Abstract
The remarkable infectivity and virulence of the 1918 influenza virus resulted in an unprecedented pandemic, raising the question of whether it is possible to develop protective immunity to this virus and whether immune evasion may have contributed to its spread. Here, we report that the highly lethal 1918 virus is susceptible to immune protection by a preventive vaccine, and we define its mechanism of action. Immunization with plasmid expression vectors encoding hemagglutinin (HA) elicited potent CD4 and CD8 cellular responses as well as neutralizing antibodies. Antibody specificity and titer were defined by a microneutralization and a pseudotype assay that could assess antibody specificity without the need for high-level biocontainment. This pseudotype inhibition assay can define evolving serotypes of influenza viruses and facilitate the development of immune sera and neutralizing monoclonal antibodies that may help contain pandemic influenza. Notably, mice vaccinated with 1918 HA plasmid DNAs showed complete protection to lethal challenge. T cell depletion had no effect on immunity, but passive transfer of purified IgG from anti-H1(1918) immunized mice provided protective immunity for naïve mice challenged with infectious 1918 virus. Thus, humoral immunity directed at the viral HA can protect against the 1918 pandemic virus.
Keywords: influenza vaccines, neutralizing antibody, vaccines
In the past century, three influenza outbreaks have caused significant increases in human fatalities throughout the world, the hallmark of pandemics. Among them, the 1918 strain was notable for its exceptional infectivity and disease severity in otherwise healthy individuals, with mortality of 40 million to 50 million people worldwide. Through molecular analysis of preserved specimens, it has been possible to characterize the gene products of this virus in an effort to determine the molecular basis for its immunopathogenesis (1–10). Recently, this virus was reconstructed fully from lung specimens stored in 1918 (1–10) and shown to cause highly lethal infection in mice. This model has provided an opportunity to analyze the genetic basis of its virulence and to explore mechanisms of immunity relevant to the development of vaccines and antivirals for this and other influenza viruses with pandemic potential. Specifically, we sought to determine whether it is possible to generate protective immunity to this virus, to define potential mechanisms of immune protection, and to ascertain whether countermeasures can be developed to contain outbreaks.
Results
Generation of Expression Vectors.
To evaluate the protective immune response against the 1918 influenza virus, synthetic plasmid expression vectors encoding HA were generated. Plasmids expressing wild-type (WT) hemagglutinin (HA) (GenBank accession no. DQ868374) or HA with a mutation in the HA cleavage site (GenBank no. DQ868375) that attenuates influenza virus during viral replication were prepared by using nonviral codons, both to ensure compatibility with mammalian codon usage and to exclude the unlikely possibility of homologous reassortment with WT influenza viruses that might generate replication-competent H1(1918) virus (Fig. 1A). Expression was confirmed in transfected human renal 293T cells by Western blot analysis using antisera from mice vaccinated with these DNA expression vectors (Fig. 1B).
Fig. 1.
Expression of viral HAs. (A) The structure of the vectors, together with the indicated specific mutations in the cleavage site, for immunogens and lentiviral vector pseudotypes. (B) Expression of the indicated viral HAs was determined by Western blot analysis with antisera reactive to the 1918 influenza HA. Expression was evaluated after transfection of the indicated plasmids in 293T cells. Arrows indicate the relevant viral HA bands.
Vaccination with DNA Vaccines and Analysis of Cellular Immune Responses.
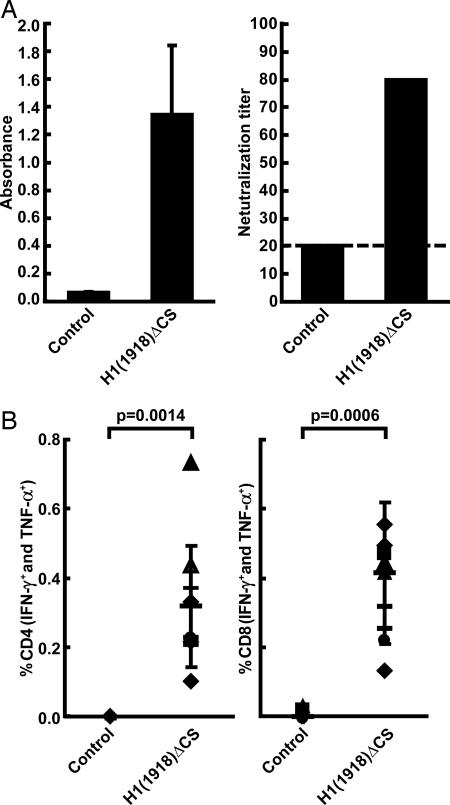
Antisera to the 1918 HA were generated by intramuscular inoculation of BALB/c mice with DNA vaccines. DNA vaccines included the WT 1918 HA as well as an attenuated HA(1918) cleavage site (ΔCS) mutant (Fig. 1A). Immunization with HA induced significant cellular and humoral responses. For example, H1(1918)ΔCS induced a >10-fold increase in H1(1918)-specific antibody measured by ELISA (Fig. 2A Left). The neutralization activity was confirmed by the microneutralization assay using live 1918 (H1N1) virus (Fig. 2A Right). Neutralization titers of ≈1:80 were observed with both the WT (data not shown) and mutant HA expression vectors using this assay, with a modest increase in the neutralization titers observed with the attenuated cleavage site mutant. Next, the cellular immune response was characterized in immunized mice. Vaccinated animals showed a marked increase in H1(1918) antigen-specific CD4 and CD8 T cells (Fig. 2B), as determined by intracellular cytokine staining to measure cells synthesizing either IFN-γ and/or TNF-α, at levels at least 62-fold and 122-fold above the background response for each T cell subset, respectively. These responses compared favorably with those observed with other viral spike proteins, including HIV, severe acute respiratory syndrome (SARS), coronavirus, and Ebola virus, all of which contain a number of predicted T cell epitopes (data not shown).
Fig. 2.
Humoral and cellular immune responses to 1918 influenza HA after DNA vaccination. (A) Antibody responses induced by DNA vaccination against the 1918 influenza HA measured by ELISA (Left) or microneutralization (Right). The microneutralization assay using dilutions of heat-inactivated sera and titers of virus neutralizing antibody were determined as the reciprocal of the highest dilution of serum that neutralized 100 plaque-forming units of virus in Madin-Darby canine kidney (MDCK) cell cultures on a 96-well plate (Right). ELISA for viral nucleocapsid protein (NP) was performed for determining the presence of the virus as the read-out. Data are presented as the mean for each group. (B) Intracellular cytokine staining was performed to analyze the T cell response to viral HA peptides. The percentage of activated T cells that produced either IFN-γ and/or TNF-α in response to stimulation with overlapping peptides in CD4 (Left) or CD8 (Right) is shown. Lymphocytes from mice (n = 5 per group) immunized with empty plasmid vector (control) or mice (n = 10 per group) immunized with the indicated plasmid at 0, 3, 6, and 12 weeks were assessed, and immune responses were measured 11 days after the final boost. Nonstimulated cells gave responses similar to controls at background levels. Symbols indicate the response of individual animals, and the median value is shown with a horizontal bar.
Immune Protection Conferred by DNA Vaccination and Mechanism of Action.
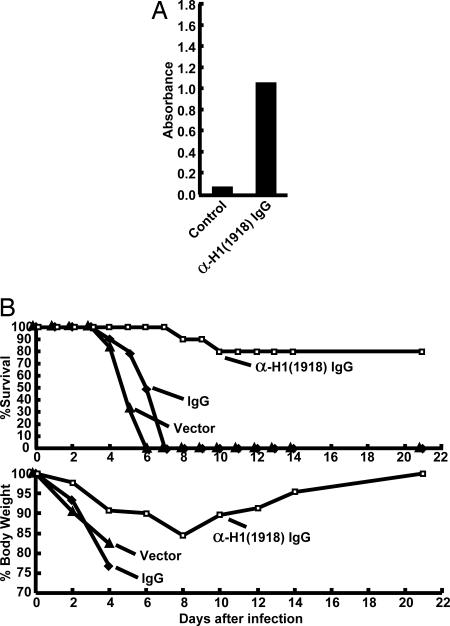
To assess the efficacy of this vaccine against lethal infection by the 1918 influenza virus, vaccinated animals were given 100 LD50 of live virus intranasally 14 days after the final DNA plasmid injection. All studies with live reconstructed 1918 virus were performed under high-containment [biosafety level 3 enhanced (BSL3)] laboratory conditions in accordance with guidelines of the National Institutes of Health and the Centers for Disease Control (ref. 11 and www.cdc.gov/flu/h2n2bsl3.htm). Both the WT and cleavage mutant H1(1918) plasmids induced complete protection against lethal viral challenge measured by survival (Fig. 3A Upper) as well as extent of weight loss compared with controls (Fig. 3A Lower). To define the mechanism of immune protection, T cell depletion was performed with monoclonal antibodies [anti-mouse CD4(GK1.5), anti-mouse CD8(2.43), or anti-mouse CD90(30-H12)] to CD4, CD8, and CD90 (Thy1.2), previously shown to deplete >99% T cells in lung and spleen (12). The same negative control DNA plasmid vectors without an insert were used for both the DNA vaccine and the depletion studies. T cell depletion of H1(1918) immunized animals did not affect survival (Fig. 3B Upper), and these mice showed weight loss comparable with nonimmune Ig-treated animals (Fig. 3B Lower). In contrast, when IgG from immunized mice purified by Protein A chromatography was passively transferred to naïve recipients, neutralizing antibodies could be detected in the recipients at levels slightly below those of the immunized mice (Fig. 4A vs. Fig. 2A Left). Importantly, passive transfer of this immune IgG conferred immune protection in 8 of 10 mice, compared with 0 of 10 animals that received IgG from control unvaccinated animals (Fig. 4B; P = 0.0007).
Fig. 3.
Immune protection conferred against lethal challenge of 1918 influenza and lack of T cell dependence. (A) Immunization with H1(1918), H1(1918)ΔCS, or negative control plasmid expression vectors was performed in mice (n = 10 per group) as described (11), and survival (Upper) and weight loss (Lower) were evaluated. The statistical significance between these groups are P = 1.08 × 10−5 and P = 1.08 × 10−5 with respect to controls by Fisher's exact test. (B) Monoclonal rat anti-mouse anti-CD4, CD8, and CD90 (T cell depletion) were used to deplete T cells in H1(1918)ΔCS immunized mice, in comparison with a control group of vaccinated animals injected with nonimmune IgG (Control IgG). Vector-immunized animals that received no depletion served as additional controls (Vector). Mice were administered IgG at −3, +3, +9, and +15 days after viral challenge. Mice (n = 10 per group) were then evaluated for survival (Upper) and weight loss (Lower). No decrease in immune protection was observed in T cell-depleted animals.
Fig. 4.
Immune mechanism of protection, showing dependence on Ig. (A) Activity of control, nonimmune, IgG (control), or anti-HA immune IgG [anti-H1(1918)], purified as described (12), was confirmed by ELISA before passive transfer into naïve recipients (n = 10 per group). (B) Passive transfer. To assess the protective effects of immune IgG, mice received immune or control IgG 24 h before infection with 100 LD50 of 1918 virus. Mice were then monitored for survival and weight loss throughout a 21-day observation period. The difference between the immune [α−H1(1918) IgG] and control IgG (IgG) groups was significant (P = 0.0007).
Development of Pseudotyped Lentiviral Reporters.
The functional activity of this HA was assessed through the use of a pseudotyped lentiviral vector in which the 1918 HA was used instead of the retroviral envelope. The HA pseudoviruses were then characterized for their susceptibility to neutralizing antibodies by using a luciferase reporter gene. Whereas H5-pseudotyped lentiviral vectors readily mediated entry, the H1(1918) strain was inactive (Fig. 5A Left vs. Center, second column). Because H5 viruses contain a cleavage site recognized more broadly by proteases, the protease cleavage site region from H5 was substituted into the relevant sequence of H1(1918) in an effort to increase its processing to a fusion-competent form. A modification that extended 11 aa (Fig. 1A; H5ΔPS2) from the cleavage site improved entry more than a slightly shorter 9-aa (Fig. 1A; H5ΔPS) addition (Fig. 5A Center, third and fourth column). Insertion of an H5 cleavage site conferred a similar increase in entry for an independent H1 strain, PR/8 (Fig. 5A Right), suggesting that such modifications could allow otherwise incompetent HAs to generate functionally active pseudotyped vectors that could be used to assess neutralizing antibody activity.
Fig. 5.
Development of HA-pseudotyped lentiviral vectors. (A) Gene transfer mediated by lentiviral vectors pseudotyped with H1(1918), H5(Kan-1), or other HAs containing the H5 protease cleavage site was measured with a luciferase reporter assay. (B) Neutralization by antisera from mice immunized with the indicated HA plasmid expression vectors or no insert (control) plasmid DNA vectors was measured with the luciferase assay with the HA-pseudotyped lentiviral vectors. Reduction of gene transfer in the presence of immune sera was observed in a dose-dependent fashion (data not shown).
Humoral immunity in mice immunized with 1918 HA plasmid DNAs was assessed by using the viral pseudotype assay. To analyze the ability of antibodies to neutralize virus, the pseudoviruses were incubated with antisera from control and HA-immune animals, and the reduction in luciferase activity was measured. Sera from animals immunized with the H1(1918) or H5(Kan-1) HA expression vectors neutralized pseudotyped lentiviral vectors encoding the homologous, but not the heterologous, HAs at dilutions of 1:400 in this assay, in contrast to nonimmune sera, which had no effect (Fig. 5B). These titers were considerably higher than those measured by microneutralization (Fig. 2A) or hemagglutination inhibition (data not shown), suggesting that the pseudotype vector inhibition assay is more sensitive. Thus, immunity in the lethal challenge model was readily measured and correlated with protection in this assay.
Discussion
In this study, gene-based vaccination was used to elicit cellular and humoral immune responses to the 1918 influenza virus HA. The humoral immune response was able to neutralize this virus, and these antibodies were necessary and sufficient to confer protective immunity to lethal challenge by virus. In contrast, although a robust T cell response was observed, this response was not required for protective immunity. Whereas slightly increased weight loss was observed in T cell-depleted animals relative to controls, this difference was also observed to some extent in control animals, likely reflecting stress associated with the additional manipulations. Thus, although it remains possible that T cells may contribute to an antiviral effect and could potentially contribute to cross-heterotypic protection to variant viruses, they are not required for immune protection for this vaccine in the mouse. In humans, immune control is likely also antibody dependent, but we cannot exclude the possibility that cellular mechanisms of protection may contribute to viral clearance. The unique circumstances that contributed to the 1918 pandemic spread are also unknown. Although there has been speculation about the types of viruses that may have circulated before the epidemic and their implications for herd immunity, neither the virus isolates nor sera from these times are available, and the present epidemiologic data do not permit further analysis, although recovery of such viruses through methods used for the 1918 rescue could be informative in the future.
The ability to use a pseudotyped lentiviral vector with viral HA allows for analysis of neutralizing antibody response with increased sensitivity in the detection of neutralizing antibodies compared with the traditional antiviral assay. In addition, the ability to perform screening in the absence of replication-competent virus allows for methods to screen for neutralizing antibodies and to generate antiviral reagents, for the 1918 pandemic influenza virus, as well as avian H5N1 influenza virus and other possibly highly pathogenic influenza viruses in a conventional biosafety level 2 laboratory. It will also be desirable in the future to compare results from this assay with hemagglutination inhibition to explore its ability to predict protective immunity in humans. However, the difficulty of preparing large quantitities of the highly pathogenic H1N1 virus make it technically infeasible at this time.
Further testing will be required to determine whether DNA vaccination can confer similar humoral immune responses in humans and to assess the response. Previous experience has shown that this mode of vaccination is much less effective in humans than in rodents and thus may not be the preferred form of the 1918 H1N1 vaccine. The results nonetheless demonstrate that humoral immune responses are protective against viral infection and provide proof of concept that immunization with H1(1918), whether by gene-based vaccines, recombinant proteins, or inactivated virus, is likely to be successful for the generation of protective immunity in humans. These data also suggest that there is no intrinsic immune resistance of this pandemic influenza virus. This knowledge, together with the enhanced ability to measure and screen for neutralizing antibody as a correlate of protection, will facilitate the development of novel protective vaccines and monoclonal antibodies for the 1918 influenza virus and for contemporary pandemic flu threats.
Materials and Methods
Immunogen and Plasmid Construction.
Plasmids encoding different versions of HA protein [A/South Carolina/1/18, GenBank AF117241; A/Thailand/1(KAN-1)/2004, GenBank AAS65615; A/PR/8/34, GenBank P03452] were synthesized by using human-preferred codons as described (12) by GeneArt (Regensburg, Germany). All of the H1 and H5 HA cleavage-site mutants were made with the original viral cleavage amino acid sequences changed to PQRETRG, ΔCS, which is originally from a low-pathogenicity H5 isolate (A/chicken/Mexico/31381/94; GenBank AAL34297), and the modification resulted in the trypsin-dependent phenotype (13) and may alter the antigenic character. To make the pseudotyped lentiviral vector for H1(1918), the original viral cleavage site was changed to IPQRERRRKKRG, ΔPS, and SPQRERRRKKRG, ΔPS2, which is originally from a highly virulent H5 [A/Thailand/1(KAN-1)/2004], and the modification should result in the trypsin-independent phenotype. Protein expression was confirmed by Western blot analysis (14) with serum from mice immunized with HA-expressing plasmid DNA.
To synthesize the coating antigen for the ELISA, codon-optimized cDNA corresponding to aa 1 to 530 was cloned into CMV/R 8κB expression vector for efficient expression in mammalian cells. This vector utilizes the CMV/R plasmid backbone (15) with several modifications at the NF-κB binding sites in the enhancer/promoter region to enhance immunogenicity of the inserts expressed in the plasmid DNA constructs. Four κB binding sites in the enhancer/promoter region were modified by two pairs to a consensus κB sequence (16) as follows: nucleotide (nt) 802 GCACCAAAATCAACGGGACTTT was changed to ACTCACCAAAATCAACGGGAATTC; nt 753 GGGGATTT was changed to GGGACTT; nt 648 GGGACTTT was changed to GGGAATTT; and nt 607 TAAATGGCCCGCCTG was changed to GAACTTCCATAAGCTT. Two additional such sites were introduced upstream of the original enhancer/promoter region as follows: nt 550 GGCAGTACATCA was changed to GGGAATTTCCA; nt 497 GGGACTTTC was changed to GGGAACTTC; nt 714 TAAATGGCGGG was changed to GAATTTCCAAA; and nt 361 GGGGTCATTAGTT was changed to GGGAACTTC. When tested in mice, a CMV/R 8κB plasmid induced a higher immunological response to HIV clade B envelope immunogen, with higher antigen-specific CD4 and CD8 T cell responses than CMV/R, and also improved antibody responses (data not shown). A trimerization sequence from bacteriophage T4 fibritin was introduced followed by a thrombin cleavage site and a His tag at the C terminus. The plasmid was then transfected into 293T cells, and the cell media containing the secreted protein was collected and purified by nickel column chromatography. The purified protein contains the following additional residues at the C terminus (RSLVPRGSPGSGYIPEAPRDGQAYVRKDGEWVLLSTFLGHHHHHH), where the thrombin site is in italics, the fibritin trimerization sequence is underlined, and the His tag is in bold. Similar modifications used for HA molecule structural studies have been described (7).
Vaccination.
Female BALB/c mice (6–8 weeks old; Jackson Laboratories, Bar Harbor, ME) were immunized intramuscularly with 15 μg of plasmid DNA in 100 μl of PBS (pH 7.4) at weeks 0, 3, and 6 for T lymphocyte depletion, IgG passive transfer, and viral challenge. T cell depletion and antibody transfer were performed as described below. An additional immunization at week 12 was performed in groups for the intracellular cytokine staining assay.
Flow Cytometric Analysis of Intracellular Cytokines.
CD4+ and CD8+ T cell responses were evaluated by using intracellular cytokine staining for IFN-γ and TNF-α as described (14) with peptide pools (15 mers overlapping by 11 aa, 2.5 μg/ml each) covering the HA protein. Cells were then fixed, permeabilized, and stained by using rat monoclonal anti-mouse CD3, CD4, CD8, IFN-γ, and TNF-α (BD-PharMingen, San Diego, CA). The IFN-γ- and TNF-α-positive cells in the CD4+ and CD8+ cell populations were analyzed with the program FlowJo (Tree Star, Ashland, OR).
ELISA for Mouse Anti-HA IgG and IgM.
The mouse anti-HA IgG and IgM ELISA titer was measured by using a described method (17). Purified HA protein was made by purification of a transmembrane-domain-truncated HA protein with a trimerization domain, thrombin cleavage site, and His tag expressed in the CMV/R 8κB expression vector. H1 or H5 protein was purified from transfected 293T cell culture supernatants as detailed in Immunogen and Plasmid Construction, also analogous to a described method (7), and used to coat the plate.
Production of HA Pseudotyped Lentiviral Vectors and Measurement of Neutralizing Activity of Immune Serum.
Influenza HA-pseudotyped lentiviral vectors expressing a luciferase reporter gene were produced as described (17). Briefly, 293T cells were cotransfected by using the following plasmids: 7 μg of pCMVΔR8.2, 7 μg of pHR′CMV-Luc, and 125 ng CMV/R 8κB H1(1918), H1(1918)(ΔPS), H1(1918)(ΔPS2), or H1(PR/8)(ΔPS), or H5(Kan-1). Cells were transfected overnight, washed, and replenished with fresh medium. Forty-eight hours later, supernatants were harvested, filtered through a 0.45-μm syringe filter, aliquotted, and used immediately or frozen at −80°C. For neutralization assays, antisera were mixed with 100 μl of pseudoviruses at various dilutions and added to 293A cells (Invitrogen, Carlsbad, CA) in 48-well dishes (30,000 cells per well). Plates were washed, and fresh medium was added 14–16 h later. Forty-eight hours after infection, cells were lysed in mammalian cell lysis buffer (Promega, Madison, WI). A standard quantity of cell lysate was used in a luciferase assay with luciferase assay reagent (Promega) according to the manufacturer's protocol.
Microneutralization Assay of 1918 (H1N1) by Mouse Immune Antisera.
Two-fold dilutions of heat-inactivated sera were tested in a microneutralization assay for the presence of antibodies that neutralized the infectivity of 100 TCID50 (50% tissue culture infectious dose) of 1918 (H1N1) viruses on MDCK cell monolayers by using two wells per dilution on a 96-well plate as described (18). After 2 days of incubation, cells were fixed, and ELISA was performed to detect the presence of viral nucleoprotein (NP) and determine the neutralization activity.
Challenge of Mice with Live 1918 (H1N1) Virus.
Two weeks after final vaccination, mice were challenged intranasally with 100 LD50 of 1918 (H1N1) virus in a volume of 50 μl. After infection, mice were monitored daily for disease signs and death for 21 days after infection. Individual body weights and death were recorded for each group on various days after inoculation. All 1918 influenza virus studies were performed under high-containment biosafety level 3 enhanced (BSL3) as described (11).
Depletion of T Cell Subsets in Vivo.
To deplete specific T cell subsets, known rat monoclonal antibodies [anti-mouse CD4 (GK1.5), anti-mouse CD8(2.43), or anti-mouse CD90(30-H12)], prepared as described (12, 19) and obtained from the National Cell Culture Center (Minneapolis, MN), were administered i.p. (1 mg each in 1 ml of PBS) 3 days before challenge and 3, 9, and 15 days after the viral challenge. For nonimmune Ig treatment (control), an isotype-matched anti-human leukocyte antigen (SFR3-D5) mononclonal antibody was used.
Passive Transfer of Ig.
IgG from mice immunized with plasmid DNA encoding HAΔCS (immune) or no insert (controls) was purified from sera by using a Montage Antibody Purification Kit (Millipore, Billerica, MA), and antibody titer was confirmed by ELISA. Briefly, 0.3 ml of purified IgG (from ≈0.7 ml of serum) was administered i.v. into each recipient naïve mouse (n = 10 per group) by tail vein injection 24 h before challenge.
Statistical Analysis.
Each individual animal immune response was counted as an individual value for statistical analysis. The significance of the cellular and humoral immune responses was calculated by Student's t test (tails = 2, type = 2) as indicated by the P value. For immune protection between groups, Fisher's exact test was used to analyze the data, and the result was indicated by the P value.
Acknowledgments
We thank Ati Tislerics and Tina Suhana for help with manuscript preparation, Martha Nason for the statistical analysis, Toni Miller and Brenda Hartman for figure preparation, and members of the G.J.N. laboratory for advice and discussions. This work was supported in part by the Intramural Research Program of the National Institutes of Health Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID), and by NIAID grants to A.G.-S.
Footnotes
The authors declare no conflict of interest.
References
- 1.Stevens J, Blixt O, Glaser L, Taubenberger JK, Palese P, Paulson JC, Wilson IA. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 3.Glaser L, Stevens J, Zamarin D, Wilson IA, Garcia-Sastre A, Tumpey TM, Basler CF, Taubenberger JK, Palese P. J Virol. 2005;79:11533–11536. doi: 10.1128/JVI.79.17.11533-11536.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid AH, Fanning TG, Janczewski TA, Lourens RM, Taubenberger JK. J Virol. 2004;78:12462–12470. doi: 10.1128/JVI.78.22.12462-12470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, et al. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 6.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Basler CF. Proc Natl Acad Sci USA. 2004;101:3166–3171. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Science. 2004;303:1866–1870. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- 8.Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, Vasisht N, Steinhauer DA, Daniels RS, Elliot A, et al. Science. 2004;303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 9.Basler CF, Reid AH, Dybing JK, Janczewski TA, Fanning TG, Zheng H, Salvatore M, Perdue ML, Swayne DE, Garcia-Sastre A, et al. Proc Natl Acad Sci USA. 2001;98:2746–2751. doi: 10.1073/pnas.031575198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid AH, Fanning TG, Janczewski TA, Taubenberger JK. Proc Natl Acad Sci USA. 2000;97:6785–6790. doi: 10.1073/pnas.100140097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, et al. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z-Y, Kong W-P, Huang Y, Roberts A, Murphy B, Subbarao K, Nabel GJ. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Liu C, Klimov A, Subbarao K, Perdue ML, Mo D, Ji Y, Woods L, Hietala S, Bryant M. J Infect Dis. 1999;179:1132–1138. doi: 10.1086/314713. [DOI] [PubMed] [Google Scholar]
- 14.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. J Virol. 2003;77:12764–12772. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, Kishko MG, Arthur JC, Miura A, Mascola JR, et al. J Virol. 2005;79:8828–8834. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung TH, Hoffmann A, Baltimore D. Cell. 2004;118:453–464. doi: 10.1016/j.cell.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z-Y, Chakrabarti BK, Xu L, Welcher B, Kong W-P, Leung K, Panet A, Mascola JR, Nabel GJ. J Virol. 2004;78:4029–4036. doi: 10.1128/JVI.78.8.4029-4036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, Nabel GJ. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]