Abstract
Pemphigus vulgaris (PV) is a potentially lethal mucocutaneous blistering disease characterized by cell-cell detachment within the stratified epithelium (acantholysis) caused by IgG autoantibodies. Intravenous immunoglobulin (IVIg) therapy effectively treats PV, but the mechanism is not fully understood. To further understand acantholysis and the efficacy of IVIg, we measured effects of IgG fractions from PV patients on keratinocyte death processes. Using IgGs from representative PV patients who improved with IVIg, we identified apoptotic and oncotic signaling pathways in in vitro and in vivo PV models. We identified two groups of PV patients, each producing autoantibodies activating predominantly either apoptotic or oncotic cell death pathway. Experimental treatments with caspase 3 or calpain inhibitors demonstrated that PV IgGs induced acantholysis through both pathways. Upstream, the apoptotic signaling involved activation of caspases 8 and 3 and up-regulation of Fas ligand mRNA, whereas calpain-mediated cell death depended on elevated intracellular free Ca2+. IVIg reduced PV IgG-mediated acantholysis and cell death and up-regulated the caspase inhibitor FLIP and the calpain inhibitor calpastatin. These results indicate that in different PV patients, IgG-induced acantholysis proceeds predominantly via distinct, yet complementary, pathways of programmed cell death differentially mediated by apoptosis and oncosis effectors, with IVIg protecting target cells by up-regulating endogenous caspase and calpain inhibitors.
Pemphigus vulgaris (PV) is a potentially lethal mucocutaneous blistering disease characterized by cell-cell detachment (acantholysis) within the stratified epithelium comprised by keratinocytes and associated with IgG autoantibodies binding to several self-antigens expressed on the keratinocyte plasma membrane, including desmosomal cadherins and acetylcholine receptors.1,2 The life-saving therapy with systemic corticosteroids targets both effectors of autoimmunity, providing for an immunosuppressive effect,3 as well as targets of autoimmunity—keratinocytes, thus exhibiting a direct anti-acantholytic effect.4 PV patients represent a heterogeneous population with regard to the natural course of their disease, clinical features, and response to therapy, perhaps due to dramatic patient-to-patient variations in the immunopathological mechanisms.5–8
Apoptosis is believed to play a role in the mechanism of keratinocyte death in PV. The occurrence of apoptosis markers has been observed in early lesions of PV patients before acantholysis.9 PVIgG and sera have been shown to induce biomolecular markers of apoptosis in keratinocyte monolayers and skin organ cultures,10–12 with caspase inhibitors abolishing the PVIgG-induced acantholysis.13,14 We have reported that ability to induce keratinocyte apoptosis determines pathogenicity of PVIgGs.15 More recently, determination of caspase 3 activity in the HaCaT culture treated with PVIgG has been proposed as a test for pathogenic activity of the autoantibodies.16
It is now well established that intravenous immunoglobulin (IVIg) therapy is an effective treatment modality of PV,17,18 but the mechanism of therapeutic action of IVIg has not been fully elucidated. The IVIg drug contains purified preparations of immunoglobulins from plasma of healthy human donors, containing predominantly polyclonal IgG, and various immunomodulatory contaminants. IVIg exhibits a plethora of biological effects, including acceleration of the clearance of autoantibodies,18 modulation of serum levels of pro-inflammatory cytokines,19 induction of immune-competent cell death,20 and an array of anti-apoptotic effects. In addition to inactivation of lytically active Fas ligand (Fas-L) in patients’ serum,21 IVIg has been shown to protect target cells from apoptosis by up-regulating Bcl-2 expression,22 interfering with the tumor necrosis factor-α (TNF-α)23 and interferon-γ24 signaling pathways, and increasing sensitivity to corticosteroid action.25 Taken together, these reports suggested that the efficacy of IVIg in PV is attributable to the combined immunosuppressive and anti-apoptotic effects.
In this study, we demonstrate for the first time that acantholysis and keratinocyte death induced by PVIgG from different patients can proceed via separate yet complementary pathways, ie, apoptosis and oncosis, and that there exist two PV patient populations, each producing IgG autoantibodies that predominantly activate either pro-apoptotic or pro-oncotic pathway. In addition, the therapeutic action of IVIg in PV results, in part, from inhibition of both extrinsic pathways of programmed cell death in keratinocytes.
Materials and Methods
Chemicals and Tissue Culture Reagents
The cell permeable chelator of intracellular free Ca2+ 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester (BAPTA/AM) and the irreversible, cell permeable broad-spectrum caspase inhibitor Z-Asp-2,6-dichlorobenzoyloxymethylketone (Z-DCB-MK) were purchased from Axxora, LLC (San Diego, CA). The cell-permeable inhibitors of predominantly caspase 3 (but also of caspase 6, caspase 7, caspase 8, and caspase 10) DEVD-CHO and Z-DEVD-FMK, as well as the potent, cell-permeable inhibitors of calpains I (m-form) and II (m-form) MDL-28170, PD-150606, and calpastatin peptide (CSP) were from Calbiochem-Novabiochem Corp. (EMD Biosciences, Inc. La Jolla, CA). The serum-free keratinocyte growth medium (KGM) containing 5 ng/ml epidermal growth factor and 50 mg/ml bovine pituitary extract was purchased from Gibco-BRL (Cambridge, MA). The DeadEnd Fluorometric TUNEL System was from Promega (Madison, WI). Mouse monoclonal antibodies to caspase 3 and Fas-receptor (Fas-R) were from Oncogene Research Products (EMD Biosciences, Inc.). The mouse monoclonal antibody to caspase 8 and rabbit polyclonal antibodies to Fas-L and the long splice form of cellular Fas-associated death domain protein-like interleukin-1-converting enzyme inhibitory protein (FLIP-l)26 were obtained from Calbiochem-Novabiochem Corp. The mouse monoclonal antibody against CSP (domain II) was from Axxora, LLC. Anti-β actin primary antibody and all secondary, fluorescein isothiocyanate-labeled antibodies were purchased from Sigma-Aldrich, Inc. (St. Louis, MO).
Pemphigus and Normal Human IgG Fractions
The results reported herein were obtained in experiments using IgG fractions isolated from sera of 30 PV patients. The in vitro and in vivo experiments used the IgG from the sera of two representative patients before (PVIgG-1b and PVIgG-2b) and after (PVIgG-1a and PVIgG-2a) IVIg therapy, IVIg samples used to treat respective PV patients (IVIgG), and sera of healthy people purchased from Sigma-Aldrich (NIgG). The diagnosis of PV was made based on the results of comprehensive clinical and histological examinations and immunological studies that included direct immunofluorescence of skin biopsies, indirect immunofluorescence of the patients’ sera on various epithelial substrates, and immunoblotting following standard protocols. The serum samples were obtained 1 week before and after a course of transfusions of IVIg in the total amount of 2 g/kg, given at daily increments of 400 mg/kg. The titers of anti-keratinocyte antibodies in the sera of PV-1 and PV-2 patients before and after IVIg therapy remained at the range of 1/640 to 1/1280, as determined by indirect immunofluorescence on the monkey esophagus substrate. Before IVIg transfusions, both patients were in acute stage of their mucocutaneous disease characterized by the appearance of flaccid blisters and erosions, and positive Nikolskiy sign.27 They received 1.2 mg/kg prednisone per day during the entire period of observation, ie, from the first to the second blood draw. No new lesions were seen after initiation of the IVIg therapy. Nikolskiy sign had turned negative by the time of second blood draw. This study had been approved by the University of California Davis Human Subjects Review Committee. All IgG fractions were isolated by FPLC protein G affinity chromatography using the FPLC System purchased from Amersham Pharmacia Biotech (Piscataway, NJ) and following the manufacturer’s protocol. Briefly, 5 ml of test serum samples in the binding buffer (20 mmol/L NaPO4, pH 7.0) were loaded on the HiTrap Protein G HP columns packed with 1 ml of protein G Sepharose high performance gel (binding capacity for human IgG ∼25 mg/ml) and incubated for 1 hour at room temperature. The IgGs were eluted with elution buffer (0.1 mol/L glycine-HCl, pH 2.7), and the purity of IgG fractions was established by gel electrophoresis (not shown). The protein concentration was measured by Bradford assay.
Experiments with Human Keratinocyte Monolayers
Normal human keratinocytes were obtained from neonatal foreskins and grown at 37°C and 5% CO2 in 25 or 75 cm2 Falcon culture flasks (Corning Glass Works, Corning, NY) in KGM containing 0.09 mmol/L Ca2+ as detailed elsewhere.28 Cell culture medium was changed every 3 days. All experiments were performed using the second to fourth passage keratinocyte monolayers at ∼80% confluence grown from at least three different foreskin donors. The IgG fractions were diluted in KGM and added to the monolayers at the final concentration of 1 mg/ml. Before exposure to PVIgG-1b or PVIgG-2b, some monolayers were pretreated for 1 hour with IgGs isolated from the IVIg batches used for treatment of the respective PV patients (IVIgG). The control monolayers were left intact. All monolayers were incubated at 5% CO2 for different periods of time (see Results) and used for RNA and protein isolation, quantitation of numbers of trypan blue dye (TBD)-positive and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL)-positive cells, or morphometric analysis of the extent of acantholysis.
Morphometric Assay of Acantholysis in Vitro
The extent of cell detachment in the monolayers was measured following the established protocol.29 Briefly, the images of at least three representative microscopic fields were recorded through a 10× objective using a computer-linked inverted phase-contrast microscope (Axiovert 135; Carl Zeiss, Inc., Thornwood, NY). The percentage of acantholysis in each field was computed by subtracting the percentage of the areas covered by the cells from the total area of the microscopic field, taken as 100%. Before being used for RNA or proteins isolation and TBD assay, the cells floating free in the culture supernatant were pelleted by centrifugation and mixed with keratinocytes that remained attached to the dish bottom.
Passive Transfer Experiments with Neonatal Mice
One-day-old pups delivered by BALB/c mice purchased from The Jackson Laboratory (Bar Harbor, ME) were used to investigate effects of test IgG samples on keratinocyte acantholysis and death, as well as the effects of the caspase inhibitor Z-DCB-MK and the calpain inhibitor MDL-28170 on the skin blistering and an extent of epidermal acantholysis induced by passive transfer of 0.1 mg/g body weight PVIgG-1b and PVIgG-2b. This study had been approved by the University of California Davis Review Committee on the Use of Animals in Research. One-day-old mice were injected intraperitoneally through a 30-gauge needle with an IgG fraction with or without test drugs and examined 24 hours later for the presence of skin erosions and blisters and Nikolskiy sign. The animals were euthanized using CO2 and used to quantify the extent of epidermal acantholysis.
Morphometric Assay of Acantholysis in Vivo
The extent of epidermal acantholysis in neonatal mice was measured microscopically, as described in the standard protocols.4,30 Briefly, the euthanized animals were snap-frozen in liquid nitrogen, cross-sectioned at the umbilicus level, embedded into the OCT compound (Miles Scientific, Naperville, IL), stained by hematoxylin and eosin, and evaluated by light microscopy. Five random microscopic fields in each skin section were captured at magnification ×10, using a Macintosh computer attached to an Axiovert 135 inverted microscope. The images were printed, and the extent of acantholysis was computed directly on the prints by measuring the length of the areas in the epidermis in which suprabasal cell detachment spread along more than four adjacent basal cells.
Real-Time Polymerase Chain Reaction (PCR) Assay
Total RNA was extracted from cultured keratinocytes at the end of exposure experiments using the guanidinium thiocyanate phenol chloroform extraction procedure as described elsewhere.31 One microgram of dried, DNase-treated RNA was reverse transcribed in 20 μl of a mix [50 mmol/L Tris (pH 8.3), 6 mmol/L MgCl2, 40 mmol/L KCl, 25 mmol/L dNTPs, 1 μg Oligo-dT (Gibco-BRL), 1 mmol/L dithiothreitol, 1 U of RNase inhibitor (Boehringer Mannheim, Germany), and 10 U of SuperScript II (Gibco-BRL)] at 42°C for 2 hours. Primers for the genes encoding caspase 3, caspase 8, Fas-L, Fas-R, FLIP-l, and CSP were designed with the assistance of the Primer Express software version 2.0 computer program (Applied Biosystems, Foster City, CA) and the service Assays-on-Design provided by Applied Biosystems. The nucleotide databases were searched to confirm gene specificity. To avoid amplification of contaminating genomic DNA, when possible, one of the two primers was placed at the junction between two exons. Primer pairs were chosen to minimize primer dimerization and to generate an amplicon between 75 and 150 bp. For each primer pair, we used no-template control and no-reverse transcriptase controls, which produced insignificant signals, suggesting that primer-dimer formation and genomic DNA contamination effects were negligible.32,33 All PCR reactions were performed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems) and the SYBR Green PCR Core Reagents kit (Applied Biosystems) in accordance to the manufacturer’s protocol, as detailed elsewhere.34 Briefly, 22.5 μl of diluted cDNA sample produced from 1 μg of total RNA was added to 25 μl of the PCR master mix. The amplification included a 2-minute 50°C step required for optimal AmpErase UNG activity, an initial denaturation step for 10 minutes at 95°C, followed by 40 cycles consisting of 15 seconds at 95°C and 1 minute at 60°C. Obtained gene expression values were normalized using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to correct minor variations in mRNA extraction and reverse transcription. The threshold cycle (Ct value), ie, the point at which target-derived fluorescence can be distinguished against background fluorescence, was determined. By constructing a standard curve, the Ct value was translated into a quantitative result.35 The data from triplicate samples were analyzed with sequence detector software (Applied Biosystems) and expressed as mean ± SD of mRNA relative to that of GAPDH. Relative expression was determined using the Ct method36 that calculates relative expression through the equation: fold induction = 2[ΔΔCt], where ΔΔCt = Ct gene of interest − Ct GAPDH.
Western Blot Assay
Proteins were isolated from the phenol-ethanol supernatant of homogenized human keratinocytes by adding 1.5 ml of isopropyl alcohol per 1 ml of TRIzol Reagent (Gibco-BRL) and analyzed via quantitative immunoblotting as described elsewhere.37 The membranes were developed using the ECL + Plus chemiluminescent detection system (Amersham Pharmacia Biotech). To visualize antibody binding, the membranes were scanned with StormFluorImager (Molecular Dynamics, Mountain View, CA), and band intensities were determined by area integration using ImageQuant software (Molecular Dynamics). To normalize data for protein content, the housekeeping protein actin was visualized in each sample with anti-β actin antibody. The results were standardized by expressing the density of each protein band under investigation in the experimental sample relative to the value determined in the control sample. The ratios obtained in three independent experiments were averaged to obtain the mean value. The protein content ratio in each control sample was always set equal to 1. The images shown represent typical appearances of protein band in the gels.
Enzymatic Assays
The enzymatic activities of calpain and caspases 3, 8 (all from Calbiochem, San Diego, CA), and 9 (R&D Systems, Minneapolis, MN) were determined using the respective fluorometric assay kits and following protocols provided by the manufacturer.
Statistical Analysis
All experiments were performed in triplicates or quadruplicates, and the results were expressed as mean ± SD. Statistical significance was determined using Student’s t-test. Differences were deemed significant if the calculated P value was <0.05.
Results
Reciprocal Effects of PVIgG and IVIgG on the Expression of Apoptosis Regulators in Keratinocytes
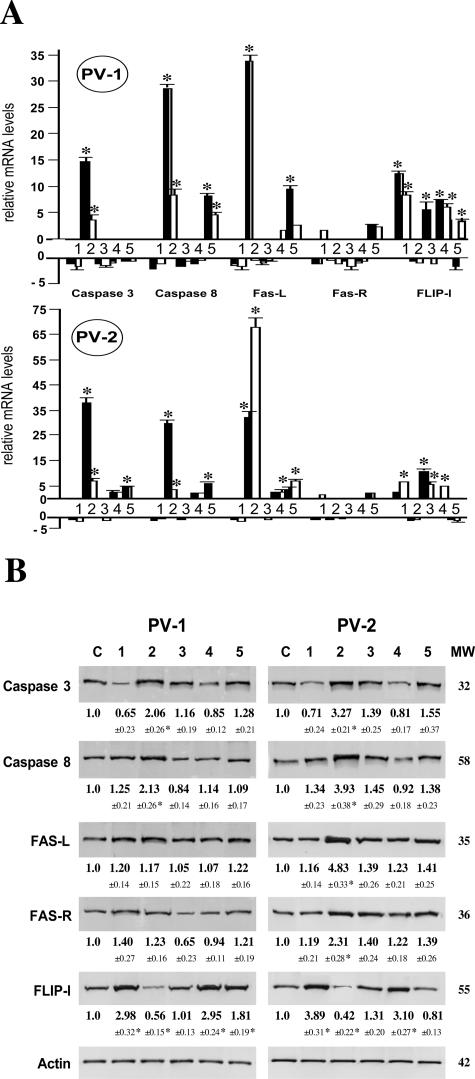
Because, on the one hand, IVIg therapy proved to be an efficient treatment modality of PV patients and, on the other hand, PVIgG-induced apoptosis reportedly is the main cause of keratinocyte detachment and epidermal blistering in PV, we tested a hypothesis that IVIg interferes with pro-apoptotic action of pemphigus antibodies. We exposed keratinocyte monolayers for 12 and 24 hours to IgG fractions isolated from the sera of two PV patients with active disease, PVIgG-1b and PVIgG-2b; the sera of these PV patients after course of IVIg therapy showing improvement, PVIgG-1a and PVIgG-2a; the IVIg batches used for transfusion of each patient, IVIgG; and normal pooled human sera, NIgG. After incubation, the effects of experimental treatments on the relative amounts of mRNA and proteins of the pro-and anti-apoptotic molecules were quantified by real-time PCR and Western blot, respectively.
At the mRNA level, after 12 hours of exposure to PVIgG-1b, keratinocytes demonstrated an up-regulated expression of caspases 3 and 8 and Fas-L by approximately 15, 30, and 35 times, respectively, whereas PVIgG-2b up-regulated expression of these pro-apoptotic genes by approximately 40, 30, and 35 times, respectively (P < 0.05) (Figure 1A). Extending incubation to 24 hours resulted in a decrease of the relative amounts of mRNA transcripts of the pro-apoptotic molecules, except for Fas-L mRNA, which increased by more than 65 times because of exposure to PVIgG-2b. The IgGs isolated from PV patients after IVIg treatment did not produce any significant alterations in the pro-apoptotic gene expression (P > 0.05) but caused a significant (P < 0.05) increase in the expression of the apoptosis inhibitor FLIP-l (Figure 1A). The relative amounts of the FLIP-l mRNA transcripts in keratinocytes incubated for 24 hours with either NIgG or IVIgG increased by six- to eightfold (P < 0.05) (Figure 1A). Therefore, not surprisingly, pretreatment of keratinocytes with IVIgG before their exposure to PVIgGs abolished, for the most part, the pro-apoptotic effects of PVIgG-1b and PVIgG-2b (Figure 1A). Thus, a dramatic change from pro- to anti-apoptotic pattern of activity of PVIgGs after IVIg transfusions could be related to the stimulatory effect of normal human IgGs on FLIP-l expression in keratinocytes.
Figure 1.
Effects of pemphigus and normal human IgG fractions on apoptosis-related molecules in keratinocytes. At ∼80% of confluence, keratinocyte monolayers from three foreskin donors (n = 3) were exposed to 1) 1 mg/ml normal pooled human sera, NIgG; 2) IgG fractions isolated from the serum of PV-1 or PV-2 patient with active disease; 3) sera of these PV patients after a course of IVIg therapy showing improvement; 4) the IVIg batches used for transfusion of each patient, IVIgG; or 5) preincubated for 1 hour with an IVIgG fraction and then exposed to corresponding PVIgG-1b or PVIgG-2b. After incubation at 5% CO2, the effects of experimental treatments on the relative amounts of mRNA and proteins of the pro- and anti-apoptotic molecules were quantified by real-time PCR and Western blot, respectively. Asterisks indicate significant (P < 0.05) differences from control. A: Real-time PCR analysis. The real-time PCR was performed using RNA isolated from keratinocytes incubated with test IgGs for either 12 hours (dashed bars) or 24 hours (open bars) exactly as described in Materials and Methods. The alterations in the gene expression levels of caspases 3 and 8, Fas-L, Fas-R, and FLIP-l are presented relative to the rates of expression of corresponding genes in control samples, taken as the baseline. B: Western blot analysis. After 24 hours of exposure, the protein levels of caspases 3 and 8, Fas-L, Fas-R, and FLIP-l were analyzed by Western blot. The gene expression ratio of 1 was assigned to control, non-treated monolayers (C). The images show typical bands appearing at the expected molecular weight (MW) indicated to the right of the gels. The ratio data underneath the bands are the means ± SD of the values obtained in three independent experiments. Specific staining was absent in the negative control experiments in which the membranes were treated without primary antibody or with irrelevant primary antibody of the same isotype and host (not shown).
The results of Western blot assay were consistent with the real-time PCR findings. At the protein level, however, the changes of the pro- and anti-apoptotic molecules reached statistical significance only after 24 hours of incubation (Figure 1B). Both PVIgG-1b and PVIgG-2b significantly increased the relative amount of caspases 3 and 8, and decreased that of FLIP-l (P < 0.05). PV-IgG2b also significantly (P < 0.05) up-regulated Fas-L and Fas-R. After patients’ IVIg transfusions and after pretreatment of keratinocytes with IVIgG in vitro, IgGs from both PV patients lost their abilities to induce significant changes of apoptosis-related proteins (Figure 1B). NIgG and IVIgG increased the relative amounts of FLIP-l by more than threefold (P < 0.05).
Taken together, these results indicated that during acute stage of their disease, PV patients produced autoantibodies that up-regulated the expression of the pro-apoptotic genes and down-regulated the expression of the apoptosis inhibitor FLIP-l and that the therapeutic action of IVIg harbored anti-apoptotic effects of normal IgG on target cells in PV, keratinocytes, including up-regulation of FLIP-l. The quantitative and qualitative differences between the effects of PVIgG-1b and PVIgG-2b suggested that the mechanisms of keratinocyte death and survival in each particular PV patient were not identical, which prompted additional studies described below.
Reciprocal Effects of PVIgG and Normal IgG Fractions on Keratinocyte Acantholysis and Cell Death
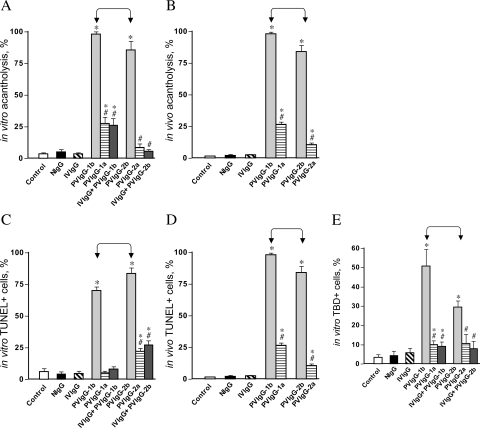
The acantholytic and pro-apoptotic effects of PVIgGs and normal human IgGs were investigated both in vitro and in vivo. Incubation of keratinocyte monolayers with PVIgG-1b or PVIgG-2b (Figure 2A) or passive transfer of these PVIgGs to neonatal BALB/c mice (Figure 2B) in both cases resulted in extensive acantholysis, associated with the appearance of gross skin blisters in neonatal mice (not shown). The acantholytic activity of PVIgG-1b exceeded that of PVIgG-2b (P < 0.05). The PVIgG fractions isolated from these patients after their treatment with IVIg produced significantly (P < 0.05) less cell detachment in the monolayers of human keratinocytes and murine epidermis (Figure 2, A and B) and did not cause gross skin blisters. Pretreatment of keratinocyte monolayers with the IVIgG samples used to treat the patients significantly (P < 0.05) decreased the acantholytic activities of patients’ IgGs (Figure 2A). Given alone, neither IVIgG nor NIgG caused any significant alterations in keratinocyte cell-cell adhesion (P > 0.05).
Figure 2.
Effects of PVIgG and normal IgGs on keratinocyte acantholysis and cell death. Keratinocyte monolayers were treated with test IgGs as described in the legend to Figure 1, except that two IVIgG samples were combined. The same IgG samples were injected intraperitoneally to 1-day-old BALB/c mice at the total dose of 0.1 mg/g body weight. Control monolayers and mice were left intact (C). The extent of acantholysis and the occurrence of cell death markers were examined in keratinocyte monolayers 48 hours after exposure and in specimens of mouse skin 24 hours after passive IgG transfer, as described in Materials and Methods. *, significant (P < 0.05) differences from control. #, statistical significance (P < 0.05) compared with the results obtained with PVIgG alone. Significant differences between specific experimental conditions are indicated in the graph with pointed square brackets. A: Extent of acantholysis in keratinocyte monolayers. B: Extent of acantholysis in mouse epidermis. C: Relative number of TUNEL+ keratinocytes in the monolayers. D: Relative number of TUNEL+ keratinocytes in mouse epidermis. E: Relative number of TBD+ keratinocytes in the monolayers.
Both PVIgG-1b and PVIgG-2b also significantly (P < 0.05) increased the number of keratinocytes featuring the DNA fragmentation detectable through labeling of DNA strand breaks by TUNEL in both cell monolayers (Figure 2C) and murine epidermis (Figure 2D). IVIg treatment of the patients significantly (P < 0.05) diminished pro-apoptotic activities of their IgGs. Likewise, preincubation of keratinocyte monolayers with IVIgG significantly (P < 0.05) decreased the number of TUNEL-positive cells in cultures treated with PVIgGs (Figure 2, C and D). The pro-apoptotic activity of PVIgG-2b exceeded that of PVIgG-1b both in in vitro and in vivo experiments (P < 0.05). Neither IVIgG nor NIgG significantly changed the number of TUNEL-positive cells, compared with untreated controls (P > 0.05).
The analysis of keratinocyte death through counting the number of TBD-positive cells revealed a significant (P < 0.05) increase in the monolayers treated with PVIgG-1b or PVIgG-2b, which could be diminished due to either preincubation of cells with IVIgG or treatment of patients with IVIg (Figure 2E). In the TBD assay, PVIgG-1b was found to be more efficient in causing keratinocyte plasma membrane disruption than PVIgG-2b (P < 0.05).
These results demonstrated that IVIg decreased the abilities of IgGs from both PV patients to cause acantholysis and cell death. The lack of correlation of the acantholytic activities of PVIgG-1b and PVIgG-2b with their abilities to increase the number of TUNEL- and TBD-positive cells further suggested that the mechanisms of acantholysis induced by each individual PVIgG may not be identical. Because TUNEL staining is considered to be a specific marker of apoptosis, although it may also be positive in oncosis, and because TBD staining is more specific for oncosis,38,39 we next sought to determine which of the two pathways of cell death are predominantly involved in mediating acantholysis caused by the PVIgGs under consideration.
Differential Effects of Caspase 3 and Calpain Inhibitors on Acantholysis Induced by IgGs from Different PV Patients
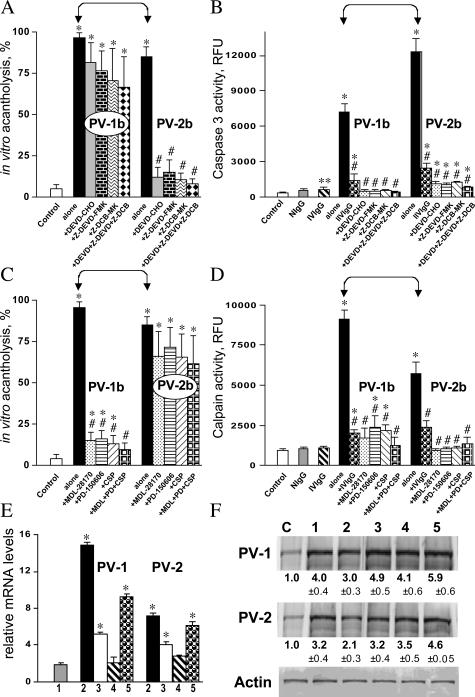
Caspase 3 has been identified as a key protease in the execution of apoptosis associated with cell shrinkage, blebbing, chromatin condensation, and DNA fragmentation,40 whereas calpains have been shown to play a critical role in oncosis by increasing plasma membrane permeability—a hallmark of oncosis.41 To evaluate the relative contribution of each pathway of extrinsic cell death, apoptosis versus oncosis, to the development of cell-cell dyshesion in PV, we investigated effects of caspase and calpain inhibitors on the extent of acantholysis in keratinocyte monolayers exposed to PVIgG-1b or PVIgG-2b. We were aware of the reports that caspase inhibitors prevented PVIgG-induced acantholysis in keratinocyte monolayers and skin organ cultures.13,14 Surprisingly, the caspase inhibitors DEVD-CHO (10 μmol/L), Z-DEVD-FMK (10 μmol/L), and Z-DCB-MK (100 μmol/L), given alone or as a mixture, inhibited acantholysis completely only in the monolayers treated with PVIgG-2b (P < 0.05) but caused only a moderate decrease of the acantholytic activity of PVIgG-1b (P > 0.05) (Figure 3A). At the same time, these inhibitors blocked caspase 3 activity induced by both PVIgGs equally efficiently (Figure 3B). It was also noted that the caspase 3 activity induced by PVIgG-2b significantly (P < 0.05) exceeded that induced by PVIgG-1b (Figure 3B).
Figure 3.
Contribution of caspase 3 and calpain to PVIgG-induced acantholysis. Effects of caspase and calpain inhibitors on the extent of acantholysis, enzymatic activities of caspase 3 and calpain, and expression of CSP were studied in experiments described in the legend to Figure 2. The monolayers were exposed to 1 mg/ml NIgG, IVIgG alone or in combination with PVIgG-1b, PVIgG-2b, PVIgG-1a, or PVIgG-2a, or with PVIgG-1b or PVIgG-2b in the presence or absence of the caspase inhibitors DEVD-CHO (10 μmol/L), Z-DEVD-FMK (10 μmol/L), and Z-DCB-MK (100 μmol/L) or calpain inhibitors MDL-28170 (10 μmol/L), PD-150606 (50 μmol/L), and CSP (50 μmol/L), given alone or as a mixture. Control monolayers (C) were left intact. *, significant (P < 0.05) differences from control. #, statistical significance (P < 0.05) compared with the results obtained with PVIgG alone. Significant differences between specific experimental conditions are indicated in the graph with pointed square brackets. A: Effects of caspase inhibitors on the extent of acantholysis in keratinocyte monolayers after 48 hours of incubation. B: Effects of caspase inhibitors on the enzymatic activity of caspase 3 in keratinocyte monolayers after 24 hours of incubation. **, P = 0.05. C: Effects of calpain inhibitors on the extent of acantholysis in keratinocyte monolayers after 48 hours of incubation. D: Effects of calpain inhibitors on the enzymatic activity of calpain in keratinocyte monolayers after 24 hours of incubation. E: Real-time PCR analysis of the relative amount of the CSP mRNA in keratinocyte monolayers after 24 hours of incubation with 1) NIgG, 2) PVIgG-1b or PVIgG-2b, 3) PVIgG-1a or PVIgG-2a, 4) an IVIgG sample, or 5) PVIgG-1b or PVIgG-2b together with the corresponding IVIgG sample given 1 hour before exposure to PVIgG. The real-time PCR was performed, and the results are expressed exactly as described in the legend to Figure 1A. F: Western blot analysis of the relative amount of the 150-kd CSP protein in keratinocyte monolayers after 24 hours of incubation. The numeric designation of experimental conditions corresponds to those in E. The experiments were performed and the results expressed exactly as described in the legend to Figure 1B. All experimental values significantly (P < 0.05) differ from those obtained in control, non-treated mono-layers (C).
The calpain inhibitors MDL-28170 (10 μmol/L), PD-150606 (50 μmol/L), and CSP (50 μmol/L) significantly (P < 0.05) decreased the extent of acantholysis induced by PVIgG-1b but produced only a minor effect on that induced by PVIgG-2b (P > 0.05) (Figure 3C). The calpain activity in keratinocyte monolayers treated with either PVIgG-1b or PVIgG-2b was significantly (P < 0.05) increased, with PVIgG-1b being almost two times as efficient as PVIgG-2b (Figure 3D). In both cases, the activity of calpains decreased significantly (P < 0.05) in the presence of calpain inhibitors tested (Figure 3D).
An increase of calpain activity in keratinocytes treated with PVIgGs was associated with an increase of the mRNA and protein levels of the endogenous inhibitor CSP (P < 0.05) (Figure 3, E and F). NIgG and IVIgG also up-regulated keratinocyte CSP gene expression at both the mRNA and the protein levels. The discrepancy between an increase of the CSP mRNA transcripts and a decrease in the CSP protein by PVIgGs relative to the effects of normal IgGs can be explained by degradation of the CSP protein by both caspases42 and calpains,43 both of which were found to be activated in keratinocytes treated with PVIgG-1b or PVIgG-2b.
These findings suggested that PVIgG-dependent acantholysis proceeds via complementary pathways that involve effectors of both apoptosis and oncosis and that the cell death pathway predominantly responsible for acantholysis may vary from patient to patient. The results also demonstrated that pretreatment of keratinocyte monolayers with IVIgG inhibits PVIgG-elevated caspase 3 and calpain activities equally efficiently, and that normal human IgGs can up-regulate CSP expression, both of which may contribute to the therapeutic activity of IVIg in PV.
Functional Analysis of Predominant Involvement of the Apoptotic and the Oncotic Pathways of Keratinocyte Death in PV Reveals the Existence of Two Patient Populations
To determine the relevance of our findings of the existence of two distinct pathophysiological pathways in the mechanism of PVIgG-induced keratinocyte death, we measured the activities of the apoptosis and oncosis effectors in the keratinocyte monolayers from three different donors incubated for 24 hours with IgG fractions isolated from the sera of 30 acute PV patients. The activities of death enzymes in each patient were expressed as times the control values obtained in keratinocyte monolayers treated with normal human IgG fraction. The results demonstrated that IgGs from 19 patients (63.7%) up-regulated caspases 3 and 8 more than calpain, whereas IgGs from 11 patients (36.7%) predominantly activated calpain (Table 1). The mean fold increase of caspase 9 activity was found to be similar in both subgroups of PV patients (P > 0.05).
Table 1.
Mean Fold Increase above the Control Level of Activities of Pro-Oncotic and Pro-Apoptotic Enzymes in Keratinocytes Incubated for 24 Hours with IgGs from 30 PV Patients versus Normal IgG (Control)
| Cell death enzyme | First group (19 PV patients) | Second group (11 PV patients) | P (between two groups) |
|---|---|---|---|
| Calpain | 12.1 ± 2.3 | 20.0 ± 1.9 | <0.05 |
| Caspase 3 | 19.3 ± 2.3 | 11.8 ± 1.9 | <0.05 |
| Caspase 8 | 19.1 ± 1.8 | 10.34 ± 1.4 | <0.05 |
| Caspase 9 | 10.2 ± 1.9 | 8.89 ± 1.7 | >0.05 |
These results indicated that two PV patient populations exist, each producing IgG autoantibodies whose binding to keratinocytes activates predominantly either apoptotic or oncotic pathway of programmed cell death. The donors of PVIgG-1 and PVIgG-2 antibodies used in this study each represented the group of PV patients producing either pro-oncotic or pro-apoptotic autoantibodies, respectively.
Additive Effects of the Apoptotic and Oncotic Events in the Mechanism of PV Acantholysis
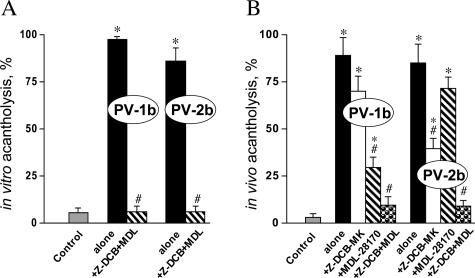
Next, we tested the hypothesis that PV acantholysis results from synergistic or additive effects of the effectors of apoptotic and oncotic pathways. In a series of in vitro and in vivo experiments, we sought to determine whether a combination of the caspase inhibitor MDL-28170 and calpain inhibitor Z-DCB-MK that had been successfully used in animal experiments44,45 could completely abolish acantholysis induced by PVIgG. Incubation of keratinocyte monolayers with a mixture of 10 μmol/L MDL-28170 and 100 μmol/L Z-DCB-MK or intraperitoneal administration of 25 μg/g body weight MDL-28170 and 10 μg/g body weight Z-DCB-MK completely abolished acantholysis induced by either PVIgG-1b or PVIgG-2b (Figure 4, A and B).
Figure 4.
Effects of a combination of caspase and calpain inhibitors on PVIgG-induced acantholysis. Effects of caspase and calpain inhibitors on the extent of acantholysis in vitro and in vivo were studied using the morphometric technique described in Materials and Methods. The monolayers were exposed to 1 mg/ml PVIgG-1b or PVIgG-2b alone or in the presence of a mixture of 100 μmol/L caspase inhibitor Z-DCB-MK and 10 μmol/L calpain inhibitor MDL-28170. The neonatal BALB/c mice were injected intraperitoneally with PVIgG-1b or PVIgG-2b (0.1 mg/g body weight), with or without MDL-28170 (25 μg/g body weight) or Z-DCB-MK (10 μg/g body weight), or a mixture of these inhibitors. Control monolayers and mice were left intact. *, significant (P < 0.05) differences from control. #, statistical significance (P < 0.05) compared with the results obtained with PVIgG alone. A: Extent of acantholysis in keratinocyte monolayers after 48 hours of incubation. B: Extent of acantholysis in mouse epidermis 24 hours after injection.
To better define the cell death pathways elicited by PV autoantibodies in vivo, we also tested anti-acantholytic activities of each inhibitor under consideration. Although the calpain inhibitor MDL-28170 significantly (P < 0.05) decreased acantholysis in the epidermis of pups injected with PVIgG-1b, the caspase inhibitor Z-DCB-MK significantly (P < 0.05) reduced extent of epidermal clefting induced by PVIgG-2b (Figure 4B). These results clearly demonstrated that acantholysis induced by PVIgG-1b and PVIgG-2b incorporates both the apoptotic and oncotic mechanisms, with PVIgG-1b inducing extrinsic cell death predominantly through an oncotic pathway and PVIgG-2b through an apoptotic pathway.
Relationship between the Caspase 3- and Calpain-Dependent Mechanisms of Acantholysis Induced by Autoantibodies from Different PV Patients
Because caspase 3 and calpain can positively regulate each others’ activity,42,46 we next sought to determine an apparent order of involvement of the effectors of apoptosis and oncosis along the pathobiological signaling pathways leading to acantholysis caused by PVIgG-1b and PVIgG-2b.
A mixture of calpain inhibitors, MDL-28170, PD-150606, and CSP, significantly (P < 0.05) inhibited caspase 3 activity elevated because of exposure of keratinocyte monolayer to PVIgG-1b but produced only minor (P > 0.05) inhibition of the PVIgG-2b effect (Figure 5A). In turn, caspase 3 inhibitors DEVD-CHO, Z-DEVD-FMK and Z-DCB-MK significantly (P < 0.05) decreased calpain activity elicited by PVIgG-2b but not PVIgG-1b (Figure 5B).
Figure 5.
Interrelationships between the apoptotic and oncotic signaling cascades of cell death in PV. The abilities of caspase and calpain inhibitors and the chelator of intracellular free calcium, BAPTA/AM, to diminish the pathobiological effects of 1 mg/ml PVIgG-1b or PVIgG-2b in keratinocyte monolayers were tested using functional assays described in Materials and Methods. A and B: *, significant (P < 0.05) differences from control, non-treated monolayers; #, statistical significance (P < 0.05) compared with the results obtained with PVIgG alone. C: *, significant differences from values with PVIgG-1b or IVIgG-2b given alone. A: Effects of a mixture of calpain inhibitors MDL-28170 (10 μmol/L), PD-150606 (50 μmol/L), and CSP (50 μmol/L) on the enzymatic activity of caspase 3 in keratinocyte monolayers after 24 hours of incubation. B: Effects of a mixture of caspase inhibitors DEVD-CHO (10 μmol/L), Z-DEVD-FMK (10 μmol/L), and Z-DCB-MK (100 μmol/L) on the enzymatic activity of calpain in keratinocyte monolayers after 24 hours of incubation. C: Effects of 10 μmol/L BAPTA/AM on the abilities of PVIgG-1b or PVIgG-2b 1) to induce acantholysis; 2) to promote TUNEL positivity and 3) TBD positivity; and 4) to elevate caspase 3 and 5) calpain activities in keratinocyte monolayers after 24 hours (TUNEL, TBD, and enzymatic activities) and 48 hours (acantholysis) of incubation.
Finally, to determine relative contribution of intracellular calcium to each pathobiological pathway, we compared the effects of PVIgG-1b and PVIgG-2b on keratinocyte death and survival in monolayers pretreated with 10 μmol/L BAPTA/AM. The abilities of both PVIgG-1b and PVIgG-2b to induce acantholysis and to elevate calpain activity were inhibited equally efficiently because of chelation of intracellular free Ca2+ in keratinocytes (Figure 5C). BAPTA/AM also significantly (P < 0.05) decreased the abilities of PVIgG-1b to up-regulate the number of TUNEL-, and TBD-positive cells and to activate caspase 3. Lessening of the latter effects of PVIgG-2b with BAPTA/AM did not reach statistical significance (P > 0.05) (Figure 5C).
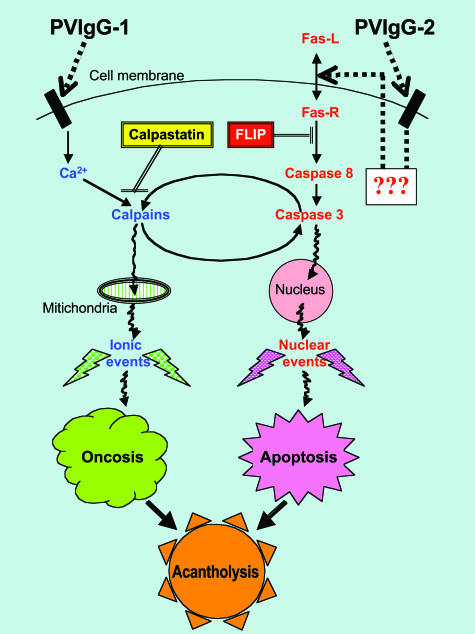
The obtained results indicated that in keratinocytes targeted by PVIgG-1b, the involvement of caspase 3 into the acantholytic process occurs downstream of calpain activation, whereas PVIgG-2b binding to keratinocytes leads to activation of caspase 3 directly, followed by involvement of calpains. The calpain-mediated execution of the extrinsic programmed cell death induced by PVIgG-1b depended on elevation of the intracellular free Ca2+. Because the morphology of keratinocyte acantholysis caused by PVIgG-1b was indistinguishable from that induced by PVIgG-2b, we believe that the acantholytic changes become visible only after the cell has entered the common step in the death pathway. The hypothetical interplay between distinct pathological signaling cascades initiated by PVIgG-1b and PVIgG-2b in keratinocytes is schematically depicted in Figure 6.
Figure 6.
Hypothetical scheme of the signaling mechanisms leading to acantholysis through predominantly oncotic and apoptotic pathways in PV-1 and PV-2 patients, respectively. The signaling cascades originating from the plasma membrane antigens targeted by PVIgG-1 and PVIgG-2 include distinct stimulatory (↓) and inhibitory (⊥) steps and involve cross-talk between the effectors of oncotic and apoptotic pathway, thus leading to acantholysis through an overlapping, synergistic mechanism.
Discussion
In this study, we demonstrated for the first time that keratinocyte detachment and death in PV stem from a synergistic action of the effectors of apoptosis and oncosis and that the therapeutic activity of IVIg in PV is mediated, in part, by stimulation of intracellular anti-apoptotic and anti-oncotic events. Fine mechanisms of antibody-induced keratinocyte detachment, death, and survival varied between different PV patients, which helps explain known patient-to-patient variations in clinical and immunopathological features of PV.
Previous studies demonstrated that PVIgG binding to keratinocytes causes cell death associated with: 1) secretion of soluble Fas-L; 2) elevated cellular amounts of Fas-R, Fas-L (soluble and membranal), Bax, p53, and c-myc; 3) co-aggregation of Fas-L and Fas-R with caspase 8 in a membranal death-inducing signaling complex; 4) enrichment in caspase 8 and activation of caspases 1 and 3; 5) reduction in levels of cellular Bcl-2; 6) increased expression of the inducible nitric oxide synthase mRNA; 7) overproduction of nitric oxide; 8) overexpression of heat shock protein 70; 9) decrease in relative amount of mRNAs encoding the cellular stress response protein GADD34; 10) down-regulation of the apoptosis-related gene TSSC3; and 11) DNA fragmentation.4,10–13,16,47 Thus, it appeared that different cell death signaling systems were predominantly engaged in different PV patients.
This study showed that IgG fractions from sera of PV patients in acute stage of disease contained anti-keratinocyte antibodies that trigger cell death, whereas both NIgG and IVIgG—anti-apoptotic and anti-oncotic effects. The time-course analysis of the expression of cell death genes using a high sensitivity real-time PCR assay demonstrated that the apoptotic pathway is activated a long time before an appearance of the first morphological evidence of cell dyshesion (acantholysis), ie, 12 hours (apoptosis initiation) versus 24 to 48 hours (acantholysis). These observations are consistent with finding in most models of apoptosis that cell death begins 12 to 24 hours after an initial trigger48 and that acantholysis develops >24 hours after exposure to PVIgG.11,13,14 Puviani et al10 observed that apoptosis can be enhanced in cells as early as 6 hours after exposure to pemphigus autoantibodies. These cumulative results argue in favor of the supposition that keratinocyte death via anoikis does not take place in PV.9 Anoikis (Greek: state of homelessness) describes a type of apoptosis of adherent cells that lose anchorage to the extracellular matrix.49
In PV, the earliest acantholytic changes occur among basal cells and between them and the first row of suprabasal keratinocytes and consist of detachment of tonofilaments from the attachment plate of the desmosomes.50 Thereafter, tonofilaments retract into the perinuclear region, followed by disintegration of the desmosomes and the appearance of variable signs of degeneration, such as vacuolization, nuclear pyknosis, and abnormalities with the mitochondria and the endoplasmic reticulum.5,51 In some mitochondria, cristae are preserved and are partially destroyed in the others.52 After separation and eventual disappearance of desmosomes, residual cell membranes often show a conspicuous “villous” (bleb) formation.53 The nuclei in the acantholytic cells enlarge, exhibit peripherally polisaded chromatin, and occupy almost the entire cytoplasm.54 In acantholytic lesions, some cells enlarge dramatically and lose a regular (oval or polygonal) shape—the so called gigantic or “monster” cells.55 These changes suggest engagement of the oncotic process in the mechanism of acantholysis.
Oncosis, derived from the Greek word for swelling, often affects many contiguous cells, with early manifestations consisting of swelling and clearing of the cytosol, plasma membrane blebs that are typically organelle free, dilation of the endoplasmic reticulum and Golgi, mitochondrial condensation followed by swelling, clumping of nuclear chromatin, cellular swelling, increased membrane permeability, and marked alterations within the cytoskeleton. Because oncosis is based on the loss of volume control related to a damage of the plasma membrane, the oncotic cells are visualized through the use of die exclusion tests.38,39 The oncotic cell death is mediated by calpains, a family of Ca2+-activated neutral cysteine proteases.41
Morphological changes in apoptosis include cell shrinkage, chromatin condensation (margination) rendering curved profile to the nucleus, ending in break of the nucleus (karyorhexis), normal or condensed mitochondria, and apoptotic body formation.38 Multiple cytoplasmic buds typically contain organelles.39 Both apoptosis and oncosis can lead to necrosis—the postmortem autolytic and degradative changes.39 Necrosis is signaled by irreversible changes in the nucleus (karyolysis, pyknosis, and karyorhexis) and in the cytoplasm (condensation and intense eosinophilia, loss of structure, and fragmentation). Some of the detached keratinocytes that have drifted into the blister cavity in PV patients have intense eosinophilia.53
Certain hallmarks of programmed cell death up-regulated by PVIgG are not unique to apoptosis. For instance, TUNEL positivity is not specific for apoptosis, because either oncotic or apoptotic necrosis shows positive results with the TUNEL assay.39 Likewise, evidence has been presented that questioned the validity of using DNA electrophoresis for identifying apoptosis.56 It is not specific for apoptosis, because it occurs in lytic necrosis as well. Apoptosis is an energy-dependent process, whereas oncosis is a form of cell death induced by energy depletion.38 A decrease in ATP seems to be most important in situations that lead to oncosis compared with apoptosis. Interference with ATP synthesis rapidly leads to de-energization of the Na+, K+-ATPase at the plasmalemma followed by an increase in intracellular Na+ and Cl− accompanied by water influx and cellular swelling. In this regard, it is worth noting that cellular ATP is decreased in keratinocytes in familial benign pemphigus (a.k.a. Hailey-Hailey disease)57 that display cytopathological features characteristic of acantholytic cells of PV.5
The morphology of acantholytic cells in PV does not match that of either typical apoptotic or oncotic cells. Because under certain circumstances, target cells have been reported to display features of both apoptosis and oncosis, such as a combination of an apoptotic nucleus and oncotic cytoplasm, termed the “apoptotic oncotic” cells,58 we believe that simultaneous engagement of the apoptosis and oncosis effector mechanisms lead to cell death via acantholysis in PV. Because in keratinocytes exposed to PVIgGs, both apoptotic and oncotic processes were activated, ie, simultaneous activation of caspase 3 and calpain, and because inhibition of caspase or calpain in both cases diminished the extent of PVIgG-induced acantholysis, we propose that the morphology of acantholytic (Tzank) cells59 in PV reflects an overlap of complementary death signaling pathways (Figure 6).
Two subgroups of PV patients were identified based on the predominant type of cell death elicited in keratinocytes due to binding of IgG autoantibodies. One subgroup of patients, represented by PVIgG-1b, produced antibodies that activated predominantly the calpain-mediated pathway. Another subgroup, represented by PVIgG-2b, produced antibodies activating the death pathway mediated predominantly by caspases 8 and 3. Binding of PVIgGs from both subgroups activated caspase 9, which may provide a common step in death signaling pathway downstream of both the calpain- and the caspase 8-mediated events.60,61 A slightly less pronounced up-regulation of caspase 9 in keratinocytes treated with the PVIgG predominantly activating the calpain-mediated pathway may be explained by an ability of calpain to cleave caspase 9 by calpain, generating the inactive form of this caspase.62
Upstream of the apoptosis and oncosis effectors, autoantibodies from two different PV patients preferentially activated distinct signaling mechanisms. Several cellular events have been shown to increase the activity of calpains: a rise in the cytosolic Ca2+ concentration, translocation of calpains to membranes, autolysis of procalpains, dissociation of the calpain subunits, decreased levels of CSP, and interaction with calpain activator proteins or phospholipids.41,63 Recent studies also showed that calpains have several phosphorylation sites and may be regulated by phosphorylation.64 An increase of calpain activity in keratinocytes treated with PVIgGs observed in this study is in keeping with observations that binding of PVIgG to keratinocytes causes a transient increase in intracellular free Ca2+, activates phospholipase C, stimulates production of inositol 1,4,5-trisphosphate, induces activation and translocation of protein kinase C from the cytosol to the particulate/cytoskeleton fractions, and increases the phosphorylation status of cellular proteins.4,30,65–70 Furthermore, it has been recently reported that inhibitors of tyrosine kinases, phospholipase C, calmodulin, and the serine/threonine kinase protein kinase C prevented PVIgG-induced acantholysis in vivo.71
TNF-α has been shown to activate calpains because of a rise in the concentration of intracellular free Ca2+.72 It is well established that TNF-α is elevated in the serum of PV patients,73–76 deposited in the lesional skin,77,78 present in the blister fluid,74 and up-regulated in keratinocytes due to PV antibody binding78,79 and that anti-TNF-α therapy with etanercept (a fusion protein of TNF-α receptor) can ameliorate recalcitrant PV.80,81
During cell injury, calpain functions as a signal to mediate the influx of extracellular Ca2+ after ATP depletion and endoplasmic reticulum Ca2+ release.82 Therefore, an overt dependence of pathobiological effects of PVIgG-1b, rather than PVIgG-2b, on intracellular Ca2+ (Figure 5C) is in agreement with preferential engagement of the calpain signaling pathway by autoantibodies produced by the PV-1 patient. In this patient, engagement of the apoptosis effectors could be a secondary phenomenon. It has been shown that activation of the death receptor pathway, such as Fas-mediated apoptotic pathway, could be the result of a positive feedback mechanism secondary to mitochondrial damage.83,84 Furthermore, a loss of adhesion was reported to increase Fas-R and Fas-L expression and to down-regulate FLIP in endothelial cells.85 Downstream of the activation of caspases and calpains, the signaling pathways intersect, and the pathobiological effects overlap. Therefore, not surprisingly, the use of calpain inhibitors attenuated PVIgG-dependent caspase 3 activity, and vice versa, caspase inhibitors decreased PVIgG-induced rise in calpain activity. Acantholysis, too, could be attenuated by inactivating either arm of the pathobiological signaling, ie, with either caspase or calpain inhibitors. Because a combination of the caspase and calpain inhibitors showed an additive anti-acantholytic effect both in vitro and in vivo, acantholysis induced by PVIgG from each patient apparently resulted from an overlap of both pathobiological pathways acting synergistically.
Calpains have a large number of substrates, including signaling molecules, membrane proteins, intracellular enzymes, and structural proteins.41 Several calpain substrates are actin-associated proteins (spectrin, talin, paxillin, vinculin, and α-actinin). These proteins play a major role in maintaining cell shape, retaining plasma membrane integrity, and supporting cell adhesion. Calpain proteolysis of these proteins may lead to structural changes seen in acantholysis. Most importantly, calpain has been shown to be involved in the cleavage of adhesion molecules such as cadherins.86 Interestingly, UV irradiation up-regulates remarkably immunohistochemical visualization of calpain in the epidermis.87 UV irradiation is a common culprit of pemphigus exacerbation88 that has been shown to induce acantholysis in patients’ skin.89
Among calpain substrates are also proteins involved in apoptosis (caspase 3, Bax, Bcl-XL, and Bid).41 Calpain has been shown to positively regulate caspase 3 and caspase 7 activities independently of caspase 8.46,90 In turn, caspase activation leads to proteolytic cleavage of numerous intracellular targets, including CSP, which up-regulates calpain activity,91 and the adhesion molecules desmogleins, desmocollins, and β- and γ-catenins.92–94 The extracellular domains of desmoglein 3 and desmocollin 3 are released from the cell surface by a metalloproteinase activity, because in the presence of caspase and/or metalloproteinase inhibitors, both cleavage reactions are almost completely inhibited. This may initiate or augment production autoantibodies to desmoglein 3 and other adhesion molecules found in PV patients (reviewed by Grando2).
The fact that the PVIgG-1b and PVIgG-2b binding to keratinocytes elicited the apoptotic and oncotic events of different intensities can be explained through a hypothesis that a unique constellation of anti-keratinocyte autoantibodies, including desmoglein and non-desmoglein antibodies, determines the predominant type of cell death in each PV patient. Patient-to-patient variations in target cell response may also play a role. This possibility, however, was not addressed in this study, because we used keratinocytes from three different foreskin donors and obtained consistent results for IgGs derived from each particular PV patient.
The results of this study showing elevated expression of FLIP-l and CSP in keratinocytes treated with normal human IgGs (NIgG or IVIgG) suggest that protection of the target cells through up-regulation of the endogenous caspase and calpain inhibitors is a novel mechanism of therapeutic action of IVIg in PV. The fact that PVIgGs also up-regulated CSP can be explained through two distinct, yet complementary mechanisms: 1) positive feedback to intracellular calpain activation; and 2) occurrence in PV sera of the CSP-inducing IgGs that were also present in the NIgG and IVIgG fractions. The mechanism of protective effects of human IgGs on targets of autoimmunity in PV observed in this study remains to be elucidated. Because this study was focused on the IgG component of the IVIg drug, other potentially therapeutic substances may also be important.
In conclusion, the present study revealed that acantholysis in PV develops as a result of synergistic and additive actions of apoptosis and oncosis effectors activated by autoantibodies produced by different PV patients with variable efficacies. Inhibition of both death signaling pathways can completely block acantholysis. The therapeutic activity of IVIg in PV harbors direct anti-acantholytic effects on keratinocytes that may stem, in part, from up-regulation of both FLIP-l and CSP. Future studies should be directed toward identification of the types of IgG autoantibodies triggering distinct cell death pathways and elucidation of the mechanisms mediating protective effects of IVIg on targets of humoral autoimmunity.
Footnotes
Address reprint requests to Sergei A. Grando, 4860 Y Street, Suite 3400, Sacramento, CA 95817. E-mail: sagrando@ucdavis.edu.
Supported in part by a research grant from the Robert Leet & Clara Guthrie Patterson Trust and the International Pemphigus Research Fund (to S.A.G.).
Presented in part at the 66th Annual Meeting of the Society for Investigative Dermatology and published in form of an abstract (Grando SA, Chernyavsky A, Karaouni A, Arredondo J: Novel mechanisms of keratinocyte death and survival, and of therapeutic action of IVIg in pemphigus. J Invest Dermatol 2005;124[Suppl]: A6).
References
- Udey MC, Stanley JR. Pemphigus: diseases of antidesmosomal autoimmunity. J Am Med Assoc. 1999;282:572–576. doi: 10.1001/jama.282.6.572. [DOI] [PubMed] [Google Scholar]
- Grando SA. Autoimmunity to keratinocyte acetylcholine receptors in pemphigus. Dermatology. 2000;201:290–295. doi: 10.1159/000051540. [DOI] [PubMed] [Google Scholar]
- Korman NJ. Pemphigus. Dermatol Clin. 1990;8:689–700. [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Kitajima Y, Pittelkow M, Grando SA. Pemphigus vulgaris IgG and methylprednisolone exhibit reciprocal effects on keratinocytes. J Biol Chem. 2004;279:2135–2146. doi: 10.1074/jbc.M309000200. [DOI] [PubMed] [Google Scholar]
- Lever WF. Springfield: Charles C. Thomas,; Pemphigus and Pemphigoid. 1965 [Google Scholar]
- Judd KP, Lever WF. Correlation of antibodies in skin and serum with disease severity in pemphigus. Arch Dermatol. 1979;115:428–432. [PubMed] [Google Scholar]
- Sison-Fonacier L, Bystryn JC. Heterogeneity of pemphigus vulgaris antigens. Arch Dermatol. 1987;123:1507–1510. [PubMed] [Google Scholar]
- Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42:422–427. doi: 10.1016/s0190-9622(00)90213-5. [DOI] [PubMed] [Google Scholar]
- Gniadecki R, Jemec GB, Thomsen BM, Hansen M. Relationship between keratinocyte adhesion and death: anoikis in acantholytic diseases. Arch Dermatol Res. 1998;290:528–532. doi: 10.1007/s004030050347. [DOI] [PubMed] [Google Scholar]
- Puviani M, Marconi A, Cozzani E, Pincelli C. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J Invest Dermatol. 2003;120:164–167. doi: 10.1046/j.1523-1747.2003.12014.x. [DOI] [PubMed] [Google Scholar]
- Pelacho B, Natal C, Espana A, Sanchez-Carpintero I, Iraburu MJ, Lopez-Zabalza MJ. Pemphigus vulgaris autoantibodies induce apoptosis in HaCaT keratinocytes. FEBS Lett. 2004;566:6–10. doi: 10.1016/j.febslet.2004.03.107. [DOI] [PubMed] [Google Scholar]
- Milner Y, Metezeau P, Kiefer H, Finemesser M, Bregegere F, Zlotkin M, Wang X, Michel B. Pemphigus an autoimmune disease of the skin: cell-cell separation versus membrane signaling and apoptosis in acantholysis. Shoenfeld Y, editor. Amsterdam: Elsevier Science BV,; The Decade of Autoimmunity. 1999:pp 606–615. [Google Scholar]
- Wang X, Bregegere F, Frusic-Zlotkin M, Feinmesser M, Michel B, Milner Y. Possible apoptotic mechanism in epidermal cell acantholysis induced by pemphigus vulgaris autoimmunoglobulins. Apoptosis. 2004;9:131–143. doi: 10.1023/B:APPT.0000018795.05766.1f. [DOI] [PubMed] [Google Scholar]
- Wang X, Bregegere F, Soroka Y, Frusic-Zlotkin M, Milner Y. Replicative senescence enhances apoptosis induced by pemphigus autoimmune antibodies in human keratinocytes. FEBS Lett. 2004;567:281–286. doi: 10.1016/j.febslet.2004.04.083. [DOI] [PubMed] [Google Scholar]
- Grando SA, Chernyavsky AI, Arredondo J. Ability to induce keratinocyte apoptosis determines pathogenicity of pemphigus IgGs (abstract 35). J Investig Dermatol. 2004;122:A6. [Google Scholar]
- Frusic-Zlotkin M, Pergamentz R, Michel B, David M, Mimouni D, Bregegere F, Milner Y. The interaction of pemphigus autoimmunoglobulins with epidermal cells: activation of the fas apoptotic pathway and the use of caspase activity for pathogenicity tests of pemphigus patients. Ann NY Acad Sci. 2005;1050:371–379. doi: 10.1196/annals.1313.040. [DOI] [PubMed] [Google Scholar]
- Ahmed AR. Intravenous immunoglobulin therapy in the treatment of patients with pemphigus vulgaris unresponsive to conventional immunosuppressive treatment. J Am Acad Dermatol. 2001;45:679–690. doi: 10.1067/mjd.2001.116339. [DOI] [PubMed] [Google Scholar]
- Bystryn JC, Jiao D, Natow S. Treatment of pemphigus with intravenous immunoglobulin. J Am Acad Dermatol. 2002;47:358–363. doi: 10.1067/mjd.2002.122735. [DOI] [PubMed] [Google Scholar]
- Bhol KC, Desai A, Kumari S, Colon JE, Ahmed AR. Pemphigus vulgaris: the role of IL-1 and IL-1 receptor antagonist in pathogenesis and effects of intravenous immunoglobulin on their production. Clin Immunol. 2001;100:172–180. doi: 10.1006/clim.2001.5061. [DOI] [PubMed] [Google Scholar]
- Prasad NK, Papoff G, Zeuner A, Bonnin E, Kazatchkine MD, Ruberti G, Kaveri SV. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J Immunol. 1998;161:3781–3790. [PubMed] [Google Scholar]
- Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- Ekberg C, Nordstrom E, Skansen-Saphir U, Mansouri M, Raqib R, Sundqvist VA, Fernandez C. Human polyspecific immunoglobulin for therapeutic use induces p21/WAF-1 and Bcl-2, which may be responsible for G1 arrest and long-term survival. Hum Immunol. 2001;62:215–227. doi: 10.1016/s0198-8859(00)00250-0. [DOI] [PubMed] [Google Scholar]
- Stangel M, Schumacher HC, Ruprecht K, Boegner F, Marx P. Immunoglobulins for intravenous use inhibit TNF alpha cytotoxicity in vitro. Immunol Invest. 1997;26:569–578. doi: 10.3109/08820139709088541. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Brocker EB, Blaser K, Akdis CA. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol. 2001;108:839–846. doi: 10.1067/mai.2001.118796. [DOI] [PubMed] [Google Scholar]
- Spahn JD, Leung DY, Chan MT, Szefler SJ, Gelfand EW. Mechanisms of glucocorticoid reduction in asthmatic subjects treated with intravenous immunoglobulin. J Allergy Clin Immunol. 1999;103:421–426. doi: 10.1016/s0091-6749(99)70466-5. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Grando SA, Grando AA, Glukhenky BT, Doguzov V, Nguyen VT, Holubar K. History and clinical significance of mechanical symptoms in blistering dermatoses: a reappraisal. J Am Acad Dermatol. 2003;48:86–92. doi: 10.1067/mjd.2003.39. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete and degrade acetylcholine. J Invest Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Chernyavsky AI, Arredondo J, Bercovich D, Orr-Urtreger A, Vetter DE, Wess J, Beaudet AL, Kitajima Y, Grando SA. Synergistic control of keratinocyte adhesion through muscarinic and nicotinic acetylcholine receptor subtypes. Exp Cell Res. 2004;294:534–549. doi: 10.1016/j.yexcr.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Pittelkow MR, Kitajima Y, Grando SA. Pemphigus vulgaris acantholysis ameliorated by cholinergic agonists. Arch Dermatol. 2004;140:327–334. doi: 10.1001/archderm.140.3.327. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Bieche I, Onody P, Laurendeau I, Olivi M, Vidaud D, Lidereau R, Vidaud M. Real-time reverse transcription-PCR assay for future management of ERBB2-based clinical applications. Clin Chem. 1999;45:1148–1156. [PubMed] [Google Scholar]
- Bieche I, Parfait B, Le Doussal V, Olivi M, Rio MC, Lidereau R, Vidaud M. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: an outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 2001;61:1652–1658. [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Marubio LM, Beaudet AL, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: regulation of gene expression through a3b2 nicotinic receptor in oral epithelial cells. Am J Pathol. 2005;166:597–613. doi: 10.1016/s0002-9440(10)62281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Nguyen VT, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab Invest. 2001;81:1653–1668. doi: 10.1038/labinvest.3780379. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997;25:82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol. 2004;44:349–370. doi: 10.1146/annurev.pharmtox.44.101802.121804. [DOI] [PubMed] [Google Scholar]
- Shi Y, Melnikov VY, Schrier RW, Edelstein CL. Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am J Physiol Renal Physiol. 2000;279:F509–F517. doi: 10.1152/ajprenal.2000.279.3.F509. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Hallin U, Andersson AL, Puka-Sundvall M, Bahr BA, McRae A, Saido TC, Kawashima S, Hagberg H. Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J Biol Chem. 1999;274:14046–14052. doi: 10.1074/jbc.274.20.14046. [DOI] [PubMed] [Google Scholar]
- Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol. 2004;43:295–301. doi: 10.1016/j.jacc.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- Baroni A, Buommino E, Paoletti I, Orlando M, Ruocco E, Ruocco V. Pemphigus serum and captopril induce heat shock protein 70 and inducible nitric oxide synthase overexpression, triggering apoptosis in human keratinocytes. Br J Dermatol. 2004;150:1070–1080. doi: 10.1111/j.1365-2133.2004.05919.x. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. Defining apoptosis. Am J Pathol. 1995;146:16–19. [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgram GF, Caulfield JB, Lever WF. An electron microscopic study of acantholysis in pemphigus vulgaris. J Invest Dermatol. 1961;36:373–382. doi: 10.1038/jid.1961.58. [DOI] [PubMed] [Google Scholar]
- Fritsch PO, Elias PM. Mechanisms of vesicle formation and classification. Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. New York: McGraw-Hill Book Company,; Dermatology in General Medicine. 1979:pp 287–295. [Google Scholar]
- Torsuyev NA, Sheklakov ND, Romanenko VN. Moscow: Meditzina; Bullous Dermatoses. 1979 (In Russian) [Google Scholar]
- McKee PH. London: Mosby-Wolfe,; Pathology of the SkinWith Clinical Correlations. 1996 [Google Scholar]
- Cohen LM, Skopicki DK, Harrist TJ, Clark WH., Jr Noninfectious vesicobullous and vesicopustular diseases. Elder D, Elenitas R, Jaworsky C, Johnson BJ, editors. Philadelphia: Lippincott-Raven,; Lever’s Histopathology of the Skin. 1997:pp 209–252. [Google Scholar]
- Sheklakov ND. Moscow: MEDGIZ; Pemphigus. 1961 (In Russian) [Google Scholar]
- Collins RJ, Harmon BV, Gobe GC, Kerr JF. Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. Int J Radiat Biol. 1992;61:451–453. doi: 10.1080/09553009214551201. [DOI] [PubMed] [Google Scholar]
- Aronchik I, Behne MJ, Leypoldt L, Crumrine D, Epstein E, Ikeda S, Mizoguchi M, Bench G, Pozzan T, Mauro T. Actin reorganization is abnormal and cellular ATP is decreased in Hailey-Hailey keratinocytes. J Invest Dermatol. 2003;121:681–687. doi: 10.1046/j.1523-1747.2003.12472.x. [DOI] [PubMed] [Google Scholar]
- Takeda M, Shirato I, Kobayashi M, Endou H. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron. 1999;81:234–238. doi: 10.1159/000045282. [DOI] [PubMed] [Google Scholar]
- Tzank A. Le cytodiagnostic immediat en dermatologie. Ann Derm Syph. 1948;8:205. [Google Scholar]
- You KR, Shin MN, Park RK, Lee SO, Kim DG. Activation of caspase-8 during N-(4-hydroxyphenyl)retinamide-induced apoptosis in Fas-defective hepatoma cells. Hepatology. 2001;34:1119–1127. doi: 10.1053/jhep.2001.29199. [DOI] [PubMed] [Google Scholar]
- Gomez-Vicente V, Donovan M, Cotter TG. Multiple death pathways in retina-derived 661W cells following growth factor deprivation: crosstalk between caspases and calpains. Cell Death Differ. 2005;12:796–804. doi: 10.1038/sj.cdd.4401621. [DOI] [PubMed] [Google Scholar]
- Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- Lyubimov H, Goldshmit D, Michel B, Oron Y, Milner Y. Pemphigus: identifying the autoantigen and its possible induction of epidermal acantholysis via Ca2+ signalling. Israel J Med Sci. 1995;31:42–48. [PubMed] [Google Scholar]
- Seishima M, Esaki C, Osada K, Mori S, Hashimoto T, Kitajima Y. Pemphigus IgG, but not bullous pemphigoid IgG, causes a transient increase in intracellular calcium and inositol 1,4,5-triphosphate in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. 1995;104:33–37. doi: 10.1111/1523-1747.ep12613469. [DOI] [PubMed] [Google Scholar]
- Esaki C, Seishima M, Yamada T, Osada K, Kitajima Y. Pharmacologic evidence for involvement of phospholipase C in pemphigus IgG-induced inositol 1,4,5-trisphosphate generation, intracellular calcium increase, and plasminogen activator secretion in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. 1995;105:329–333. doi: 10.1111/1523-1747.ep12319948. [DOI] [PubMed] [Google Scholar]
- Osada K, Seishima M, Kitajima Y. Pemphigus IgG activates and translocates protein kinase C from the cytosol to the particulate/cytoskeleton fractions in human keratinocytes. J Invest Dermatol. 1997;108:482–487. doi: 10.1111/1523-1747.ep12289726. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Owada MK, Kitajima Y. A pathogenic autoantibody, pemphigus vulgaris-IgG, induces phosphorylation of desmoglein 3, and its dissociation from plakoglobin in cultured keratinocytes. Eur J Immunol. 1999;29:2233–2240. doi: 10.1002/(SICI)1521-4141(199907)29:07<2233::AID-IMMU2233>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling: inhibition of p38MAPK prevents pemphigus vulgaris IgG induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carpintero I, Espana A, Pelacho B, Lopez Moratalla N, Rubenstein DS, Diaz LA, Lopez-Zabalza MJ. In vivo blockade of pemphigus vulgaris acantholysis by inhibition of intracellular signal transduction cascades. Br J Dermatol. 2004;151:565–570. doi: 10.1111/j.1365-2133.2004.06147.x. [DOI] [PubMed] [Google Scholar]
- Chang I, Cho N, Kim S, Kim JY, Kim E, Woo JE, Nam JH, Kim SJ, Lee MS. Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol. 2004;172:7008–7014. doi: 10.4049/jimmunol.172.11.7008. [DOI] [PubMed] [Google Scholar]
- Ujihara M, Hamanaka S, Matsuda S, Numa F, Kato H. Pemphigus vulgaris associated with autoimmune hemolytic anemia and elevated TNF alpha. J Dermatol. 1994;21:56–58. doi: 10.1111/j.1346-8138.1994.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Alecu M, Alecu S, Coman G, Galatescu E, Ursaciuc C. ICAM-1, ELAM-1, TNF-alpha and IL-6 in serum and blister liquid of pemphigus vulgaris patients. Roum Arch Microbiol Immunol. 1999;58:121–130. [PubMed] [Google Scholar]
- Ameglio F, D’Auria L, Cordiali-Fei P, Trento E, D’Agosto G, Mastroianni A, Giannetti A, Giacalone B. Anti-intercellular substance antibody log titres are correlated with serum concentrations of interleukin-6, interleukin-15 and tumor necrosis factor-alpha in patients with Pemphigus vulgaris relationships with peripheral blood neutrophil counts, disease severity and duration and patients’ age. J Biol Regul Homeost Agents. 1999;13:220–224. [PubMed] [Google Scholar]
- D’Auria L, Bonifati C, Mussi A, D’Agosto G, De Simone C, Giacalone B, Ferraro C, Ameglio F. Cytokines in the sera of patients with pemphigus vulgaris: interleukin-6 and tumour necrosis factor-alpha levels are significantly increased as compared to healthy subjects and correlate with disease activity. Eur Cytokine Netw. 1997;8:383–387. [PubMed] [Google Scholar]
- Lopez-Robles E, Avalos-Diaz E, Vega-Memije E, Hojyo-Tomoka T, Villalobos R, Fraire S, Domiguez-Soto L, Herrera-Esparza R. TNF-alpha and IL-6 are mediators in the blistering process of pemphigus. Int J Dermatol. 2001;40:185–188. doi: 10.1046/j.1365-4362.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- Feliciani C, Toto P, Amerio P, Mohammad S, Coscione PS, Amerio P, Shivji G, Wang B, Sauder SN. In vitro and in vivo expression of interleukin-1a and tumor necrosis factor-a mRNA in pemphigus vulgaris: interleukin-1a and tumor necrosis factor-a are involved in acantholysis. J Invest Dermatol. 2000;114:71–77. doi: 10.1046/j.1523-1747.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Feliciani C, Toto P, Wang B, Sauder DN, Amerio P, Tulli A. Urokinase plasminogen activator mRNA is induced by IL-1alpha and TNF-alpha in in vitro acantholysis. Exp Dermatol. 2003;12:466–471. doi: 10.1034/j.1600-0625.2002.120415.x. [DOI] [PubMed] [Google Scholar]
- Berookhim B, Fischer HD, Weinberg JM. Treatment of recalcitrant pemphigus vulgaris with the tumor necrosis factor alpha antagonist etanercept. Cutis. 2004;74:245–247. [PubMed] [Google Scholar]
- Lin MH, Hsu CK, Lee JY. Successful treatment of recalcitrant pemphigus vulgaris and pemphigus vegetans with etanercept and carbon dioxide laser. Arch Dermatol. 2005;141:680–682. doi: 10.1001/archderm.141.6.680. [DOI] [PubMed] [Google Scholar]
- Harriman JF, Liu XL, Aleo MD, Machaca K, Schnellmann RG. Endoplasmic reticulum Ca(2+) signaling and calpains mediate renal cell death. Cell Death Differ. 2002;9:734–741. doi: 10.1038/sj.cdd.4401029. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann J. Molecular mechanisms of “detachment-induced apoptosis-Anoikis”. Apoptosis. 2002;7:247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-FLIP and implications for anoikis. J Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Fujio Y, Yamada-Honda F, Funai H, Wada A, Kawashima S, Awata N, Shibata N. Elevated calcium level induces calcium-dependent proteolysis of A-CAM (N-cadherin) in heart-analysis by detergent-treated model. Biochem Biophys Res Commun. 1995;217:649–653. doi: 10.1006/bbrc.1995.2823. [DOI] [PubMed] [Google Scholar]
- Miyachi Y, Yoshimura N, Suzuki S, Hamakubo T, Kannagi R, Imamura S, Murachi T. Biochemical demonstration and immunohistochemical localization of calpain in human skin. J Invest Dermatol. 1986;86:346–349. doi: 10.1111/1523-1747.ep12285556. [DOI] [PubMed] [Google Scholar]
- Ruocco V, Pisani M. Induced pemphigus. Arch Dermatol Res. 1982;274:123–140. doi: 10.1007/BF00510366. [DOI] [PubMed] [Google Scholar]
- Reis VM, Toledo RP, Lopez A, Diaz LA, Martins JE. UVB-induced acantholysis in endemic Pemphigus foliaceus (Fogo selvagem) and Pemphigus vulgaris. J Am Acad Dermatol. 2000;42:571–576. [PubMed] [Google Scholar]
- Ruiz-Vela A, Gonzalez de Buitrago G, Martinez AC. Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 1999;18:4988–4998. doi: 10.1093/emboj/18.18.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L, Allen H. Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch Biochem Biophys. 1998;356:187–196. doi: 10.1006/abbi.1998.0748. [DOI] [PubMed] [Google Scholar]
- Brancolini C, Sgorbissa A, Schneider C. Proteolytic processing of the adherens junctions components beta-catenin and gamma-catenin/plakoglobin during apoptosis. Cell Death Differ. 1998;5:1042–1050. doi: 10.1038/sj.cdd.4400443. [DOI] [PubMed] [Google Scholar]
- Steinhusen U, Badock V, Bauer A, Behrens J, Wittman-Liebold B, Dorken B, Bommert K. Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J Biol Chem. 2000;275:16345–16353. doi: 10.1074/jbc.M001458200. [DOI] [PubMed] [Google Scholar]
- Weiske J, Schoneberg T, Schroder W, Hatzfeld M, Tauber R, Huber O. The fate of desmosomal proteins in apoptotic cells. J Biol Chem. 2001;276:41175–41181. doi: 10.1074/jbc.M105769200. [DOI] [PubMed] [Google Scholar]