Abstract
The most common inherited form of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease affecting adult motoneurons, is caused by dominant mutations in the ubiquitously expressed Cu2+/Zn2+ superoxide dismutase (SOD1). Recent studies suggest that glia may contribute to motoneuron injury in animal models of familial ALS. To determine whether the expression of mutant SOD1 (mSOD1G93A) in CNS microglia contributes to motoneuron injury, PU.1−/− mice that are unable to develop myeloid and lymphoid cells received bone marrow transplants resulting in donor-derived microglia. Donor-derived microglia from mice overexpressing mSOD1G93A, an animal model of familial ALS, transplanted into PU.1−/− mice could not induce weakness, motoneuron injury, or an ALS-like disease. To determine whether expression of mSOD1G93A in motoneurons and astroglia, as well as microglia, was required to produce motoneuron disease, PU.1−/− mice were bred with mSOD1G93A mice. In mSOD1G93A/PU.1−/− mice, wild-type donor-derived microglia slowed motoneuron loss and prolonged disease duration and survival when compared with mice receiving mSOD1G93A expressing cells or mSOD1G93A mice. In vitro studies confirmed that wild-type microglia were less neurotoxic than similarly cultured mSOD1G93A microglia. Compared with wild-type microglia, mSOD1G93A microglia produced and released more superoxide and nitrite+nitrate, and induced more neuronal death. These data demonstrate that the expression of mSOD1G93A results in activated and neurotoxic microglia, and suggests that the lack of mSOD1G93A expression in microglia may contribute to motoneuron protection. This study confirms the importance of microglia as a double-edged sword, and focuses on the importance of targeting microglia to minimize cytotoxicity and maximize neuroprotection in neurodegenerative diseases.
Keywords: bone marrow transplant, neuroprotection, superoxide dismutase, nitric oxide, motoneurons
Amyotrophic lateral sclerosis (ALS) causes an adult-onset neurodegenerative disease that selectively kills upper and lower motoneurons, resulting in paralysis and death (1). Transgenic animals (2–5) overexpressing mutant human Cu2+/Zn2+ superoxide dismutase (mSOD1) develop a progressive motoneuron disease that resembles the clinical and pathological features of human familial ALS. Presently, the mechanisms by which mSOD1 causes selective motoneuron death are not clearly defined. Elegant data from chimeric mice, using the original mSOD1 promoter, in the same genomic location, and with the same transgene copy number, showed that mSOD1-overexpressing neurons surrounded by normal glia remained relatively intact, whereas normal neurons surrounded by mSOD1-overexpressing glia showed signs of injury (6). However, that study did not distinguish which glia, whether astroglia, oligodendroglia, or microglia, were responsible for neuronal injury. Because activated microglia and macrophages are found in the spinal cords of transgenic mice overexpressing the G93A form of mSOD1 (mSOD1G93A) (7), it is likely that microglia and other immune system components can influence motoneuron injury. To evaluate the effects of mSOD1G93A expression in microglia in ALS pathogenesis, we sought to express mSOD1G93A exclusively in those cells. A bone marrow transplant (BMT) after γ-irradiation can replace the peripheral immune system, but although the transplanted cells can develop into a few microglia, they will not replace the resident parenchymal microglia. To resolve this issue, we used PU.1 knockout (PU.1−/−) mice because, at birth, these mice lack macrophages, neutrophils, T and B cells, and most importantly, CNS microglia (8, 9). Even with antibiotics, PU.1−/− mice survive for >20 days only if a BMT is given at birth, and the BMT results in donor-derived immune cells and CNS microglia (8, 9).
Results
Growth of PU.1 Mice.
In accord with a previous report (9), birth weights of PU.1−/− mice were not different from either PU.1 heterozygous (PU.1+/−) mice or nontransgenic (B6/SJL mice) wild-type (WT - PU.1+/+) mice. However, regardless of the donor bone marrow, surviving PU.1−/− mice grew slower than PU.1+/− or WT mice (Fig. 8A, which is published as supporting information on the PNAS web site; P < 0.001). PU.1−/− mice that received mSOD1G93A bone marrow grew at a rate significantly slower than PU.1−/− mice transplanted with WT nontransgenic (B6/SJL mice) bone marrow (Fig. 8B; P = 0.046). Systemic expression of mSOD1G93A in the PU.1−/− (mSOD1G93A/PU.1−/−) mice did not slow growth (Fig. 8C). All surviving transplanted PU.1−/− mice had a mean life span of 331 ± 20 days.
Bone Marrow-Derived Cells in Neonatal PU.1 Mice.
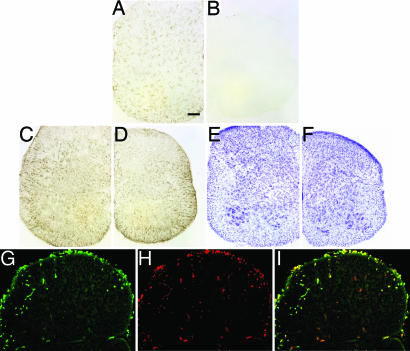
CD11b microglial signal was apparent in spinal cord sections from 11-day-old PU.1+/− mice (Fig. 1A), but was absent in nontransplanted PU.1−/− spinal cords (Fig. 1B). In contrast, GFAP (Fig. 1 C and D) and cresyl violet (Fig. 1 E and F) staining demonstrated that the astrocytic and neuronal morphology and populations were similar between nontransplanted PU.1−/− mice and their PU.1+/− littermates. Cells positive for CD11b and GFP were observed in the peripheral white matter of the spinal cords as early as 11 days of age after GFP BMT of PU.1−/− mice (Fig. 1 G–I). In these 11-day-old mice, CD11b staining revealed that the microglia in PU.1−/− mice had several morphological differences, including more prominent, rounded cell bodies and shorter/thicker processes, which may be indicative of a less differentiated state. Thus, the complete overlap of GFP and CD11b immunofluorescence confirmed the model system.
Fig. 1.
Identification and characterization of donor-derived microglia in spinal cord sections from neonatal PU.1−/− mice. Presence (A) and absence (B) of CD11b signal on microglia in PU.1+/− mouse and PU.1−/− mouse without BMT, respectively. GFAP immunohistochemical and cresyl violet staining did not reveal any differences in astrocyte numbers or morphology, and motoneuron numbers or morphology, in spinal cord sections of 11-day-old PU.1+/− (C and E) and PU.1−/− (D and F) mice that did not receive a BMT. Green GFP (G) and red CD11b (H) signals from an 11-day-old PU.1−/− mouse. (I) Merged image of G and H. (Scale bar, 100 μm.)
Bone Marrow-Derived Cells in Adult PU.1 Mice.
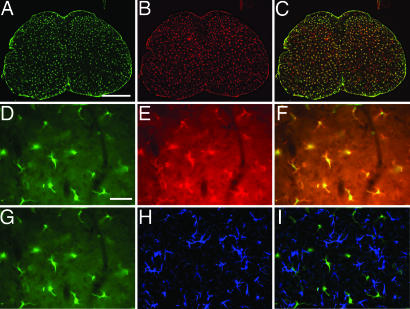
Parenchymal GFP+ cells that also expressed CD11b were observed in spinal cord sections from 40-day-old PU.1−/− mice with GFP BMT (Fig. 2A–C). Although there was variability in terms of expression levels of both GFP and CD11b, essentially all GFP and CD11b signals overlapped (Fig. 2 D–F). Furthermore, donor cells did not develop into astrocytes because no observable GFP+ cells expressed GFAP (Fig. 2 G–I). Thus, donor-derived bone marrow cells do colonize the spinal cords of PU.1−/− mice and generate microglia, but do not develop into detectable numbers of astrocytes.
Fig. 2.
Identification and characterization of donor-derived microglia in spinal cord sections from adult PU.1−/− mice. Green GFP (A) and red CD11b (B) signals from a 40-day-old PU.1−/− mouse. (C) Merged image of A and B. Higher magnification of the GFP (D) and red CD11b (E) signals from a 40-day-old PU.1−/− mouse. (F) Merged image of D and E. (G) Same image as D of the green GFP signal in spinal cord sections from 40-day-old PU.1−/− mice that received BMT from GFP donors. (H) Blue GFAP immunofluorescence indicating the presence of astrocytes. (I) Merged image of G and H demonstrating that the blue GFAP signal does not overlap the green GFP signal. (Scale bars, 100 μm in A–C and 500 μm in D–I.)
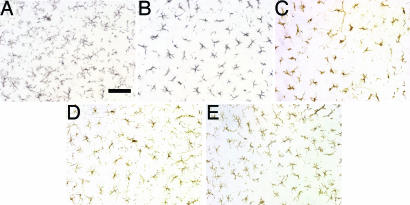
CD11b immunoreactivity was apparent on microglia in lumbar spinal cord sections from 40-day-old PU.1+/− mice (Fig. 3A) and did not differ from WT mice (data not shown). In 40- and 140-day-old PU.1−/− mice transplanted with either WT or mSOD1G93A bone marrow, the microglia differed morphologically from those in PU.1+/− mice; the cell bodies of the microglia were rounded with shorter/thicker processes (Fig. 3 B and C). Microglia in lumbar spinal cord sections from PU.1−/− mice 370 days after BMT also displayed shorter and less ramified processes when compared with PU.1+/− mice (Fig. 3D); these differences were much less prominent than in younger BMT mice. Although fewer in number, at 570 days after BMT, the microglia from PU.1−/− mice receiving WT or mSOD1G93A bone marrow were morphologically indistinguishable from microglia observed in spinal cord sections from either PU.1+/− or WT mice (Fig. 3E). However, there were no differences in the number of microglia between PU.1 mice receiving BMT from either WT or mSOD1G93A donor mice. Immunostaining for F4/80, another microglial marker, documented similar differences as those observed with CD11b (Fig. 9 A–C, which is published as supporting information on the PNAS web site).
Fig. 3.
Adult microglia in adult PU.1 mice. CD11b positive microglia in a spinal section from 40-day-old PU.1+/− (A) and PU.1−/− (B) mice, and 140- (C), 370- (D), and 570-day-old (E) PU.1−/− mice after BMT. (Scale bar, 100 μm.)
PU.1−/− mice that received bone marrow from mSOD1G93A donors did not develop weakness or other signs of motoneuron disease. GFAP immunoreactivity was apparent on astrocytes in lumbar spinal cord sections from 140-, 370-, and 570-day-old PU.1−/− mice after BMT from WT or mSOD1G93A donors and did not differ from PU.1+/− or WT mice (Fig. 9 D–F). Cresyl violet staining of lumbar spinal cord sections from PU.1−/− mice receiving WT or mSOD1G93A BMT did not reveal any motoneuron loss when compared with either WT or PU.1+/− mice (Fig. 9 G–I). Therefore, mSOD1G93A expression in microglia and other immune cells alone does not induce motoneuron disease.
Characteristics of Disease Progression in SOD1G93A/PU.1−/− Transgenic Mice.
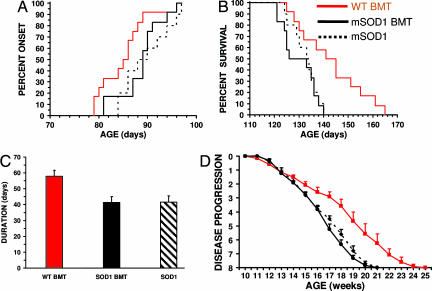
Doubly mSOD1G93A/PU.1−/− transgenic mice were developed and transplanted with bone marrow from WT or control mSOD1G93A donors. Disease progression was evaluated and recorded three times per week by a blinded examiner (Appendix, which is published as supporting information on the PNAS web site). Using previously described symptoms (3, 10), onset of disease was noted after three consecutive evaluations of tremulousness and/or gait abnormalities. mSOD1G93A/PU.1+/− (seven males, five females) had similar onset and survival times as mSOD1G93A mice (four males, six females; onset, P = 0.665; survival, P = 0.907) (Fig. 4A–C). Furthermore, mSOD1G93A/PU.1−/− mice receiving a BMT from mSOD1G93A mice (six males, six females) had onset, survival, and duration times that were not different from mSOD1G93A mice (onset, P = 0.492; survival, P = 0.550; duration, P = 0.994). Therefore, bone marrow from mSOD1G93A mice reconstituted mSOD1G93A/PU.1−/− mice with a disease phenotype identical to that of mSOD1G93A mice. Because onset, survival, and duration times were not different between mSOD1G93A/PU.1−/− mice transplanted with mSOD1G93A bone marrow and mSOD1G93A mice, the daily antibiotic injections given during the first 3 weeks after transplantation did not alter disease progression. Thus, the antibiotic injections and transplantation procedure were not confounding variables in this study.
Fig. 4.
WT BMT prolongs survival and duration in mSOD1G93A/PU.1−/− mice. (A) The onset times of mSOD1G93A mice (n = 10), and mSOD1G93A/PU.1−/− mice transplanted with WT (n = 12), or mSOD1G93A (n = 12), bone marrow were not different. (B) WT bone marrow significantly prolongs survival in mSOD1G93A/PU.1−/− mice. (C) Disease duration was 40% longer in mSOD1G93A/PU.1−/− mice receiving WT BMT. (D) Disease progression was significantly delayed in mSOD1G93A/PU.1−/− mice receiving WT BMT. Significance was evaluated by using a log rank sum test (A and B), Student's t test (C), and ANOVA with repeated measures (D).
The mean survival and duration times were significantly different in mSOD1G93A/PU.1−/− mice transplanted with WT bone marrow (six males, six females) when compared with either mSOD1G93A/PU.1−/− mice transplanted with mSOD1G93A bone marrow (survival, P = 0.011; duration, P = 0.002) or mSOD1G93A mice (survival, P = 0.035; duration, P = 0.0007). The WT BMT mice had disease durations that were 40% longer than those transplanted with mSOD1G93A bone marrow. However, mSOD1G93A/PU.1−/− mice transplanted with WT bone marrow had a mean onset time that was not different from either mSOD1G93A/PU.1−/− mice transplanted with mSOD1G93A bone marrow (P = 0.074) or mSOD1G93A mice (P = 0.066). Additionally, because disease onset in mSOD1G93A/PU.1−/− mice receiving WT BMT was not different from those animals receiving mSOD1G93A bone marrow, disease progression is age-dependent and not influenced by the rate of growth due to the PU.1 mutation.
Disease Progression After BMTs.
Disease progression was not different between mSOD1G93A mice and mSOD1G93A/PU.1−/− mice receiving a BMT from mSOD1G93A donors (P = 0.511; Fig. 4D). However, mSOD1G93A/PU.1−/− mice transplanted with WT bone marrow had a significantly slower disease progression than mSOD1G93A mice, and mSOD1G93A/PU.1−/− mice transplanted with bone marrow from mSOD1G93A (P = 0.00006 and P = 0.0008). Thus, WT bone marrow transplanted into mSOD1G93A/PU.1−/− mice slows disease progression.
Bone Marrow Transplants Slow Motoneuron Loss in the Lumbar Region of SOD1G93A/PU.1−/− Transgenic Mice.
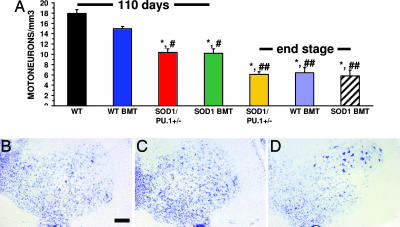
At 110 days of age, mSOD1G93A/PU.1+/− transgenic mice and mSOD1G93A/PU.1−/− transgenic mice receiving mSOD1G93A BMT showed signs of moderate hindlimb weakness. In contrast, mSOD1G93A/PU.1−/− transgenic mice receiving BMT from WT donors had little evidence of weakness at 110 days of age. At this age and at the level of the lumbar enlargement, significant neuronal loss was observed in both mSOD1G93A/PU.1+/− transgenic mice and mSOD1G93A/PU.1−/− transgenic mice receiving mSOD1G93A BMT compared with WT mice (Fig. 5A–D; P = 0.003 and 0.006, respectively). mSOD1G93A/PU.1+/− mice and mSOD1G93A/PU.1−/− mice receiving mSOD1G93A BMT had reduced numbers of motoneurons (41.9% and 43.0%, respectively) compared with WT mice. There was a statistical difference in motoneuron number between mSOD1G93A/PU.1−/− transgenic mice receiving WT BMT when compared with either mSOD1G93A/PU.1+/− transgenic mice (P = 0.006) or with mSOD1G93A/PU.1−/− transgenic mice receiving mSOD1G93A BMT (P = 0.021). However, at 110 days of age, mSOD1G93A/PU.1−/− transgenic mice receiving WT BMT had lost only 16.2% of their motoneurons, which was not significantly different from WT mice (P = 0.16).
Fig. 5.
Increased motoneuron numbers after WT BMT. (A) Motoneuron counting. n = 3 for each group; ∗, statistically different from WT mice; #, statistically different from mSOD1G93A/PU.1−/− mice with WT BMT at 110 days of age; ##, statistically different from either mSOD1G93A/PU.1+/− mice or mSOD1G93A/PU.1−/− mice with mSOD1G93A BMT at 110 days of age. (B–D) Cresyl violet staining of a spinal cord section from a 110-day-old mSOD1G93A/PU.1+/− mouse (B), a 110-day-old mSOD1G93A/PU.1−/− mouse with mSOD1G93A BMT (C), and a 110-day-old mSOD1G93A/PU.1−/− mouse with WT BMT (D). Error bars indicate standard error of the mean. Significance was evaluated by using a one-way ANOVA (A). (Scale bar, 100 μm in B–D.)
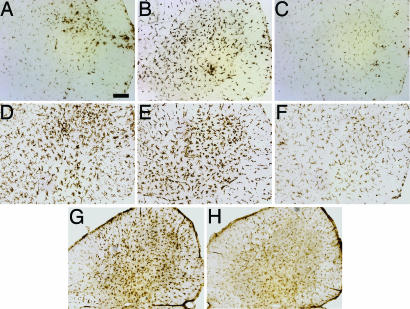
At 110 days of age, lumbar spinal cord sections from mSOD1G93A/PU.1−/− mice transplanted with WT BMT contained less CD68 immunoreactivity, a myeloid-specific LAMP protein expressed on phagocytic cells, than sections from mice transplanted with mSOD1G93A BMT (Fig. 6A–C). Immunostaining for CD11b documented similar differences as those observed with CD68 (Fig. 6 D–F). These data also correlated well with CD40 signal, a marker of mature dendritic cells and activated macrophages; there was less CD40 signal in mSOD1G93A/PU.1−/− mice with WT BMT than in mice receiving mSOD1G93A BMT (Fig. 6 G and H).
Fig. 6.
Characterization of microglia in vivo. (A–H) Immunohistochemical staining of lumbar spinal cord from 110-day-old mice. CD68 signal from microglia/macrophages of mSOD1G93A/PU.1+/− (A), and mSOD1G93A/PU.1−/− with either mSOD1G93A BMT (B) or WT BMT (C) mice. CD11b signal from microglia/macrophages of mSOD1G93A/PU.1+/− (D), and mSOD1G93A/PU.1−/− with either mSOD1G93A BMT (E) or WT BMT (F) mice. CD40 signal from mSOD1G93A/PU.1−/− with either mSOD1G93A (G) or WT BMT (H). (Scale bar, 100 μm.)
Increased Toxicity from mSOD1G93A Microglia.
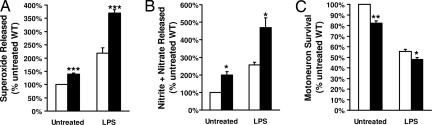
In vitro, WT microglia released less neurotoxins than mSOD1G93A microglia. Untreated primary cultures of mSOD1G93A microglia released more superoxide (40.0%) and nitrite+nitrate (99.6%) than untreated primary cultures of WT microglia (P = 0.002 and 0.025, respectively), demonstrating that mSOD1G93A microglia are more activated than WT microglia in culture (Fig. 7A and B). The level of nitrite+nitrate was determined as an indicator of nitric oxide (NO) production. Forty-eight hours after treatment with 1 μg/ml lipopolysaccharide (LPS), mSOD1G93A microglia produced 68.8% more superoxide and 82.8% more nitrite+nitrate than similarly treated WT microglia (P = 0.001 and 0.035, respectively). This finding demonstrates that mSOD1G93A microglia are more activatable than WT microglia.
Fig. 7.
Characterization of microglia in vitro. Open bar, WT microglia; filled bar, mSOD1G93A microglia. Superoxide (A) or nitrite+nitrate (B) released in cultures of primary microglia before and after treatment with LPS. (C) Survival of motoneuron when cocultured with microglia before and after treatment with LPS. Significance was evaluated by using an ANOVA. Error bars indicate standard error of the mean. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
One possible pathway for activated microglia to initiate motoneuron injury involves the production of superoxide and NO, which then combine to form the highly neurotoxic compound peroxynitrite (11). When cocultured with primary motoneurons, untreated WT microglia, by producing less of these neurotoxins, induced 21.8% less neuronal death than untreated mSOD1G93A microglia (P = 0.01; Fig. 7C). Although LPS-treated WT microglia did induce neuronal death when cocultured with motoneurons, LPS-treated WT microglia induced significantly less neuronal death than LPS treated mSOD1G93A microglia (15.6%; P = 0.024). Thus, in this in vitro model, mSOD1G93A microglia are not only more activated and activatable as demonstrated by the increased production and release of superoxide and nitrite+nitrate, they are also capable of inducing more neuronal death.
Discussion
The present study attempted to determine the effect of mSOD1G93A expression in microglia in the pathogenesis of motoneuron injury in a mouse model of mSOD1G93A mediated familial ALS. mSOD1 expressed primarily in astrocytes was previously established not to induce the development of motoneuron disease in mice (12). Furthermore, mSOD1 expression in neurons alone either does not result in motoneuron disease (13, 14) or dramatically delays disease onset,‖ suggesting that other cells expressing mSOD1, such as microglia, contribute to the disease process. Indeed, studies of chimeric mice carrying a mixture of WT and mSOD1-expressing cells in the spinal cord suggest that expression of mSOD1 in glia contributes to motoneuron injury (6). However, the identities of the specific glia involved with injuring motoneurons were not characterized. The major benefit of the PU.1−/− model is that it enables us to express mSOD1G93A in microglia as well as other myeloid and lymphoid cells. With this model, we demonstrated that microglia in the spinal cords of BMT PU.1−/− mice are donor-derived and develop into morphologically mature microglia (8). More importantly, when PU.1−/− mice were transplanted with bone marrow from mSOD1G93A transgenic mice, no clinical or pathological evidence of disease was noted in these animals. Thus, the expression of mSOD1G93A in microglia is not capable of inducing motoneuron disease.
The fact that the expression of mSOD1 in astrocytes or microglia alone does not give rise to disease suggests that the presence of mSOD1 in motoneurons and surrounding glia may be required to mediate injury. To determine the effects of mSOD1G93A expression in microglia in vivo when mSOD1G93A is also expressed in astrocytes and motoneurons, PU.1−/− mice were bred with mSOD1G93A transgenic mice. When the resulting mSOD1G93A/PU.1−/− mice received a BMT from mSOD1G93A mice, disease onset, duration, and survival were identical to mSOD1G93A transgenic mice, demonstrating that disease progression was not influenced by the transplantation procedure or the daily injections of Baytril. Disease progression was also documented to be age-dependent and not influenced by rates of initial growth.
To determine whether WT donor cells, cells not expressing mSOD1G93A, alter disease progression, mSOD1G93A/PU.1−/− mice received BMT from genotypically identical WT mice. After transplantation into mSOD1G93A/PU.1−/− mice, WT donor cells significantly slowed motoneuron loss, prolonged disease duration, and survival. Thus, after transplantation of mSOD1G93A/PU.1−/− mice with WT donor cells, these cells developed into central nervous system (CNS) microglia and promoted neuroprotection.
The mechanism of the neuroprotective benefits of WT microglia is unclear. A recent study demonstrated that microglia activated by LPS or ALS IgG immune complexes can induce WT motoneuron injury by a glutamate- and calcium-mediated mechanism (11). Another report also suggests that LPS-treated primary cultured adult WT microglia were less activated, as demonstrated by significantly less TNF-α release, when compared with cultured mSOD1G93A microglia (15). Similarly, Lee et al. (16) reported that WT microglia were less sensitive to LPS treatment than microglia derived from mutant presenilin mice; microglia expressing mutant presenilin exhibited a heightened sensitivity to LPS, as demonstrated by superinduction of inducible nitric oxide synthase and activation of mitogen-activated protein kinase. In addition, a recent study using cultured embryonic motoneurons from SOD1G93A, SOD1G37R, and SOD1G85R transgenic mice found that these motoneurons had a 10- to 100-fold increased sensitivity to extracellular agonists of the Fas receptor or to NO compared with WT mouse motoneurons (17).
To investigate possible neuroprotective mechanism of WT microglia, WT and mSOD1G93A-expressing microglia were compared in vitro. The present study using cultured primary microglia demonstrated that WT microglia were less activated and activatable than microglia expressing mSOD1G93A. Furthermore, activated WT-derived microglia secreted significantly less NO as well as superoxide anion, and induced less motoneuron injury, compared with mSOD1G93A expressing microglia. Additionally, WT microglia secrete relatively more insulin-like growth factor-1 (IGF-1) than mSOD1G93A microglia (unpublished data), thereby possibly providing for a more neuroprotective environment. These data are in accord with the study demonstrating that IGF-1 is neuroprotective when delivered intramuscular via injections with an AAV vector (18). Thus, the in vivo benefit observed after WT BMT of mSOD1G93A/PU.1−/− mice could possibly be related to the decreased secretion of free radicals and increased secretion of IGF-1 from WT microglia. These in vitro and in vivo data suggest that parenchymal WT microglia may contribute significantly to protection in which mSOD1G93A is expressed in motoneurons.
Although the differences in disease onsets between groups were not statistically significant, because of the slightly earlier onset in SOD1G93A/PU.1−/− mice receiving WT BMT, there exists the possibility that WT BMT may be neurotoxic earlier in the disease process. However, this cannot be the case because the number of motoneurons at 110 days of age was not different from the number of motoneurons in WT mice. Additionally, as illustrated by the disease progression graph (Fig. 4B), disease onsets were similar between groups. Furthermore, this graph also demonstrates that the progression is slower in the SOD1G93A/PU.1−/− mice receiving BMT from WT donors compared with both SOD1G93A mice and SOD1G93A/PU.1−/− mice receiving SOD1G93A BMT. Boillee et al. (19) used transgenic mice with a different SOD1 mutation, mSOD1G37R, and a different technique to reduce the expression of mSOD1G37R (i.e., the Cre-Lox system), to reach a similar conclusion, namely that the reduction of mSOD1 in microglia prolongs disease duration and survival. Furthermore, they suggest that the onset of disease may be more related to the expression of mSOD1 in motoneurons, whereas, in accord with our data, duration of disease may be related to expression of mSOD1 in microglia.
Because BMT colonizes the peripheral immune system of mSOD1G93A/PU.1−/− transgenic mice, the neuroprotection observed after WT BMT could be partially conferred by peripheral immune cells and their interaction with parenchymal microglia. The present study suggests that WT parenchymal microglia could contribute significantly to motoneuron protection mSOD1G93A/PU.1−/− mice in which mSOD1G93A is expressed in motoneurons. Two recently published studies of γ-irradiated mSOD1 mice provide conflicting data as to whether BMT can alter disease course in mSOD1 transgenic mice. The first study reported an alteration of disease onset with minimal changes in disease duration after WT BMT (20). On the other hand, the second study reported that BMT with WT GFP+ cells did not affect the rate of disease progression in mSOD1 transgenic mice despite an increase in GFP+ cells in spinal cord with less than 20% also expressing F4/80, an immunohistological epitope expressed on microglia (21). Our own experiments are in accord with the report of Solomon et al. (21) with no change in disease onset, survival, or duration after GFP+ BMT of γ-irradiated mSOD1G93A transgenic mice (unpublished data). The difference between the two conflicting BMT studies of mSOD1 mice and the current BMT experiments using mSOD1G93A/PU.1−/− mice is that we were able to replace all parenchymal microglia with WT cells. Such data suggest that the presence of WT microglia throughout the parenchyma may be required for the observed neuroprotective effects. However, what is unclear is whether the presence of WT microglia in only the spinal cord parenchyma is sufficient to mediate such neuroprotective effects or that peripheral immune cells may contribute to the observed neuroprotection. Studies from optic or facial nerve injury models do suggest that peripheral immune cells and as well as central microglia may be involved in neuroprotection (22, 23). The potential modulation of CNS innate immune microglia by peripheral immune cells clearly requires detailed examination. The data presented in the current study support immunomodulatory therapies directed at minimizing cytotoxicity and maximizing neuroprotection in neurodegenerative diseases.
Materials and Methods
PU.1 and SOD1G93A Mice.
All transgenic animals were bred and maintained in our animal facility. Genomic tail DNA was isolated by using a standard protocol (24). Within 12 h of birth, PU.1 litters were injected with antibiotic (Baytril, Bayer HealthCare, Shawnee Mission, KS) and each pup was uniquely identified. The presence or absence of the PU.1 gene was then determined by PCR using tail DNA (9). More detailed methods can be found in Supporting Text, which is published as supporting information on the PNAS web site. All mice were housed in microisolator cages within a modified pathogen-free barrier facility, and had access to food and water ad libitum. All animal protocols were approved by the Methodist Research Institute's Institutional Animal Care and Research Advisory Committee in compliance with National Institutes of Health guidelines.
BMT.
The donor bone marrow was obtained from WT and mSOD1G93A mice or from mice overexpressing GFP (The Jackson Laboratory, Bar Harbor, MA, stock no. 003291), 6–12 weeks old and transplanted into the PU.1−/− or the mSOD1G93A/PU.1−/− pups within 24 h of birth, as described (25, 26). More detailed methods can be found in Supporting Text.
Dual Immunofluorescence and Immunohistochemistry.
For immunohistochemistry, free-floating sections were incubated with rat anti-mouse Ab CD68 (1:2,000), F4/80 (1:2,000), CD40 (1:1,000), or CD11b (1:500) (Serotec, Raleigh, NC). Sections were incubated with biotinylated goat anti-rat IgG (1:200, Vector Laboratories, Burlingame, CA), further incubated with biotin-avidin complex conjugated to HRP (Vector Laboratories), and visualized with the Immunopure Metal enhanced DAB substrate kit (Pierce, Rockford, IL). More detailed methods can be found in Supporting Text.
Stereological Analysis of Motoneurons Number in Lumbar Spinal Cords.
Stereological counting of motoneurons number in lumbar spinal cord sections was performed as described. More detailed methods can be found in Supporting Text.
Primary Microglia and Motoneuron Cultures.
Primary microglial cultures were prepared from 8- to 9-day-old mice and treated with LPS as described (28). Greater than 95% of the floating cells were microglia as determined by OX42 (Chemicon, Temecula, CA) immunocytochemical staining. Microglia monocultures were plated at 30,000 cells per well. Primary motor neuron cultures were prepared from embryonic day 13–14 rat spinal cords by metrizamide gradient centrifugation as described (11, 29, 30). Microglia (10,000 cells per well) were added to motoneurons (10,000 cells per well) 1 day after motoneuron plating. Microglia were activated with 1 μg/ml LPS. Forty-eight hours after treatment, motoneuron were fixed, stained and counted as described (11). More detailed methods can be found in Supporting Text.
Supplementary Material
Acknowledgments
We thank E. Simpson for expert advice and A. Huang and S. Wen for technical assistance. This work was supported by grants from the National Institutes of Health, the Muscular Dystrophy Association, the Curtis and Doris K. Hankamer Foundation, and The Houston Endowment.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- BMT
bone marrow transplant.
Footnotes
The authors declare no conflict of interest.
Joarsma, D., Teuling, E., Hoogenraad, C. C. (2006) Soc. Neurosci. Abstr. 36, abstr. 508.1 (abstr.).
References
- 1.Cleveland DW, Rothstein JD. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 4.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 5.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, et al. Proc Natl Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 7.Alexianu ME, Kozovska M, Appel SH. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 8.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 9.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, et al. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 10.Weydt P, Hong SY, Kliot M, Moller T. NeuroReport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Xie W, Le W, Beers DR, He Y, Henkel JS, Simpson EP, Yen AA, Xiao Q, Appel SH. J Neuropathol Exp Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]
- 12.Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. J Neurosci. 2000;20:660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lino MM, Schneider C, Caroni P. J Neurosci. 2002;22:4825–4832. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weydt P, Yuen EC, Ransom BR, Moller T. Glia. 2004;48:179–182. doi: 10.1002/glia.20062. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Chan SL, Mattson MP. Neuromol Med. 2002;2:29–45. doi: 10.1385/NMM:2:1:29. [DOI] [PubMed] [Google Scholar]
- 17.Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 19.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 20.Corti S, Locatelli F, Donadoni C, Guglieri M, Papadimitriou D, Strazzer S, Del Bo R, Comi GP. Brain. 2004;127:2518–2532. doi: 10.1093/brain/awh273. [DOI] [PubMed] [Google Scholar]
- 21.Solomon JN, Lewis CA, Ajami B, Corbel SY, Rossi FM, Krieger C. Glia. 2006;7:744–753. doi: 10.1002/glia.20331. [DOI] [PubMed] [Google Scholar]
- 22.Shaked I, Porat Z, Gersner R, Kipnis J, Schwartz M. J Neuroimmunol. 2004;146:84–93. doi: 10.1016/j.jneuroim.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beers DR, Ho BK, Siklos L, Alexianu ME, Mosier DR, Mohamed AH, Osaka Y, Kozovska ME, McAlhany RE, Smith RG, et al. J Neurochem. 2001;79:499–509. doi: 10.1046/j.1471-4159.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 25.Belmont JW, Henkel-Tigges J, Chang SM, Wager-Smith K, Kellems RE, Dick JE, Magli MC, Phillips RA, Bernstein A, Caskey CT. Nature. 1986;322:385–387. doi: 10.1038/322385a0. [DOI] [PubMed] [Google Scholar]
- 26.Belmont JW, Henkel-Tigges JS, Wager-Smith K, Chang SMW, Caskey CT. Ann Clin Res. 1986;18:322–326. [PubMed] [Google Scholar]
- 27.Gundersen HJ, Jensen EB. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 28.Le W, Rowe D, Ortiz I, He Y, Appel SH. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson CE, Bloch-Gallego E, Camu W. In: Neural Cell Culture: A Practical Approach. Cohen J, Wilkin G, editors. Oxford: IRL Press; 1995. [Google Scholar]
- 30.Vandenberghe W, Van Den Bosch L, Robberecht W. NeuroReport. 1998;9:2791–2796. doi: 10.1097/00001756-199808240-00020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.