Abstract
The molecular pathology of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas has not been well characterized, and there are no reliable markers to predict the presence of an associated invasive carcinoma in IPMNs. Using oligonucleotide microarrays, we performed a large-scale gene expression profiling of 12 IPMNs with or without an associated invasive carcinoma. A subset of genes identified was validated for the gene expression patterns in a large panel of IPMNs by reverse-transcription polymerase chain reaction and/or immunohistochemistry. A total of 673 transcripts were identified as expressed at significantly higher levels (P < 0.05 and at fivefold or greater) in IPMNs relative to normal pancreatic ductal epithelial samples. Of interest, many of the genes identified as overexpressed in IPMNs have also been previously reported to be highly expressed in infiltrating ductal adenocarcinoma of the pancreas. By analyzing genes overexpressed selectively in IPMNs with an associated invasive carcinoma (n = 7), we also identified a panel of genes potentially associated with the invasive phenotype of the neoplasms. Immunohistochemical validation revealed that claudin 4, CXCR4, S100A4, and mesothelin were expressed at significantly high frequency in invasive IPMNs than in noninvasive IPMNs. Notably, the expression of at least two of the four proteins was observed in 73% of 22 invasive IPMNs but in none of 16 noninvasive IPMNs (P < 0.0001). Our findings suggest that preoperative assessment of gene expression profiles may be able to differentiate invasive from noninvasive IPMNs.
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas was originally identified as a distinct pancreatic neoplasm with a characteristic endoscopic finding of extrusion of mucin through the ampulla of Vater.1 Since the recognition of this entity by the World Health Organization in 1996, IPMNs have been identified with increased frequency and the unique clinical, radiological, and pathological features of IPMNs have been defined.2–5 IPMNs are characterized as having grossly identifiable proliferations of mucin-producing neoplastic epithelium within dilated pancreatic ducts and ductules.6 The intraductal components of IPMNs display a broad spectrum of dysplasia ranging from adenoma to borderline to carcinoma in situ, and ∼30% of IPMNs are associated with an infiltrating adenocarcinoma.3,6–8
Although the overall prognosis for patients with IPMNs is better than it is for patients with conventional infiltrating ductal adenocarcinoma of the pancreas, a subset of IPMNs recur, and some patients die of their disease even after surgical resection.2,3,8–12 Among several prognostic factors reported for patients with IPMNs, the presence of an associated invasive carcinoma has been considered the best predictor for a poor clinical outcome.9,11,13–15 In a recent retrospective study with a large series of IPMNs, the 5-year survival for patients with invasive carcinoma was 36% compared with 84.5% for patients without invasive carcinoma.8 However, there are no reliable imaging techniques or molecular markers to predict the presence of such an invasive component in IPMNs.
In contrast to the progress made in our understanding of the molecular genetics of conventional pancreatic ductal adenocarcinoma,16 less is known about the molecular events associated with IPMNs. Genetic alterations in IPMNs have been reported to involve activating point mutations in the K-ras oncogene,17,18 overexpression of the HER-2/neu (c-erbB2) gene product,17,19 loss of heterozygosity at several chromosomal loci,20 and inactivating mutations in the p5317 and STK11/LKB1 tumor-suppressor genes.21 Biallelic inactivation of the p16 and Smad4/DPC4 genes is relatively uncommon in IPMNs and is primarily confined to those neoplasms with high-grade dysplasia or invasive carcinoma.22,23 Recently, we and other investigators have demonstrated that aberrant CpG island hypermethylation of tumor-suppressor genes is a frequent event in IPMNs.24,25
The emergence of microarray technology has enabled us to analyze global gene expression patterns in neoplasms by determining the expression of a large number of transcripts simultaneously. Many investigators have applied microarrays to analyze gene expression profiles in invasive pancreatic adenocarcinoma.26–29 By contrast, only one report has described gene expression patterns in IPMNs investigated using cDNA microarrays.30 Characterization of genes differentially expressed in IPMNs may provide significant insights into the molecular basis of this distinct type of neoplasm. Additionally, the discovery of molecular markers that reliably predict the behavior of IPMNs would be of immediate benefit in the clinical setting. We therefore performed a large-scale gene expression profiling in IPMNs using oligonucleotide microarrays (Affymetrix GeneChip) containing more than 13,000 full-length genes.
Materials and Methods
Tissue Samples and Cells
Twelve fresh-frozen tissues of IPMNs collected from patients undergoing pancreatic resection at the Johns Hopkins Hospital were selected for the present study based on the availability of sufficient quantities of neoplastic cells. For each case, hematoxylin and eosin (H&E)-stained slides were carefully reviewed and the diagnosis of IPMN was confirmed according to recently established criteria.6 Normal pancreatic duct epithelial cells were selectively microdissected from frozen sections of two resected pancreata using a laser-capture microdissection system (PixCell II; Arcturus, Mountain View, CA). A nonneoplastic cell line established from normal human pancreatic ductal epithelium (HPDE) was kindly provided by Dr. Ming-Sound Tsao (University of Toronto, Toronto, Ontario, Canada). Tissue microarrays (TMAs) of IPMNs were constructed from a total of 38 formalin-fixed, paraffin-embedded blocks of IPMNs using a manual tissue puncher/arrayer (Beecher Instruments, Silver Spring, MD). For each case, two to eight cores punched from the representative areas of IPMNs were arrayed on the TMA blocks. This study was performed with approval of the Johns Hopkins Medical Institutions Joint Committee for Clinical Investigation.
RNA Extraction and Preparation for Array Hybridization
All frozen sections of IPMNs were evaluated with H&E staining and trimmed to enrich the population of neoplastic cells. In IPMNs with an associated infiltrating adenocarcinoma, the intraductal component was selectively dissected for analysis so that we were able to obtain a high neoplastic cellularity of ∼80 to 90%. Total RNA was isolated from homogenized frozen IPMNs using Trizol reagent (Invitrogen, Carlsbad, CA), and was purified using the RNeasy mini kit (Qiagen, Valencia, CA). Total RNA was extracted from two frozen samples of microdissected normal ductal epithelial cells using the Picopure RNA isolation kit (Arcturus) according to the manufacturer’s instructions, and was subjected to two rounds of linear amplification using the RiboAmp RNA amplification kit (Arcturus).
Oligonucleotide Array Hybridization
First- and second-stranded cDNA was synthesized from 10 μg of total RNA using T7-(dT)24 primer (Genset Corp., South La Jolla, CA) and SuperScript Choice system (Invitrogen). Labeled cRNA was synthesized from the purified cDNA by in vitro transcription reaction using the BioArray HighYield RNA transcript labeling kit (Enzo Diagnostics, Inc., Farmingdale, NY) at 37°C for 6 hours. The cRNA was fragmented at 94°C for 35 minutes in a fragmentation buffer (40 mmol/L Tris-acetate, pH 8.1, 100 mmol/L potassium acetate, 30 mmol/L magnesium acetate). One of the fragmented cRNA samples was run on the Test3 chip (Affymetrix, Santa Clara, CA) to ensure the quality of target samples. The fragmented cRNA samples were then hybridized to the human genome U133A chips (Affymetrix) at 45°C for 16 hours. The washing and staining procedure was performed in the Affymetrix Fluidics Station according to the manufacturer’s instructions. The probes were then scanned using a laser scanner, and signal intensity for each transcript (background-subtracted and adjusted for noise) and detection call (present, absent, or marginal) were determined using Microarray Suite software 5.0 (Affymetrix).
Analysis of Microarray Data
Hierarchical cluster analysis was performed using dChip (DNA-chip analyzer) software (www.dChip.org) after filtering genes with the greatest variation across all samples (SD/mean >2). The analysis of genes differentially expressed between IPMNs and control (normal pancreatic duct epithelial) samples was performed with fold-change analysis and t-test using the Data Mining Tool software (Affymetrix). Expression data for all probe sets was filtered out to identify transcripts expressed at significantly higher levels (P < 0.05 by t-test and at least fivefold greater) in IPMNs compared to control samples. We also compared gene expression profiles between IPMNs with an associated invasive carcinoma (n = 7) and IPMNs without an associated invasive carcinoma (n = 5) and identified transcripts that were significantly (P < 0.05 by t-test and at least threefold greater) overexpressed selectively in IPMNs with an associated invasive carcinoma relative to control samples. Although it has been demonstrated that expression changes more than twofold are significant in Affymetrix microarrays,31 we used a more stringent cutoff to reduce the number of false-positives.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
We performed RT-PCR to confirm the expression patterns of selected genes in 12 IPMNs (all of which were subjected to array analysis) and in the nonneoplastic HPDE cells. Four μg of total RNA was reverse-transcribed using Superscript II (Invitrogen). PCR reaction was performed as follows: 95°C for 5 minutes; then 35 cycles of 95°C for 20 seconds, 60°C for 20 seconds, and 72°C for 20 seconds; and a final extension of 4 minutes at 72°C. Primer sequences are available on request. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also amplified in the same PCR reaction to ensure the cDNA integrity.
Immunohistochemistry
Immunohistochemical analysis was performed to validate the differential expression of selected genes on archival IPMN tissue sections or TMAs containing 38 IPMNs. Five-μm sections were cut onto coated slides and deparaffinized by routine techniques. Antigen retrieval was performed in 10 mmol/L of sodium citrate buffer (pH 6.0) heated at 95°C in a steamer for 20 minutes. After blocking endogenous peroxidase activity with a 3% aqueous H2O2 solution for 5 minutes, the sections were incubated with primary antibodies for 30 to 60 minutes. The antibodies used and their dilutions were as follows: S100A4 (1:500; DAKO, Carpinteria, CA), prostate stem cell antigen (PSCA) (1:200, clone 1G8; kindly provided from Dr. Robert E. Reiter, Dept. of Urology, Univ. of California), decay accelerating factor for complement (1:50, CD55, H-319; Santa Cruz Biotechnology, Santa Cruz, CA), TIMP1 (1:20, H-150; Santa Cruz), mesothelin (1:20, clone 5B2; Novocastra Laboratories, Newcastle, UK), CXCR4 (1:50, clone 12G5; Zymed Laboratories, South San Francisco, CA), and claudin 4 (1:400, clone 3E2C1; Zymed Laboratories). Labeling was detected with the Envision Plus Detection Kit (DAKO) following the protocol as suggested by the manufacturer, and all sections were counterstained with hematoxylin. Slides or individual cores on TMAs were scored as either positive (positive staining in >10% of neoplastic cells) or negative. To validate TMA, cases were considered positive if at least one tissue core showed positive immunostaining and negative if none of the tissue cores showed positive labeling.
Statistical Analysis
Statistical analysis was performed using Fisher’s exact probability test or Mann-Whitney U nonparametric test. Differences were considered significant at P < 0.05.
Results
Clinicopathological Characteristics of IPMN Patients
The 12 IPMN cases analyzed for gene expression profiling included six men and six women with a mean age of 72 years (range, 60 to 79 years). These neoplasms arose from the head of the pancreas in nine (75%) cases, body of the pancreas in two cases, and diffusely involved the entire gland in one case. The maximum diameter of the neoplasms ranged from 2.5 to 9.0 cm (mean, 5.1 cm). The intraductal components were classified as carcinoma in situ in all IPMNs, and seven (58%) of the IPMNs were associated with an infiltrating ductal (tubular) adenocarcinoma. Lymph node metastases were identified in five (71%) of the seven invasive IPMNs, and metastatic disease to the liver was observed in one invasive IPMN at the time of surgery.
Identification of Genes Overexpressed in IPMNs
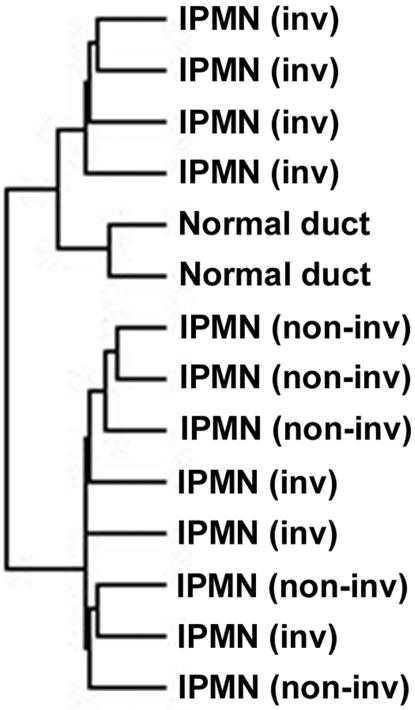
Using high-density oligonucleotide microarrays (Affymetrix human genome U133A chips) with 18,462 gene/expressed sequence tag transcripts, we performed a gene expression profiling in 12 IPMNs and 2 microdissected histologically normal pancreatic duct epithelial samples. For IPMN samples, the intraductal component was selectively dissected for microarray analysis and was estimated to achieve an average neoplastic cellularity of ∼80 to 90% (Figure 1). Analysis of the gene expression profiles of 12 IPMNs and the 2 normal-appearing pancreatic duct samples identified 369 transcripts that had the greatest variation in transcription among the 14 samples (SD/mean >2). Hierarchical cluster analysis of these 369 transcripts identified two major clusters: the first containing four IPMNs and two normal ductal epithelial samples, and the second containing eight IPMNs (Figure 2). The two normal ductal epithelial samples were clustered in the closest branches in the dendrogram. Four of the seven IPMNs with an associated invasive carcinoma were clustered in the first major branch with remarkable similarity, whereas all of the five IPMNs without an invasive carcinoma were clustered together in the second major cluster, suggesting different gene expression patterns between these two groups.
Figure 1.
An example of the intraductal component of IPMN frozen sections dissected for microarray analysis showing a high neoplastic cellularity (stained with hematoxylin).
Figure 2.
Hierarchical cluster analysis of 12 IPMNs with or without an associated invasive carcinoma and 2 normal pancreatic ductal epithelial samples.
The Affymetrix data mining tool was then used to identify transcripts expressed at significantly higher levels (P < 0.05 by t-test and at least fivefold greater) in IPMNs compared to normal ductal epithelial samples. Furthermore, we eliminated transcripts whose expression call was absent in more than 6 of 12 IPMNs. Using these stringent criteria, a total of 673 transcripts were identified as significantly overexpressed in IPMNs relative to nonneoplastic pancreatic ductal epithelium [Table 1, and full list of transcripts is available at our website (http://www.pathology2.jhu.edu/pancreas/IPMN)]. We also identified 72 transcripts that were significantly (P < 0.05 and at least fivefold) underexpressed in IPMNs compared to control samples, including known genes such as P-glycoprotein (mdr1) (available at http://www.pathology2.jhu.edu/pancreas/IPMN). The large panel of overexpressed transcripts included several genes whose expression pattern in IPMNs has been confirmed in previous publications, such as those encoding for mucins (MUC1 and MUC5AC).32–35 On the other hand, the majority of the genes identified here have not been previously implicated in IPMNs. Functional classification of these genes revealed several groups associated with important biological processes, including immune response (immunoglobulin genes, interferon-related genes, anti-hepatitis A IgG, and others), calcium homeostasis (S100P, S100A4, S100A6, S100A11, annexin 14, and CACNL1A1), cytoskeletal organization (profilin 1, tropomyosin 2, and plectin 1), cell adhesion (E-cadherin and protocadherin1), cell motility (dynamin 2 and hyaluronan-mediated motility receptor), cell-cycle checkpoint (stratifin and GADD34), cell proliferation (melanoma inhibitory activity and insulin-like growth factor binding protein 2), cell death (death-associated protein (DAP) and cell death-regulatory protein GRIM19), signal transudation (tetraspanin TM4-C, TRAF5, and signal sequence receptor, delta), and extracellular matrix remodeling [TIMP1 and urokinase-type plasminogen activator receptor (uPAR)]. Notably, some of the most highly expressed genes in IPMNs (MUC5AC, pepsinogen C, claudin-18, and cathepsin E) encode proteins known to be primarily expressed in normal mucosa of the stomach, suggesting gastric-type differentiation during the evolution of IPMNs.
Table 1.
Highly Expressed Genes in IPMNs as Compared with Normal Pancreatic Ductal Epithelium*
| Fold change | P value | GenBank | Gene name (symbol) |
|---|---|---|---|
| 378.82 | 0.000 | NM_005980 | S100 calcium-binding protein P (S100P) |
| 168.59 | 0.000 | NM_002654 | Pyruvate kinase, muscle (PKM2) |
| 149.71 | 0.004 | AW192795 | MUC5AC |
| 140.93 | 0.001 | BC005332 | Similar to immunoglobulin kappa constant |
| 118.13 | 0.000 | NM_005022 | Profilin 1 (PFN1) |
| 106.82 | 0.026 | S55735 | Immunoglobulin A1-A2 lambda hybrid GAU heavy chain |
| 99.54 | 0.014 | NM_005823 | Mesothelin (MSLN) |
| 72.14 | 0.003 | NM_002639 | Serine (or cysteine) proteinase inhibitor, clade B (ovalbumin), member 5 (SERPINB5) |
| 71.14 | 0.000 | NM_004480 | Fucosyltransferase 8 (alpha (1,6) fucosyltransferase) (FUT8) |
| 66.83 | 0.000 | NM_031286 | SH3BGRL3-like protein (SH3BGRL3) |
| 65.48 | 0.000 | NM_007263 | Coatomer protein complex, subunit epsilon (COPE) |
| 62.38 | 0.002 | AF133425 | Tetraspanin TM4-C |
| 53.21 | 0.003 | M63438 | Ig rearranged gamma chain |
| 51.09 | 0.002 | BC000329 | Stratifin |
| 50.67 | 0.022 | M87789 | Anti-hepatitis A IgG variable region, constant region, complementarity-determining regions |
| 48.81 | 0.038 | NM_002961 | S100 calcium-binding protein A4 (S100A4) |
| 48.77 | 0.035 | NM_002630 | Progastricsin (pepsinogen C) (PGC) |
| 47.80 | 0.021 | NM_006533 | Melanoma inhibitory activity (MIA) |
| 46.77 | 0.005 | NM_000597 | Insulin-like growth factor binding protein 2 (36kD) (IGFBP2) |
| 44.86 | 0.002 | NM_002862 | Phosphorylase, glycogen; brain (PYGB) |
| 43.36 | 0.000 | NM_000975 | Ribosomal protein L11 (RPL11) |
| 42.47 | 0.000 | NM_000099 | Cystatin C (amyloid angiopathy and cerebral hemorrhage) (CST3) |
| 40.60 | 0.000 | NM_021029 | Ribosomal protein L44 (RPL44) |
| 39.29 | 0.002 | NM_016286 | Carbonyl reductase (LOC51181) |
| 38.46 | 0.000 | AF231056 | BRG1-Associated Factor 250a (BAF250a) |
| 37.85 | 0.022 | NM_016369 | Claudin 18 (CLDN18) |
| 36.13 | 0.001 | NM_001970 | Eukaryotic translation initiation factor 5A (EIF5A) |
| 35.44 | 0.000 | NM_002743 | Protein kinase C substrate 80K-H (PRKCSH) |
| 33.80 | 0.012 | NM_024727 | Hypothetical protein FLJ23259 (FLJ23259) |
| 33.17 | 0.000 | NM_014501 | Ubiquitin carrier protein (E2-EPF) |
| 32.70 | 0.001 | M60333 | MHC class II HLA-DRA |
| 32.32 | 0.001 | NM_003289 | Tropomyosin 2 (beta) (TPM2) |
| 32.14 | 0.039 | NM_005672 | Prostate stem cell antigen (PSCA) |
| 31.94 | 0.019 | X57812 | Rearranged immunoglobulin lambda light chain |
| 31.45 | 0.018 | NM_000295 | Serine (or cysteine) proteinase inhibitor, clade A, member 1 (SERPINA1) |
| 29.93 | 0.027 | NM_002084 | Glutathione peroxidase 3 (plasma) (GPX3) |
| 29.64 | 0.004 | BC004242 | Similar to E1B-55kDa-associated protein 5, clone MGC:10422 |
| 28.85 | 0.000 | U63131 | CDC37 homolog |
| 28.23 | 0.000 | NM_006815 | Coated vesicle membrane protein (RNP24) |
| 28.14 | 0.013 | BC001288 | Similar to decay accelerating factor for complement (CD55, Cromer blood group system) |
| 27.73 | 0.000 | NM_006713 | Activated RNA polymerase II transcription cofactor 4 (PC4) |
| 25.91 | 0.000 | NM_014047 | HSPC023 protein (HSPC023) |
| 25.70 | 0.003 | X75208 | HEK2 mRNA for protein tyrosine kinase receptor |
| 25.52 | 0.000 | NM_004082 | Dynactin 1 (p150, Glued (Drosophila) homolog) (DCTN1) |
| 24.56 | 0.006 | NM_004235 | Kruppel-like factor 4 (gut) (KLF4) |
| 24.25 | 0.000 | NM_005184 | Calmodulin 3 (phosphorylase kinase, delta) (CALM3) |
| 23.74 | 0.001 | NM_000574 | Decay accelerating factor for complement (CD55, Cromer blood group system) (DAF) |
| 23.13 | 0.000 | M68956 | Myristoylated alanine-rich C-kinase substrate |
| 22.58 | 0.000 | NM_018133 | Hypothetical protein FLJ10546 (FLJ10546) |
| 22.27 | 0.001 | NM_004669 | Chloride intracellular channel 3 (CLIC3) |
| 22.20 | 0.000 | NM_025076 | Hypothetical protein FLJ23591 (FLJ23591) |
| 21.49 | 0.000 | NM_000980 | Ribosomal protein L18a (RPL18A) |
| 21.47 | 0.029 | NM_005518 | 3-Hydroxy-3-methylglutaryl-Coenzyme A synthase 2 (mitochondrial) (HMGCS2) |
| 21.42 | 0.019 | NM_002345 | Lumican (LUM) |
| 21.04 | 0.011 | NM_000120 | Epoxide hydrolase 1, microsomal (xenobiotic) (EPHX1) |
| 20.97 | 0.000 | NM_000992 | Ribosomal protein L29 (RPL29) |
| 20.63 | 0.000 | NM_002229 | Jun B proto-oncogene (JUNB) |
| 20.09 | 0.000 | NM_021242 | Hypothetical protein STRAIT11499 (STRAIT11499) |
| 20.07 | 0.000 | NM_007085 | Follistatin-like 1 (FSTL1) |
| 19.90 | 0.005 | NM_004363 | Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) |
| 19.77 | 0.000 | M32221 | Saposin proteins A-D |
| 19.66 | 0.001 | NM_002149 | Hippocalcin-like 1 (HPCAL1) |
| 19.40 | 0.000 | NM_001311 | Cysteine-rich protein 1 (intestinal) (CRIP1) |
| 19.29 | 0.000 | NM_002721 | Protein phosphatase 6, catalytic subunit (PPP6C) |
| 19.14 | 0.000 | BC002356 | Nucleobindin 1 |
| 19.02 | 0.000 | NM_014371 | Neighbor of A-kinase anchoring protein 95 (NAKAP95) |
| 18.87 | 0.000 | NM_005770 | Small EDRK-rich factor 2 (SERF2) |
| 18.54 | 0.000 | NM_000101 | Cytochrome b-245, alpha polypeptide (CYBA) |
| 18.52 | 0.000 | NM_002949 | Mitochondrial ribosomal protein L12 (MRPL12) |
Table 1.
Continued
| 18.47 | 0.000 | NM_005620 | S100 calcium-binding protein A11 (calgizzarin) (S100A11) |
|---|---|---|---|
| 18.29 | 0.000 | NM_013366 | Anaphase-promoting complex subunit 2 (APC2) |
| 18.27 | 0.002 | U82164 | Transmembrane protein CD99 type II |
| 18.20 | 0.000 | Z54367 | Plectin 1, intermediate filament binding protein, 500kD |
| 18.15 | 0.003 | NM_002346 | Lymphocyte antigen 6 complex, locus E (LY6E) |
| 18.10 | 0.000 | AF067173 | Mago homolog |
| 18.02 | 0.000 | NM_003564 | Transgelin 2 (TAGLN2) |
| 17.87 | 0.002 | D89324 | Alpha (1,31,4) fucosyltransferase |
| 17.71 | 0.000 | NM_014624 | S100 calcium-binding protein A6 (calcyclin) (S100A6) |
| 17.43 | 0.001 | NM_005762 | KRAB-associated protein 1 (TIF1B) |
| 17.29 | 0.037 | NM_001216 | Carbonic anhydrase IX (CA9) |
| 17.13 | 0.001 | NM_001540 | Heat shock 27kD protein 1 (HSPB1) |
| 17.06 | 0.003 | NM_001423 | Epithelial membrane protein 1 (EMP1) |
| 16.97 | 0.000 | NM_001355 | D-dopachrome tautomerase (DDT) |
| 16.93 | 0.000 | NM_007121 | Nuclear receptor subfamily 1, group H, member 2 (NR1H2) |
| 16.91 | 0.001 | U38654 | RAB27A, member RAS oncogene family |
| 16.79 | 0.000 | NM_015392 | Neural proliferation, differentiation and control, 1 (NPDC1) |
| 16.75 | 0.000 | NM_000281 | 6-pyruvoyl-tetrahydropterin synthasedimerization cofactor of hepatocyte nuclear factor 1 alpha (TCF1) |
| 16.68 | 0.001 | NM_007061 | Serum constituent protein (MSE55) |
| 16.58 | 0.000 | NM_022744 | Hypothetical protein FLJ13868 (FLJ13868) |
| 16.44 | 0.006 | NM_019093 | UDP glycosyltransferase 1 family, polypeptide A3 (UGT1A3) |
| 15.80 | 0.000 | BC002906 | Similar to uridine monophosphate kinase, clone MGC:10318 |
| 15.78 | 0.002 | NM_012101 | Ataxia-telangiectasia group D-associated protein (ATDC) |
| 15.63 | 0.000 | NM_016275 | Selenoprotein T (LOC51714) |
| 15.62 | 0.000 | NM_018227 | Hypothetical protein FLJ10808 (FLJ10808) |
| 15.61 | 0.011 | NM_005451 | Enigma (LIM domain protein) (ENIGMA) |
| 15.40 | 0.011 | NM_015869 | Peroxisome proliferative activated receptor, gamma (PPARG) |
| 15.26 | 0.000 | NM_030571 | Hypothetical protein MGC10924 similar to Nedd4 WW-binding protein 5 (MGC10924) |
| 15.09 | 0.000 | NM_020145 | SH3-containing protein SH3GLB2 (LOC56904) |
| 15.08 | 0.001 | AB011110 | KIAA0538 protein |
| 14.89 | 0.001 | NM_001500 | GDP-mannose 4,6-dehydratase (GMDS) |
This table includes a partial list of genes (the highest ranked 100 known genes) from a total of 673 transcripts overexpressed in IPMNs as compared with normal ductal epithelial samples.
Of interest, many of the genes identified by our approach have also been implicated in infiltrating ductal adenocarcinoma of the pancreas. For example, among the most highly expressed 100 known genes identified in IPMNs, at least 21 genes have been previously reported to be highly expressed in pancreatic ductal adenocarcinoma. These genes included S100P,27,36 pyruvate kinase, muscle (PKM2),36 mesothelin,37,38 SERPINB5,36 stratifin,29,36 S100A4,38,39 ubiquitin carrier protein (E2-EPF),36 tropomyosin 2 (TPM2),36 prostate stem cell antigen (PSCA),40 coated vesicle membrane protein,38 decay accelerating factor for complement (CD55),29,36 CEACAM5,36 S100A11,28 S100A6,36 and cysteine-rich protein 1.38 Importantly, aberrant expression of many of these genes has been also associated with the malignant phenotype in a variety of nonpancreatic neoplasms, suggesting that these genes may play central roles in the development and progression of IPMNs.
Confirmation of Expression Patterns of Selected Genes by RT-PCR and Immunohistochemistry
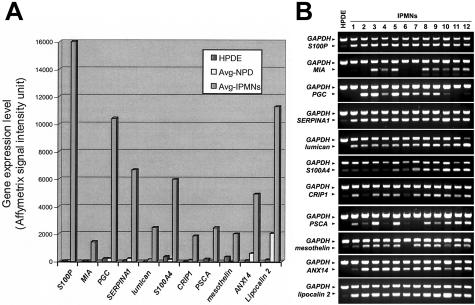
Thirteen genes were chosen from the list of overexpressed transcripts for further confirmation of their expression patterns. We first performed RT-PCR to examine the mRNA expression of 11 genes in 12 IPMNs (all of them were analyzed by microarray). The 11 genes examined were S100P, melanoma inhibitory activity (MIA), pepsinogen C (PGC), SERPINA1, lumican, S100A4, cysteine-rich protein 1 (CRIP1), PSCA, mesothelin, annexin 14 (ANX14), and lipocalin 2. As a control, we used the nonneoplastic pancreatic ductal epithelial cell line (HPDE), which demonstrated the gene expression patterns similar to those of microdissected normal ductal epithelium by microarray analysis (Figure 3A). All 11 genes were confirmed by RT-PCR to be strongly expressed at mRNA levels in most of the 12 IPMNs in contrast to weak or absent expression in HPDE (Figure 3B). Of note, the RT-PCR analyses on these genes paralleled the results from microarrays. For example, RT-PCR demonstrated abundant mRNA expression of MIA in eight IPMNs whose expression of the corresponding gene was called present by microarray analysis. By contrast, no detectable MIA transcript was found in the remaining four IPMNs and all four of these had an absent call for this gene in the gene expression microarrays.
Figure 3.
A: Microarray expression levels of 11 genes selected for RT-PCR validation in the nonneoplastic ductal cell line (HPDE), microdissected normal ductal epithelium (Avg-NPD), and IPMNs (Avg-IPMNs). B: RT-PCR analysis of 11 selected genes in HPDE and 12 IPMNs. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serves as a RNA control.
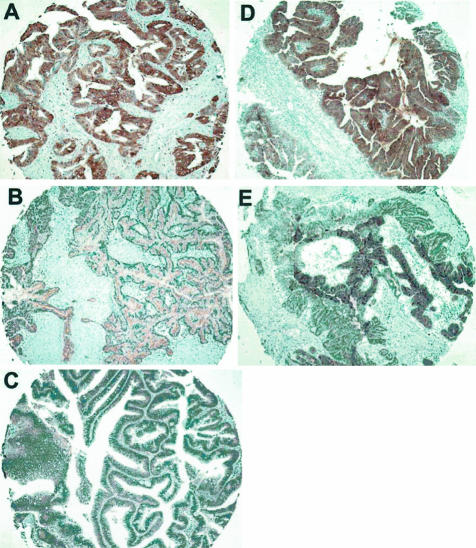
We next verified the protein expression of five selected genes by immunohistochemistry on TMAs representing 38 IPMNs and on five archival tissue sections containing normal pancreatic ducts. The proteins tested included S100A4, decay accelerating factor for complement (CD55), TIMP1, mesothelin, and PSCA. Representative results are shown in Figure 4. For each of these proteins, labeling of normal ductal epithelium was weak or minimal. By contrast, moderate to strong labeling was identified in the neoplastic epithelium of IPMNs at varying frequencies. There was no stromal labeling for any of these genes. To confirm the accuracy of the TMA immunohistochemistry, we labeled full tissue sections of five IPMNs and confirmed that the cores adequately represented the labeling pattern of each neoplasm. Overall, positive immunolabeling was identified in 22% of all of the IPMNs tested for S100A4, 49% for CD55, 70% for TIMP1, 25% for mesothelin, and 39% for PSCA. When the labeling pattern of the noninvasive component of the IPMNs was compared between IPMNs with an associated with invasive carcinoma (n = 22) and IPMNs not associated with an invasive carcinoma (n = 16), the expression of S100A4 and mesothelin was found to be more frequency detected in IPMNs with an associated invasive carcinoma than in noninvasive IPMNs (S100A4: 38% versus 0%, P = 0.006; mesothelin: 43% versus 0%; P = 0.005, Fisher’s exact probability test). There were no significant differences in the labeling frequencies of other proteins between invasive and noninvasive IPMNs.
Figure 4.
Immunohistochemical validation of five overexpressed genes on IPMN tissue microarrays. Shown are representative tissue cores stained positive for S100A4 (A), CD55 (B), TIMP1 (C), PSCA (D), and mesothelin (E).
Identification of Genes Associated with the Invasive Phenotype of IPMNs
Immunohistochemical analysis suggests that mesothelin and S100A4 expression appeared to be restricted to IPMNs with an associated invasive carcinoma. To identify additional genes whose expression was primarily restricted to IPMNs with an associated invasive carcinoma, we first compared the expression profiles between seven IPMNs with an associated invasive carcinoma and two control samples. This analysis identified 1755 transcripts significantly (P < 0.05 by t-test and at least threefold greater) overexpressed in IPMNs with an associated carcinoma relative to nonneoplastic pancreatic duct epithelium. From the 1755 transcripts identified, 1408 transcripts that were also expressed at high levels (threefold or greater) in IPMNs without an associated invasive carcinoma were excluded, leaving 347 transcripts that were preferentially overexpressed in IPMNs with an associated invasive carcinoma. We also excluded transcripts whose expression call was absent in four or more of seven IPMNs with an associated invasive carcinoma. Consequently, we identified 187 transcripts that were overexpressed in IPMNs with an associated invasive carcinoma but not in IPMNs without an associated invasive carcinoma (Table 2, and full list of transcripts is available at http://www.pathology2.jhu.edu/pancreas/IPMN). In support for our approach, this panel of transcripts included several genes previously implicated in tumor invasion, metastasis, and/or angiogenesis such as chemokine receptor 4 (CXCR4, fusin),41 cyclin D2,42 extracellular matrix protein 1 (ECM1),43 alpha2 integrin,44 lamin B1,45 and S100A4.46,47
Table 2.
Representative Subset of Genes Overexpressed Selectively in Invasive IPMNs
| Fold change | P value | GenBank | Gene name (symbol) |
|---|---|---|---|
| 3.14 | 0.016 | NM_001675 | Activating transcription factor 4 (tax-responsive enhancer element B67) (ATF4) |
| 3.04 | 0.001 | NM_006367 | Adenylyl cyclase-associated protein (CAP) |
| 3.54 | 0.001 | NM_004045 | ATX1 (antioxidant protein 1, yeast) homolog 1 (ATOX1) |
| 3.24 | 0.015 | NM_001207 | Basic transcription factor 3 (BTF3) |
| 4.05 | 0.001 | NM_001211 | Budding uninhibited by benzimidazoles 1 (yeast homolog), beta (BUB1B) |
| 3.54 | 0.024 | NM_001904 | Catenin (cadherin-associated protein), beta 1 (88kD) (CTNNB1) |
| 3.39 | 0.032 | AJ224869 | Chemokine (C-X-C motif), receptor 4 (fusin, CXCR4) |
| 4.48 | 0.015 | NM_001274 | CHK1 (checkpoint, S.pombe) homolog (CHEK1) |
| 5.09 | 0.017 | NM_001305 | Claudin 4 (CLDN4) |
| 3.37 | 0.017 | NM_001992 | Coagulation factor II (thrombin) receptor (F2R) |
| 3.09 | 0.023 | NM_004701 | Cyclin B2 (CCNB2) |
| 3.70 | 0.046 | NM_001759 | Cyclin D2 (CCND2) |
| 4.33 | 0.043 | NM_004354 | Cyclin G2 (CCNG2) |
| 3.88 | 0.028 | NM_013989 | Deiodinase, iodothyronine, type II (DIO2) |
| 5.66 | 0.017 | NM_001343 | Disabled (Drosophila) homolog 2 (mitogen-responsive phosphoprotein) (DAB2) |
| 3.00 | 0.012 | AF147209 | Double-stranded RNA-binding nuclear protein DRBP76 |
| 3.60 | 0.001 | NM_006442 | DR1-associated protein 1 (negative cofactor 2 alpha) (DRAP1) |
| 3.19 | 0.000 | NM_001428 | Enolase 1, (alpha) (ENO1) |
| 3.80 | 0.023 | NM_004454 | Ets variant gene 5 (ets-related molecule) (ETV5) |
| 4.77 | 0.006 | U65932 | Extracellular matrix protein 1 (ECM1) |
| 3.94 | 0.016 | AF207990 | Fer-1 like protein 3 (FER1L3) |
| 3.11 | 0.000 | BC000717 | GAP-associated tyrosine phosphoprotein p62 (Sam68) |
| 7.41 | 0.004 | NM_014330 | Growth arrest and DNA-damage-inducible 34 (GADD34) |
| 3.20 | 0.022 | NM_002105 | H2A histone family, member X (H2AFX) |
| 5.27 | 0.014 | NM_006644 | Heat shock 105kD (HSP105B) |
| 5.49 | 0.019 | NM_006145 | Heat shock 40kD protein 1 (HSPF1) |
| 3.24 | 0.003 | NM_014707 | Histone deacetylase 9 (HDAC9) |
| 5.03 | 0.046 | NM_017409 | Homeo box C10 (HOXC10) |
| 3.02 | 0.006 | NM_004907 | Immediate early protein (ETR101) |
| 3.09 | 0.016 | NM_002166 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein (ID2) |
| 9.60 | 0.032 | NM_001552 | Insulin-like growth factor-binding protein 4 (IGFBP4) |
| 3.52 | 0.006 | NM_002203 | Integrin, alpha 2 (CD49B, alpha 2 subunit of VLA-2 receptor) (ITGA2) |
| 3.20 | 0.017 | NM_006435 | Interferon induced transmembrane protein 2 (1-8D) (IFITM2) |
| 3.66 | 0.003 | NM_005531 | Interferon, gamma-inducible protein 16 (IFI16) |
| 3.01 | 0.002 | NM_006084 | Interferon-stimulated transcription factor 3, gamma (48kD) (ISGF3G) |
| 6.64 | 0.003 | NM_005573 | Lamin B1 (LMNB1) |
| 3.11 | 0.019 | U16797 | LERK-5 (EPLG5) |
| 5.36 | 0.031 | NM_002288 | Leukocyte-associated Ig-like receptor 2 (LAIR2) |
| 3.12 | 0.001 | AB018009 | L-type amino acid transporter 1 |
| 3.13 | 0.038 | NM_021960 | Myeloid cell leukemia sequence 1 (BCL2-related) (MCL1) |
| 3.02 | 0.017 | U26162 | Myosin regulatory light chain |
| 3.73 | 0.012 | AB042278 | Nucleophosmin/B23 |
| 3.89 | 0.002 | NM_012250 | Oncogene TC21 (TC21) |
| 3.42 | 0.010 | NM_002646 | Phosphoinositide-3-kinase, class 2, beta polypeptide (PIK3C2B) |
| 4.15 | 0.015 | U16996 | Protein tyrosine phosphatase |
| 4.38 | 0.040 | AF152318 | Protocadherin gamma A1 (PCDH-gamma-A1) |
| 3.44 | 0.045 | NM_015714 | Putative lymphocyte G0G1 switch gene (G0S2) |
| 3.95 | 0.007 | AF098158 | Restricted expressed proliferation associated protein 100 |
| 80.71 | 0.036 | NM_002961 | S100 calcium-binding protein A4 (S100A4) |
| 3.02 | 0.001 | NM_016542 | Serinethreonine protein kinase MASK (LOC51765) |
| 3.92 | 0.001 | NM_006516 | Solute carrier family 2 (facilitated glucose transporter), member 1 (SLC2A1) |
| 5.62 | 0.031 | NM_005842 | Sprouty (Drosophila) homolog 2 (SPRY2) |
| 3.37 | 0.009 | AB032261 | Stearoyl-CoA desaturase |
| 3.33 | 0.011 | AJ270770 | T-cell transcription factor-4 (TCF-4) |
| 4.79 | 0.040 | BC002827 | Tropomyosin 4 |
| 4.18 | 0.023 | NM_000043 | Tumor necrosis factor receptor superfamily, member 6 (TNFRSF6) |
| 3.21 | 0.019 | NM_006291 | Tumor necrosis factor, alpha-induced protein 2 (TNFAIP2) |
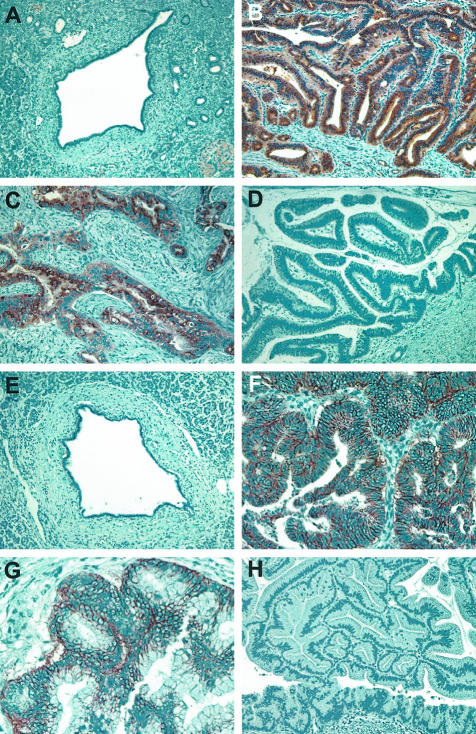
Among the genes identified as overexpressed in IPMNs with an associated invasive carcinoma, the chemokine receptor CXCR4 has recently attracted considerable interest for its potential role in cell motility, adhesion, invasion, and metastasis of various cancer types.48–50 We therefore determined the expression pattern of CXCR4 in an expanded series of 51 IPMNs (27 IPMNs with an associated invasive carcinoma and 24 IPMNs without an associated invasive carcinoma) by immunohistochemical labeling with an anti-CXCR4 monoclonal antibody. Expression of CXCR4 was not identified in normal ductal epithelium, but was localized to neoplastic epithelial cells in 16 (31%) of 51 IPMNs (Figure 5). CXCR4 expression was detected at higher frequency in IPMNs with an associated invasive carcinoma than in IPMNs without an associated invasive carcinoma (44% versus 17%, P = 0.04, Fisher’s exact probability test).
Figure 5.
Immunohistochemical validation of two selected genes (CXCR4 and claudin 4) associated with the invasive phenotype of IPMNs. CXCR4 is not expressed in normal ductal epithelium (A). Strong immunolabeling of CXCR4 is detected in two cases of invasive IPMNs (B and C), but not in an IPMN associated with colloid carcinoma (D). Claudin 4 is not expressed in normal ductal epithelium (E). Diffuse labeling for claudin 4 is shown in the neoplastic epithelium of invasive IPMNs (F and G), in contrast to negative staining in noninvasive IPMN (H). Note the discrete membrane immunoreactivity of claudin 4 in the neoplastic cells demonstrated by a high-power view (G).
We then determined the expression pattern of claudin 4, which was identified as overexpressed in IPMNs with an associated invasive carcinoma, in 51 IPMNs by immunohistochemistry. This gene encodes for a transmembrane protein known to function as a high-affinity receptor for Clostridium perfringens enterotoxin (CPE),51 and claudin 4 has been shown to be overexpressed in invasive adenocarcinoma of the pancreas,27,29,38,52 suggesting a role for this gene in the invasive phenotype during pancreatic neoplastic progression. Normal duct epithelium did not label for claudin 4, whereas membranous immunolabeling was detectable in neoplastic epithelium in 34 (67%) of 51 IPMNs (Figure 5). Remarkably, positive claudin 4 immunostaining was identified in 100% (27 of 27) of IPMNs with an associated invasive carcinoma but only in 29% (7 of 24) of IPMNs without an associated invasive carcinoma (P < 0.0001), supporting our microarray data.
Protein Expression Profiling of IPMNs
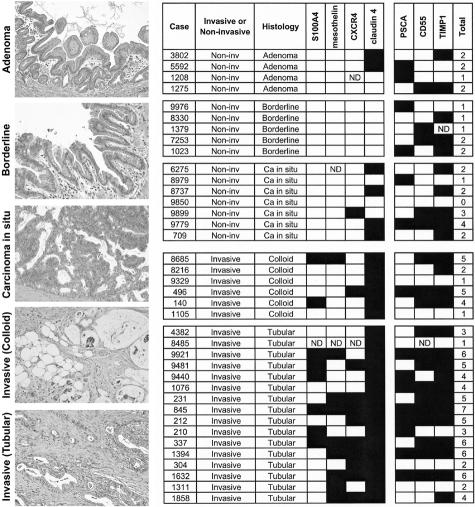
Finally, we investigated by immunohistochemical analyses the protein expression of seven genes (S100A4, CD55, TIMP1, mesothelin, PSCA, claudin 4, and CXCR4) overexpressed in IPMNs using a panel of 38 IPMNs arrayed on TMAs. These IPMNs were classified into adenoma, borderline, carcinoma in situ, and IPMNs with an associated invasive carcinoma according to the established criteria (Figure 6).6 Among the IPMNs without an associated invasive carcinoma, there was no significant difference observed in the protein expression patterns of these genes between adenoma (n = 4), borderline (n = 5), and carcinoma in situ IPMNs (n = 7). We also classified the invasive component of IPMNs with an associated invasive carcinoma into colloid-type carcinoma (n = 6) or tubular-type carcinoma (n = 16) to determine whether different gene expression patterns existed in these subtypes of invasive carcinoma associated with IPMNs. The expression of mesothelin and CXCR4 was more frequently detected in the noninvasive components of IPMNs with an associated tubular carcinoma than in those with an associated colloid carcinoma, although the difference did not reach statistical significance. Overall, the average number of overexpressed genes was significantly higher in IPMNs with an associated invasive carcinoma than in IPMNs without an associated invasive carcinoma (4.0 versus 1.8; Mann-Whitney U nonparametric test, P = 0.0005). Notably, the expression of at least two of the four proteins (S100A4, mesothelin, claudin 4, and CXCR4) was commonly observed in IPMNs with an associated invasive carcinoma but was not seen in IPMNs without an associated invasive carcinoma; 16 (73%) of 22 invasive IPMNs expressed two or more of these four proteins, compared to none of 16 noninvasive IPMNs (P < 0.0001) (Figure 6). These results suggest that the assessment of gene expression profiles of IPMNs could potentially be used in the preoperative setting to help identify IPMNs with an associated invasive carcinoma.
Figure 6.
Examples for histological classification of IPMNs (left, H&E staining) and expression profiles of a panel of 38 IPMNs on TMAs determined by immunohistochemical analyses of all of the seven genes tested (right). Filled box and open box indicate positive and negative immunolabeling, respectively. ND, not determined.
Discussion
In the present study, we have characterized a global gene expression profile of IPMNs using a high-throughput approach based on Affymetrix oligonucleotide microarrays featuring more than 18,000 unique transcripts. Although a gene expression study of IPMNs has been recently reported using a cDNA array of ∼5000 genes,30 the oligonucleotide microarray platform enabled us to identify a large number of previously unreported genes overexpressed in IPMNs. To identify overexpressed genes, we compared gene expression profiles from IPMNs with those of nonneoplastic pancreatic ductal epithelial cells selectively microdissected by laser-capture microdissection and only defined overexpression if there was a mean fivefold difference in expression levels between groups and if this difference was statistically significant. Because the intraductal components of IPMNs dissected for our microarray analysis were highly enriched (∼80% to 90%) for neoplastic epithelium, its with microdissected pancreatic ductal cells minimized the detection of “falsely elevated” genes that commonly arise when one compares tissue types with different proportions of epithelial and other cell types. Using RT-PCR and immunohistochemistry, we were able to confirm the expression patterns of all of the 13 selected genes identified as overexpressed by gene expression microarray analysis. Notably, many of the overexpressed genes identified by our analyses participate in important biological processes and have been previously implicated in a variety of other tumor types, suggesting that many of these genes contribute to the neoplastic evolution, malignant transformation, and invasive progression of IPMNs.
IPMNs are usually slow-growing neoplasms and generally less aggressive than conventional ductal adenocarcinoma of the pancreas, leading to the hypothesis that the molecular alterations of IPMNs differ from those of infiltrating ductal adenocarcinoma.3 In support for this view, previous studies have shown that inactivation of several tumor-suppressor genes such as p53 and DPC4/Smad4 occur less frequently in IPMNs than in infiltrating pancreatic ductal adenocarcinoma.17,22 By contrast, a recent immunohistochemical study demonstrated that inactivation of STK11/LKB1 was more common in IPMNs than in pancreatic ductal adenocarcinoma.53 Therefore, it is possible that IPMNs and pancreatic adenocarcinoma may differ in their expression profiles particularly those genes downstream of pancreatic cancer tumor suppressor genes. Currently, however, little is known about the difference in gene expression profiles between IPMNs and infiltrating pancreatic ductal adenocarcinoma because of the lack of information on gene expression patterns in IPMNs. In the present study, we find that many of the genes identified as overexpressed in IPMNs have also been previously reported to be highly expressed in invasive pancreatic ductal adenocarcinoma. This raises the possibility that these genes are common targets involved in the neoplastic progression of pancreatic ductal system and that IPMN and pancreatic adenocarcinoma share some gene expression pathways. Although IPMNs and pancreatic intraepithelial neoplasias (PanINs) share similar histological features and both are recognized as precursors to invasive adenocarcinoma,7,54 accumulating evidence suggests that IPMNs and PanINs represent two distinct types of intraductal neoplasia. For example, IPMNs and PanINs display different staining patterns for DPC4/Smad422,55 and MUC2,33,35,56 suggesting a fundamental difference in the molecular pathways leading to these intraductal neoplasias of the pancreas.
We also found that some of the most highly expressed genes in IPMNs are primarily expressed in normal mucosa of the stomach, including MUC5AC, pepsinogen C, claudin-18, and cathepsin E. The concomitant overexpression of multiple gastric-related genes suggests differentiation into a gastric-type epithelial phenotype during the neoplastic evolution of IPMNs. Importantly, expression of these gastric-type markers has been linked to a better prognosis in pancreatic and other neoplasms. For example, it has been shown that patients with pancreatic neoplasms expressing MUC5AC mRNA have a significantly better prognosis than those with no MUC5AC mRNA expression.32 Furthermore, pepsinogen C is known to be a strong predictor for favorable outcome in patients with various cancers, including breast,57 gastric,58 and pancreatic cancer.59 Based on these findings, it is possible that consistent overexpression of these gastric-type proteins in IPMNs is related to their indolent biological behavior and relatively favorable outcome.
Among the genes identified as overexpressed in IPMNs, genes encoding secreted proteins may represent promising candidates to be exploited for diagnosis and/or follow-up of patients with IPMNs. In support for this hypothesis, we have recently demonstrated that HIP/PAP-I, a secreted protein overexpressed in the acini adjacent to the invasive pancreatic ductal adenocarcinoma, can be detected at high levels in pancreatic juice and serum samples from patients with pancreatic cancer.60 In our present analysis, we found the overexpression of several genes encoding secreted proteins [such as melanoma inhibitory activity (MIA), lumican, and putative secreted protein XAG] in IPMNs. Among them, MIA has been recently shown to be a potential serological marker for metastatic or recurrent melanoma.61,62 Further studies are required to evaluate the diagnostic value of these secreted proteins in IPMNs.
Widespread use of screening ultrasound and computed tomography has led to the identification of increasing numbers of patients with asymptomatic cystic lesions of the pancreas, including IPMNs.63 Accurate preoperative assessment of the grade of malignancy in IPMNs is critical to determine the optimal management for these patients and to avoid unnecessary radical pancreatic resection in those patients with low-grade IPMNs (adenoma) especially for patients with concomitant disease for whom surveillance would be the preferred approach to management. At present, however, neither imaging nor laboratory markers can adequately differentiate between benign and malignant IPMNs or detect an invasive focus preoperatively. In the present study, we attempted to identify genes associated with the invasive phenotype of IPMNs. To eliminate the contamination of the nonneoplastic tissues (especially of abundant desmoplastic stroma observed in the invasive component associated with IPMNs), we analyzed the intraductal components for gene expression profiling. Therefore, it is likely that some genes that are expressed specifically in the invasive components of IPMNs will be missed using this strategy. We hypothesized that the gene expression profile of the intraductal (noninvasive) components of IPMNs may reflect the invasive potential of these neoplasms. This hypothesis is supported by recent reports that metastatic potential of breast and other solid tumors could be reliably predicted from the gene expression profile of the primary tumor.45,64 Indeed, we were able to identify a large panel of genes overexpressed selectively in the intraductal components of IPMNs with an associated invasive carcinoma, and many of these genes are functionally important for tumor invasion and metastasis, including S100A4, CXCR4, and claudin 4. For example, inhibition of S100A4 expression in osteosarcoma cells with a hammerhead ribozyme results in decreased invasive properties in vitro,46 whereas transfection of S100A4 produces metastatic variants of an orthotopic model of bladder cancer.47 Interaction between the chemokine receptor CXCR4 and its ligand CXCL12/SDF-1 has been shown to play a critical role in invasion and metastasis of different types of cancers including breast,48 prostate,49 and small cell lung cancer.50 Of particular interest, claudin 4 has been identified as a high-affinity epithelial receptor for CPE.51 Recently, it has been shown that claudin 4 is overexpressed in pancreatic ductal adenocarcinoma and treatment with CPE results in an acute cytotoxic effect specifically in claudin 4-expressing pancreatic cancer cells.52 Although the therapeutic significance of claudin 4 has not been determined, our findings suggest that claudin 4 and other invasion-associated genes identified could be molecular targets for therapy as well as for diagnosis in patients with IPMNs with an associated invasive carcinoma.
In summary, we have identified a large set of overexpressed genes that shed light on the molecular basis underlying the pathogenesis and progression of IPMNs. Further characterization of these genes/expressed sequence tags may refine our understanding of IPMNs and enhance our ability to diagnose and manage these patients appropriately.
Note Added in Proof
We have recently added three normal ductal epithelial samples and reanalyzed the gene expression data (available at http://www.pathology2.jhu.edu/pancreas/IPMN).
Footnotes
Address reprint requests to Michael Goggins, M.D., Departments of Pathology, Medicine, and Oncology, the Johns Hopkins Medical Institutions, 632 Ross Bldg., 720 Rutland Ave., Baltimore, MD 21205-2196. E-mail: mgoggins@jhmi.edu.
Supported by the Specialized Program of Research Excellence (SPORE) in Gastrointestinal Malignancies (grant CA62924), the Michael Rolfe Foundation, and a gift to support pancreatic cancer research from Susan Gurney.
References
- Ohhashi K, Murakami F, Maruyama M. Four cases of mucous secreting pancreatic cancer. Prog Dig Endosc. 1982;203:348–351. [Google Scholar]
- Loftus EV, Jr, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, DiMagno EP. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996;110:1909–1918. doi: 10.1053/gast.1996.v110.pm8964418. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas: pathology and molecular genetics. J Gastrointest Surg. 2002;6:656–659. doi: 10.1016/s1091-255x(02)00057-4. [DOI] [PubMed] [Google Scholar]
- Schmitz-Winnenthal FH, Z’Graggen K, Volk C, Schmied BM, Buchler MW. Intraductal papillary mucinous tumors of the pancreas. Curr Gastroenterol Rep. 2003;5:133–140. doi: 10.1007/s11894-003-0082-y. [DOI] [PubMed] [Google Scholar]
- Solcia E, Capella C, Kloppel G. Tumors of the pancreas. Rosai J, Sobin LH, editors. Washington, DC: Armed Forces Institute of Pathology,; Atlas of Tumor Pathology, series 3. 1997:pp 31–144. [Google Scholar]
- Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT, Wiersema MJ, Farnell MB, Sarr MG. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- Azar C, Van de Stadt J, Rickaert F, Deviere M, Baize M, Kloppel G, Gelin M, Cremer M. Intraductal papillary mucinous tumours of the pancreas. Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–464. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nishio K, Nagao M, Ikeda N, Kanokogi H, Yamada T, Nakano H. Pattern of recurrence after resection for intraductal papillary mucinous tumors of the pancreas. World J Surg. 1998;22:874–878. doi: 10.1007/s002689900485. [DOI] [PubMed] [Google Scholar]
- Cuillerier E, Cellier C, Palazzo L, Deviere J, Wind P, Rickaert F, Cugnenc PH, Cremer M, Barbier JP. Outcome after surgical resection of intraductal papillary and mucinous tumors of the pancreas. Am J Gastroenterol. 2000;95:441–445. doi: 10.1111/j.1572-0241.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Mukai K, Sakamoto M, Hasebe T, Shimada K, Kosuge T, Kinoshita T, Hirohashi S. Invasive carcinoma derived from intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic and immunohistochemical study of eight cases. Virchows Arch. 2001;439:6–13. doi: 10.1007/s004280100438. [DOI] [PubMed] [Google Scholar]
- Yamao K, Ohashi K, Nakamura T, Suzuki T, Shimizu Y, Nakamura Y, Horibe Y, Yanagisawa A, Nakao A, Nimuara Y, Naito Y, Hayakawa T. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepato-Gastroenterol. 2000;47:1129–1134. [PubMed] [Google Scholar]
- Gigot JF, Deprez P, Sempoux C, Descamps C, Metairie S, Glineur D, Gianello P. Surgical management of intraductal papillary mucinous tumors of the pancreas: the role of routine frozen section of the surgical margin, intraoperative endoscopic staged biopsies of the Wirsung duct, and pancreaticogastric anastomosis. Arch Surg. 2001;136:1256–1262. doi: 10.1001/archsurg.136.11.1256. [DOI] [PubMed] [Google Scholar]
- Raimondo M, Tachibana I, Urrutia R, Burgart LJ, DiMagno EP. Invasive cancer and survival of intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 2002;97:2553–2558. doi: 10.1111/j.1572-0241.2002.06022.x. [DOI] [PubMed] [Google Scholar]
- Goggins M, Kern SE, Offerhaus JA, Hruban RH. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol. 1999;10:4–8. [PubMed] [Google Scholar]
- Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, Pellegata NS, Ranzani GN, Rickaert F, Kloppel G. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- Z’Graggen K, Rivera JA, Compton CC, Pins M, Werner J, Fernandez-del Castillo C, Rattner DW, Lewandrowski KB, Rustgi AK, Warshaw AL. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491–498. doi: 10.1097/00000658-199710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Sasano H, Shimosegawa T, Koizumi M, Yamazaki T, Mochizuki F, Kobayashi N, Okano T, Toyota T, Sawai T. An immunohistochemical study of the c-erbB-2 oncogene product in intraductal mucin-hypersecreting neoplasms and in ductal cell carcinomas of the pancreas. Cancer. 1993;72:51–56. doi: 10.1002/1097-0142(19930701)72:1<51::aid-cncr2820720112>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fujii H, Inagaki M, Kasai S, Miyokawa N, Tokusashi Y, Gabrielson E, Hruban RH. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151:1447–1454. [PMC free article] [PubMed] [Google Scholar]
- Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–2022. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biankin AV, Biankin SA, Kench JG, Morey AL, Lee CS, Head DR, Eckstein RP, Hugh TB, Henshall SM, Sutherland RL. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861–868. doi: 10.1136/gut.50.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Ueki T, Fukushima N, Iacobuzio-Donahue CA, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Aberrant methylation of CpG islands in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2002;123:365–372. doi: 10.1053/gast.2002.34160. [DOI] [PubMed] [Google Scholar]
- House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- Crnogorac-Jurcevic T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, Jones M, Tyson K, Bassi C, Scarpa A, Lemoine NR. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Maitra A, Shen-Ong GL, van Heek T, Ashfaq R, Meyer R, Walter K, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol. 2002;160:1239–1249. doi: 10.1016/S0002-9440(10)62551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Maitra A, Olsen M, Lowe AW, Van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq R, Jaffee E, Ryu B, Jones J, Eshleman JR, Yeo CJ, Cameron JL, Kern SE, Hruban RH, Brown PO, Goggins M. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol. 2003;162:1151–1162. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745–1754. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodicka L, Dong H, Mittmann M, Ho MH, Lockhart DJ. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nature Biotechnol. 1997;15:1359–1367. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T, Sato E. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54. doi: 10.1046/j.1440-1827.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- Luttges J, Zamboni G, Longnecker D, Kloppel G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–948. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, Porchet N, Couvelard A, Degott C, Flejou JF. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–637. doi: 10.1002/path.1146. [DOI] [PubMed] [Google Scholar]
- Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- Ryu B, Jones J, Blades NJ, Parmigiani G, Hollingsworth MA, Hruban RH, Kern SE. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- Rosty C, Ueki T, Argani P, Jansen M, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 2002;160:45–50. doi: 10.1016/S0002-9440(10)64347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- Liu SC, Bassi DE, Zhang SY, Holoran D, Conti CJ, Klein-Szanto AJ. Overexpression of cyclin D2 is associated with increased in vivo invasiveness of human squamous carcinoma cells. Mol Carcinog. 2002;34:131–139. doi: 10.1002/mc.10057. [DOI] [PubMed] [Google Scholar]
- Han Z, Ni J, Smits P, Underhill CB, Xie B, Chen Y, Liu N, Tylzanowski P, Parmelee D, Feng P, Ding I, Gao F, Gentz R, Huylebroeck D, Merregaert J, Zhang L. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. EMBO J. 2001;15:988–994. doi: 10.1096/fj.99-0934com. [DOI] [PubMed] [Google Scholar]
- Lochter A, Navre M, Werb Z, Bissell MJ. Alpha1 and alpha2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol Biol Cell. 1999;10:271–282. doi: 10.1091/mbc.10.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- Bjornland K, Winberg JO, Odegaard OT, Hovig E, Loennechen T, Aasen AO, Fodstad O, Maelandsmo GM. S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti-S100A4 ribozyme. Cancer Res. 1999;59:4702–4708. [PubMed] [Google Scholar]
- Levett D, Flecknell PA, Rudland PS, Barraclough R, Neal DE, Mellon JK, Davies BR. Transfection of S100A4 produces metastatic variants of an orthotopic model of bladder cancer. Am J Pathol. 2002;160:693–700. doi: 10.1016/S0002-9440(10)64889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304–6311. [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl P, Buchholz M, Rolke M, Kunsch S, Lohr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678–684. doi: 10.1053/gast.2001.27124. [DOI] [PubMed] [Google Scholar]
- Sahin F, Maitra A, Argani P, Sato N, Maehara N, Montgemery E, Goggins M, Hruban RH, Gloria HS. Expression of the tumor suppressor gene STK11/LKB1 in pancreatic and biliary neoplasms. Mod Pathol. 2003;16:686–691. doi: 10.1097/01.MP.0000075645.97329.86. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–2006. [PubMed] [Google Scholar]
- Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, Kim YS. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- Vizoso F, Sanchez LM, Diez-Itza I, Merino AM, Lopez-Otin C. Pepsinogen C is a new prognostic marker in primary breast cancer. J Clin Oncol. 1995;13:54–61. doi: 10.1200/JCO.1995.13.1.54. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Vizoso F, Rodriguez JC, Merino AM, Gonzalez LO, Quintela I, Andicoechea A, Truan N, Diez MC. Expression and prognostic significance of pepsinogen C in gastric carcinoma. Ann Surg Oncol. 2000;7:508–514. doi: 10.1007/s10434-000-0508-9. [DOI] [PubMed] [Google Scholar]
- Truan N, Vizoso F, Fresno MF, Fernandez R, Quintela I, Alexandre E, Martinez A. Expression and clinical significance of pepsinogen C in resectable pancreatic cancer. Int J Biol Markers. 2001;16:31–36. doi: 10.1177/172460080101600104. [DOI] [PubMed] [Google Scholar]
- Rosty C, Christa L, Kuzdzal S, Baldwin WM, Zahurak ML, Carnot F, Chan DW, Canto M, Lillemoe KD, Cameron JL, Yeo CJ, Hruban RH, Goggins M. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 2002;62:1868–1875. [PubMed] [Google Scholar]
- Schaller UC, Bosserhoff AK, Neubauer AS, Buettner R, Kampik A, Mueller AJ. Melanoma inhibitory activity: a novel serum marker for uveal melanoma. Melanoma Res. 2002;12:593–599. doi: 10.1097/01.cmr.0000043146.28051.b8. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Hatta N, Wakamatsu K, Takehara K, Ito S, Takata M. Melanoma inhibitory activity (MIA) as a serum marker for early detection of post-surgical relapse in melanoma patients: comparison with 5-S-cysteinyldopa. Melanoma Res. 2002;12:319–323. doi: 10.1097/00008390-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Castillo CF, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]