from hematopoietic cells, especially macrophages/neutrophils, plays a critical role in inflammatory reactions20,33 and Listeria infection.34 For mycobacterial infection a partial protective effect has been shown by membrane TNF in the same noncleavable membrane TNF mouse35 and in a different transgenic mouse model.13 The partial protection generated in mem-TNF mice could indicate local cell-to-cell TNF signaling by membrane-expressed TNF on T cells or macrophages at the site of infection, leading to a partial activation of the immune cells. Indeed, for the resistance to intracellular pathogens TNF must be produced locally,36 whereas exogenous systemically administered TNF is ineffective.37 Several biological functions of membrane TNF signaling through both TNFR1 and TNFR2 have been reported previously in vitro9 and in vivo using transgenic mice expressing membrane TNF.38,39 Because TNFR2−/− mice are resistant (data not shown) and TNF-R1−/− mice are highly sensitive to LM infection,2,3 the data suggest that membrane TNF signals through TNF-R1 to confer protection to LM infection. Further, membrane TNF has been shown to be involved in reverse (outside-to-inside) signaling. On ligation of the receptor, mem-TNF-expressing cells are activated to express E-selectin.40 Thus, membrane TNF in T cells might function as a bipolar positive regulator of inflammation, either transmitting signals as a ligand to target cells or receiving signals through membrane TNF itself into T cells.
Although the exact mechanism of how protection is acquired through membrane TNF is unclear, membrane TNF on activated T cells might be sufficient for activation of macrophages at low-dose infection, resulting in the up-regulation of NOS2 expression, which is crucial for bacterial killing.28 However, during high-dose infection, secreted TNF and distal signaling, especially for leukocyte recruitment, appears to be required for a full protective host response.
In response to mycobacterial infection, TNF−/− mice develop an uncontrolled type 1 immune response with increased IL-12 and IFN-γ production and tissue destruction.26 The IL-12 overproduction and the spreading of the microabscesses seen in LM-infected TNF−/− mice was abrogated in mem-TNF mice, indicating that the membrane form of TNF is sufficient to control LM infection. However, to what extent an exaggerated type 1 response may contribute to uncontrolled LM infection in the complete absence of TNF is presently unknown.
Interestingly, mem-TNF and TNF−/− mice develop an LM-specific T-lymphocyte response as shown on restimulation of splenocytes with HKLM and LL0 189-201, producing comparable IFN-γ production to that of wild-type mice. Protective immunity to virulent LM after infection with attenuated strains has been described for TNF-R1−/− mice31 and MyD88-deficient mice.41 Similarly, MyD88-deficient mice are able to mount protective response to mycobacterial infection on vaccination.42 Using TNF-deficient mice, we show that the TNF/TNFR1 pathways may not be absolutely necessary to develop adaptive immunity to LM on infection with an attenuated strain. This is in line with previous reports showing a critical role of cell-mediated immunity of CD8 T cells in controlling infection.31,32 To assess whether membrane-expressed TNF on lymphocytes could confer protection, lymphocytes from immunized mice were transferred into TNF−/− mice. Splenocytes from mem-TNF mice, but not TNF−/− mice, conferred protection. The mice survived and were able to clear the bacteria from the organs. Interestingly, TNF-deficient splenocytes, although primed to LM antigens after vaccination and competent for producing IFN-γ on ex vivo restimulation, were unable to confer protection to TNF-deficient mice. It is likely that aside from the T-cell effector functions, membrane-expressed TNF on T cells activates macrophages and augments their bactericidal properties. CD8 T cells contribute to cell-mediated immunity to LM, as shown previously by antibody depletion of T-cell subpopulations.41 Therefore our data suggest that membrane-expressed TNF on T cells is sufficient to reconstitute TNF deficiency and confer host protection.
In summary, we show here for the first time that membrane TNF participates in cell-mediated immunity to LM. In the absence of secreted TNF, membrane-bound TNF endows macrophages with enhanced capacity to kill LM. Protective immunity can be adoptively transferred by immune lymphocytes from vaccinated mem-TNF mice to naïve TNF−/− mice, suggesting that membrane-expressed TNF on lymphocytes is likely responsible for protective immune responses. The findings are significant, especially in the context of TNF-neutralizing therapies using antibodies or soluble receptors. Such strategies have been recently introduced and are now widely used in severe inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, or psoriasis, in which the major complications are represented by opportunistic infections including Listeria and tuberculosis.15–18 Delineating the respective role of membrane-bound versus soluble TNF in host response to infection might open new avenues for better targeted, second generation anti-TNF therapies that spare the anti-microbial host defense.
Acknowledgments
We thank Dr. R. Guler, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Rondebosch, South Africa, for critical comments.
Footnotes
Address reprint requests to Bernhard Ryffel, CNRS, IEM 2815, 3B rue de la Ferollerie, Orleans, France. E-mail: bryffel@cnrs-orleans.fr.
Supported by the Fondation de la Recherche Medicale, Ligue Contre le Cancer, and by a bursary from the French Ministry of Education, France.
Present address of J.D.S.: Eli Lilly and Company, Indianapolis, IN 46285.
References
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Ehlers S, Holscher C, Scheu S, Tertilt C, Hehlgans T, Suwinski J, Endres R, Pfeffer K. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol. 2003;170:5210–5218. doi: 10.4049/jimmunol.170.10.5210. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Cerretti DP. Characterization of the tumour necrosis factor alpha-converting enzyme, TACE/ADAM17. Biochem Soc Trans. 1999;27:219–223. doi: 10.1042/bst0270219. [DOI] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML, Gifford GE. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987;138:957–962. [PubMed] [Google Scholar]
- Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olleros ML, Guler R, Corazza N, Vesin D, Eugster HP, Marchal G, Chavarot P, Mueller C, Garcia I. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette-Guerin infection in the absence of secreted TNF and lymphotoxin-alpha. J Immunol. 2002;168:3394–3401. doi: 10.4049/jimmunol.168.7.3394. [DOI] [PubMed] [Google Scholar]
- Olleros ML, Guler R, Vesin D, Parapanov R, Marchal G, Martinez-Soria E, Corazza N, Pache JC, Mueller C, Garcia I. Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-Guerin and Mycobacterium tuberculosis infections. Am J Pathol. 2005;166:1109–1120. doi: 10.1016/S0002-9440(10)62331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuls SR, Hoek RM, Ngo VN, McNeil T, Lucian LA, Janatpour MJ, Korner H, Scheerens H, Hessel EM, Cyster JG, McEvoy LM, Sedgwick JD. Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity. 2001;15:533–543. doi: 10.1016/s1074-7613(01)00215-1. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford) 2005;44:714–720. doi: 10.1093/rheumatology/keh567. [DOI] [PubMed] [Google Scholar]
- Mohan AK, Cote TR, Siegel JN, Braun MM. Infectious complications of biologic treatments of rheumatoid arthritis. Curr Opin Rheumatol. 2003;15:179–184. doi: 10.1097/00002281-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Mohan AK, Cote TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004;39:295–299. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old LJ. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Eugster HP, Le Hir M, Shakhov A, Di Padova F, Maurer C, Quesniaux VF, Ryffel B. Correction or transfer of immunodeficiency due to TNF-LT alpha deletion by bone marrow transplantation. Mol Med. 1996;2:247–255. [PMC free article] [PubMed] [Google Scholar]
- Torres D, Barrier M, Bihl F, Quesniaux VJ, Maillet I, Akira S, Ryffel B, Erard F. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun. 2004;72:2131–2139. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Garcia I, Guler R, Vesin D, Olleros ML, Vassalli P, Chvatchko Y, Jacobs M, Ryffel B. Lethal Mycobacterium bovis bacillus Calmette Guerin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab Invest. 2000;80:1385–1397. doi: 10.1038/labinvest.3780146. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge-Dufour J, Marino MW, Horton MR, Jungbluth A, Burdick MD, Strieter RM, Noble PW, Hunter CA, Pure E. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–13811. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudget JS. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, Russell JH, Karr R, Chaplin DD. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Eugster HP, Muller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, di Padova F, Aguet M, Zinkernagel R, Bluethmann H, Ryffel B. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- White DW, Badovinac VP, Fan X, Harty JT. Adaptive immunity against Listeria monocytogenes in the absence of type I tumor necrosis factor receptor p55. Infect Immun. 2000;68:4470–4476. doi: 10.1128/iai.68.8.4470-4476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Badovinac VP, Kollias G, Harty JT. Cutting edge: antilisterial activity of CD8+ T cells derived from TNF-deficient and TNF/perforin double-deficient mice. J Immunol. 2000;165:5–9. doi: 10.4049/jimmunol.165.1.5. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Hoek RM, Wiekowski MT, Lira SA, Sedgwick JD. Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J Immunol. 2002;169:7054–7062. doi: 10.4049/jimmunol.169.12.7054. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Forster I, Clausen BE, Tessarollo L, Ryffel B, Kuprash DV, Nedospasov SA. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Saunders BM, Tran S, Ruuls S, Sedgwick JD, Briscoe H, Britton WJ. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J Immunol. 2005;174:4852–4859. doi: 10.4049/jimmunol.174.8.4852. [DOI] [PubMed] [Google Scholar]
- Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954–6961. doi: 10.1128/iai.68.12.6954-6961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kusters S, Tiegs G, Alexopoulou L, Pasparakis M, Douni E, Kunstle G, Bluethmann H, Wendel A, Pfizenmaier K, Kollias G, Grell M. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur J Immunol. 1997;27:2870–2875. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso-Hinard C, De Kesel T, Buurman WA, Moore MW, Dayer JM, Fiers W, Bluethmann H, Grau GE. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- Harashima S, Horiuchi T, Hatta N, Morita C, Higuchi M, Sawabe T, Tsukamoto H, Tahira T, Hayashi K, Fujita S, Niho Y. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. J Immunol. 2001;166:130–136. doi: 10.4049/jimmunol.166.1.130. [DOI] [PubMed] [Google Scholar]
- Way SS, Kollmann TR, Hajjar AM, Wilson CB. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J Immunol. 2003;171:533–537. doi: 10.4049/jimmunol.171.2.533. [DOI] [PubMed] [Google Scholar]
- Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]