Abstract
Dendritic cell-lysosomal associated membrane protein (DC-LAMP)/CD208, a member of the lysosomal associated membrane protein (LAMP) family, is specifically expressed by human DCs on activation. However, its mouse counterpart could not be detected in mature DCs. The present study demonstrates that DC-LAMP is constitutively expressed by mouse, sheep, and human type II pneumocytes. Confocal and immunoelectron microscopy showed that mouse DC-LAMP protein co-localizes with lbm180, a specific marker for the limiting membrane of lamellar bodies that contain surfactant protein B, as well as with intracellular MHC class II molecules that accumulate in the same organelles. Expression of DC-LAMP was also occasionally detected at the cell surface of type II pneumocytes. Interestingly, human bronchioloalveolar carcinoma tumor cells, which correspond to transformed type II pneumocytes, express DC-LAMP. Similar observations were made in the Jaagsiekte sheep retrovirus-associated ovine pulmonary adenocarcinoma, a model of human bronchioloalveolar carcinoma. This study establishes that DC-LAMP is constitutively expressed in normal type II pneumocytes. Furthermore, DC-LAMP appears to be a marker of transformed type II pneumocytes as well, an observation that may help the study and the classification of human lung adenocarcinomas.
The lysosomal associated membrane protein (LAMP) family consists of a group of heavily glycosylated proteins accounting for approximately half of the protein content in the lysosomal membrane. They all contain a conserved intracytoplasmic tyrosine-based lysosome-targeting motif YXXφ (where φ represents a bulky hydrophobic residue).1 Several members of this family (LAMP-1 to LAMP-3 and CD68) were cloned in a broad range of species.1 Although LAMP-1 and LAMP-2 are ubiquitously expressed,2 CD68 is mainly restricted to monocytes and macrophages.3 The latest human LAMP protein identified, DC-LAMP/CD208, was originally described as a molecule specifically expressed in mature dendritic cells (DCs).4 DC-LAMP appears transiently on DC activation at the limiting membrane of the MHC class II-containing intracellular compartments (MIIC)4,5 involved in MHC class II peptide loading and transport to the cell surface.5 On further maturation, MHC class II molecules and DC-LAMP segregate: MHC class II molecules are translocated to the cell surface membrane, whereas DC-LAMP concentrates in perinuclear lysosomes. On the basis of these observations, it was proposed that DC-LAMP could play a role in the sorting of the MIIC membrane-associated molecules and the transfer of MHC class II molecules to the cell surface.4 Human, monkey, and mouse DC-LAMP mRNAs were shown to be expressed in the lung4,6–8 but the cellular source was not determined. Of note, murine DC-LAMP was not detected in mouse DCs.8 In the present study, using specific monoclonal antibodies, we establish that DC-LAMP is specifically expressed by mouse, sheep, and human type II pneumocytes (PnIIs). PnIIs are peripheral pulmonary cells acting as stem cells for repopulation of lung by alveolar type I and type II cells during normal tissue turnover and after injury.9 Beside this progenitor function, PnIIs are also responsible for pulmonary surfactant synthesis, secretion, and recycling.10 Detailed analysis of DC-LAMP expression in PnIIs suggests that this molecule may play a role in the conditioning and/or the secretion of surfactant, and that it represents a promising tool for the study of PnIIs and for the diagnosis of lung adenocarcinomas.
Materials and Methods
Northern Blot
Commercially available mouse tissue mRNA-loaded membrane (MB no. 2020; OriGene Technologies Inc., Rockville, MD) was hybridized with the full-length cDNA of mouse DC-LAMP labeled by random priming with [32P]-dCTP as described elsewhere.11 Scanning was performed using a PhosporImager (Bio-Rad Laboratories Inc., Hercules, CA).
Mice Lung Single-Cell Preparation
Six-week-old specific pathogen-free BALB/c, 129sv, and C57/BL6 female mice were obtained from Charles River (Iffa Credo, L’Arbresle, France). All mice experiments were done following protocols approved by the institutional animal committee. Lung single-cell suspensions were obtained from manually minced organs after 30 minutes of digestion with 1 mg/ml collagenase (Sigma-Aldrich, St. Louis, MO), crushing through a 0.22-μm cell strainer (BD Labware Falcon, Franklin Lakes, NJ) and final incubation in NH4Cl solution (Stem Cell Technologies, Vancouver, Canada). Lung cells were then either analyzed as total lung cells or subjected to subsequent depletion using rat anti-mouse CD45 monoclonal antibody (mAb) (30-F11; BD Pharmingen, San Diego, CA) and goat anti-rat IgG-coated Dynabeads (Dynal, Oslo, Norway) to enrich the preparation for nonhematopoietic cells. Beads and attached cells were removed with a Dynal magnet. CD45-depleted lung cells were then washed in phosphate-buffered saline (PBS)/0.5 mmol/L ethylenediaminetetraacetic acid (Sigma-Aldrich), spun down, and resuspended either in PBS for cytofluorometric analysis or in serum-free Dulbecco’s modified Eagle’s medium-F12 (Invitrogen, Carlsbad, CA) for subsequent confocal and immunoelectron microscopy (IEM) studies.
Human and Sheep Lung Tissue Sections
Human lung tumor biopsies were obtained from the Centre Hospitalier Lyon Sud tissue collection (Lyon, France). Sheep tissues were obtained postmortem from naturally Jaagsiekte sheep retrovirus (JSRV)-infected animals. Presence of typical lesions associated with ovine pulmonary adenocarcinoma was confirmed by histopathological examination.
Antibody Generation
Rat anti-mouse DC-LAMP mAbs were obtained by immunizing rats with COP5 cells transfected with mouse DC-LAMP, cDNA and hybridoma supernatants were screened by immunocytochemistry and flow cytometry on mouse DC-LAMP-transfected cells, as described in detail elsewhere.8
Immunohistological Studies
Balb/C mouse lungs were collected and instilled intratracheally with Cryojung tissue-freezing medium (Leica GmbH, Nussloch, Germany) before snap-freezing in liquid nitrogen. Tissue cryosections (7-μm thick) were fixed in acetone. DC-LAMP single labeling was performed on mouse lung cryosections using standard immunohistochemistry as described elsewhere.8 Double-immunohistofluorescence stainings were performed on cryosections after saturation in PBS/2% bovine serum albumin/10% goat normal serum, using a panel of antibodies [mouse anti-rat lbm180 (3C9; Bender MedSystems Diagnostics GmbH, Vienna, Austria), rat anti-mouse CD44v6 (BMS145, Bender MedSystem), rat anti-mouse I-A/I-E (M5/114, BD Pharmingen) or corresponding isotype-matched control IgG]. Binding was detected with appropriate goat anti-species F(ab)′2 coupled to Alexa 488 (Molecular Probes, Eugene, OR). After incubation in 10% rat serum, rat anti-mouse DC-LAMP antibodies coupled in the laboratory to Alexa 594 or unrelated isotype-matched control IgG were applied. After washes in PBS and water, slides were mounted in Fluoromount (Southern Biotechnology Associates, Birmingham, AL) and analyzed with an Axioskop microscope (Zeiss, Germany).
Bouin-fixed, paraffin-embedded sheep and human lung sections were deparaffinized in toluene for 30 minutes, rehydrated in successive baths of alcohol (100% then 95%) and rinsed in water. Antigen retrieval was performed with microwave (15 minutes at 750 W) in citrate buffer (DAKO, Glostrup, Denmark). Endogenous peroxidases were blocked with 0.3% H2O2. After saturation in protein blocking reagent solution (DAKO), staining was performed with rat anti-mouse DC-LAMP-specific mAb (clone 1010E1, which cross-reacts with sheep and human DC-LAMP proteins) diluted in DAKO diluent (DAKO). Revelation was then performed using the Ultratech HRP and Ultratech DAB kits (Immunotech, Marseille, France) according to manufacturer’s instructions. Slides were counterstained with Harry’s hematoxylin before mounting.
Confocal Laser Microscopy (CLM)
CD45-depleted lung cells were resuspended in serum-free medium and allowed to adhere on 12-mm diameter coverslips (Polylabo, Strasbourg, France) precoated with poly-l-lysine (Sigma-Aldrich). After saturation with 10% fetal calf serum-containing medium at 37°C, cells were fixed in 3.7% paraformaldehyde, washed in PBS/10% fetal calf serum/2% bovine serum albumin, then in PBS, and finally permeabilized with PBS/0.05% saponin (Sigma-Aldrich)/0.2% bovine serum albumin. Staining with rat anti-mouse DC-LAMP mAb was performed for 1 hour at 37°C, and detected with goat anti-rat IgG F(ab)′2 coupled to Alexa 594 (Molecular Probes). After saturation with 10% rat serum (DAKO), co-staining was performed with either fluorescein isothiocyanate-coupled rat anti-mouse I-Ad (2G9, BD Pharmingen), or with mouse anti-rat lbm180 (3C9, Bender MedSystem), the latter being detected with goat anti-mouse IgG F(ab)′2 coupled to Alexa 488 (Molecular Probes). Double staining of MHC class II and lbm180 was performed first with rat anti-mouse I-Ad (2G9, BD Pharmingen) detected with goat anti-rat IgG F(ab)′2 coupled to Alexa 594 (Molecular Probes), and after saturation with rat serum, binding of mouse anti-rat lbm180 (3C9, Bender MedSystem) was achieved and detected with goat anti-mouse IgG F(ab)′2 coupled to Alexa 488 (Molecular Probes). After washes in PBS and water, coverslips were mounted onto glass slides in Fluoromount and analyzed with a TSC NT microscope (Leica).
IEM
CD45-depleted lung cells were fixed in 2% paraformaldehyde/0.2% glutaraldehyde and processed for ultrathin cryosectioning and immunogold labeling as described.12 Immunogold labeling was performed with the following antibodies: rat anti-mouse DC-LAMP, mouse anti-rat lbm180 (3C9, Bender MedSystems), rat anti-mouse MHC class II I-Ad (2G9, BD Pharmingen), rabbit polyclonal antisera against the cytoplasmic tails of mouse MHC class II α- and β-chain (generous gift from AY Rudensky, Washington University, Seattle, WA) and rabbit anti-bovine mature surfactant protein B.13
Flow Cytometry Analysis
Double-extracellular/intracellular staining on lung cell suspensions was performed using standard techniques. Briefly, cells were incubated with biotin-coupled mAbs and subsequently with R-phycoerythrin (R-PE)-coupled streptavidin (DAKO). After washes in PBS and permeabilization with the Fix and Perm kit (BD Pharmingen), cells were incubated with rat anti-mouse DC-LAMP mAb coupled to Alexa 488 or with isotype-matched negative control. Fluorescence was analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Results
Mouse DC-LAMP Is Expressed in the Lung
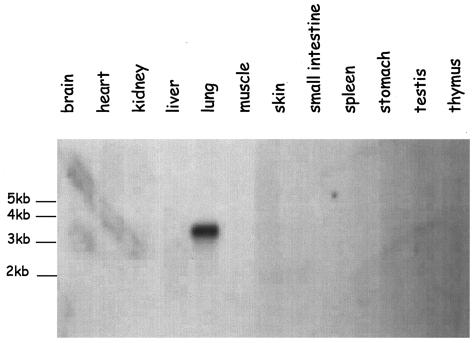
The mouse homologue of human DC-LAMP has recently been cloned from a lung cDNA library because the mRNA had been detected in this tissue by Northern blot using the human DC-LAMP cDNA as a probe.8 In the present study, mouse DC-LAMP mRNA expression was analyzed by Northern blot on commercially available membrane loaded with mRNA from 12 mouse tissues (Figure 1) using the mouse full-length DC-LAMP cDNA as a probe. A strong single 3.2-kb band was detected in the lung, similar to what has been previously observed in human4,6 and mouse.8 No mRNA expression could be detected in any other organ, including brain, heart, kidney, liver, skin, muscles, small intestine, spleen, stomach, testis, and thymus. The absence of DC-LAMP in mouse lymphoid tissues contrasts with human data and was explained by the lack of expression in mature mouse DCs.8
Figure 1.
Mouse DC-LAMP expression analysis. Commercially available Northern blot membrane probed with 32P-labeled mouse DC-LAMP cDNA. Among the 12 tissues analyzed, only lung expresses detectable amounts of DC-LAMP mRNA, which appears as a single 3.2-kb band.
Type II Pneumocytes Constitutively Express DC-LAMP
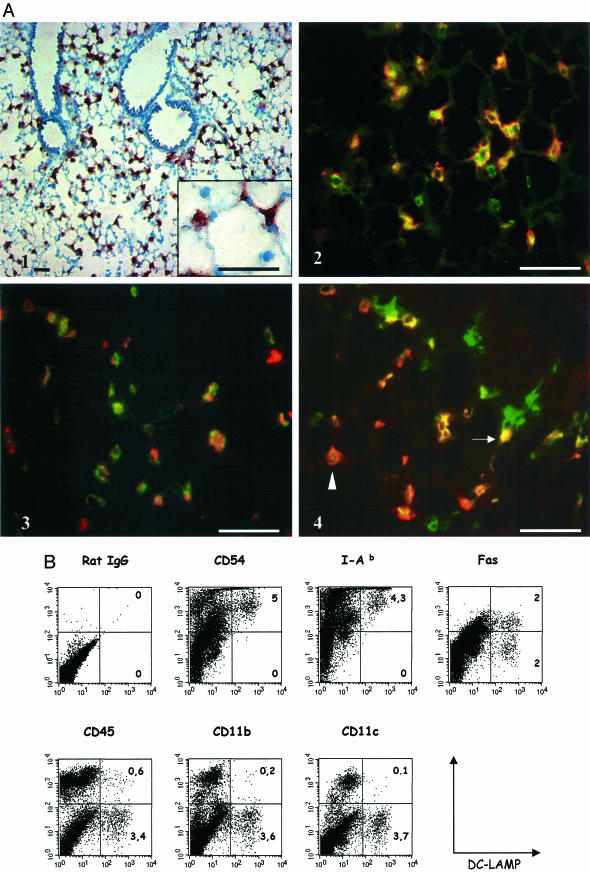
To extend the analysis of mouse DC-LAMP distribution, mouse lung tissue sections were labeled with rat monoclonal antibodies raised against the recombinant mouse protein. A strong signal was detected within a population of peripheral lung cells (Figure 2A1) with no labeling of bronchial epithelium. Both the localization and the cuboidal shape of DC-LAMP-positive cells protruding into the lumina of the alveolae (Figure 2A1, inset) suggested they could correspond to PnIIs. To formally identify the DC-LAMP-expressing cells, double-immunofluorescent labeling was performed on mouse lung cryosections (Figure 2, A2 to A4). Almost all DC-LAMP-positive cells were found to co-express the lamellar body (LB) limiting membrane marker lbm180/ABCA3 (Figure 2A2), thereby confirming that these cells were PnIIs.14,15 Most DC-LAMP+ cells were also shown to express the v6 isoform of the CD44 antigen (Figure 2A3), which is specific in the lung for bronchial epithelium and alveolar type II cells.16 Thus, the vast majority of PnIIs in normal noninflammatory lung express DC-LAMP, and this constitutive expression is in striking contrast with the tight regulation described in human DCs.4 PnIIs have previously been reported to express MHC class II molecules and to be able to present antigens to CD4+ T cells in vitro.17–19 Double labeling of lung tissue sections revealed a heterogeneous MHC class II molecule expression in ∼50% of DC-LAMP+ cells (Figure 2A4). Of note, MHC class II-positive cells not staining for DC-LAMP likely represented alveolar macrophages and/or peripheral DCs.
Figure 2.
Characterization of DC-LAMP-expressing lung cells. A: Mouse lung cryosections were stained with anti-DC-LAMP mAb, revealed with AEC substrate, and counterstained with hematoxylin (A1). DC-LAMP is expressed exclusively by a population of alveolar epithelial cells protruding in the lumen (inset). A2 to A4: Double-immunohistofluorescent stainings were performed on mouse lung cryosections. A2: Most lbm180-positive cells (green) co-express DC-LAMP (red). A3: A majority of DC-LAMP-positive cells (red) also co-express CD44v6 (green). A4: Approximately half of DC-LAMP+ cells (red) also express MHC class II (green), but at levels ranging from high (arrow) to low (arrowhead). B: Isolated mouse total lung cells prepared as described in Materials and Methods were surface-stained with various mAbs, permeabilized, and double stained with anti-DC-LAMP mAb. Fluorescence-activated cell sorting data showed that DC-LAMP+ cells express high levels of CD54 and class II (I-Ab) molecules but not CD45, CD11b, or CD11c. Approximately half of the DC-LAMP+ population expresses Fas (CD95). Numbers in the right quadrants indicate percentage of total lung cells. Original magnifications: ×100 (A1); ×400 (A2 to A4). Scale bars, 40 μm.
Further characterization of DC-LAMP+ lung cells was obtained by flow cytometry analysis (Figure 2B). The proportion of DC-LAMP+ cells among the total cells recovered after lung digestion varied from 1 to 10% (mean value, 3.8%). The lack of expression of CD45 (leukocyte common antigen restricted to hematopoietic cells), CD11b, and CD11c (leukocyte-restricted integrins) by DC-LAMP+ cells clearly establishes that they are neither DCs nor alveolar macrophages. Instead, all DC-LAMP+ cells co-expressed high levels of CD54 (ICAM-1) and MHC class II molecules, in agreement with previous reports on PnIIs.17,18,20 The lower proportion (∼50%) of DC-LAMP+ PnIIs found to express MHC class II molecules by immunohistological analysis likely reflects the difference of sensitivity between the two methods. Furthermore, flow cytometry analysis also showed that half of the DC-LAMP+ population expressed CD95 (Fas antigen), in agreement with previous reports on PnIIs.21 Similar results were obtained with the three strains of mice tested (data not shown). In conclusion, the immunohistological and flow cytometry analysis performed to characterize the DC-LAMP+ lung cells were in agreement with the established PnII phenotype.
DC-LAMP Accumulates Mostly at the Limiting Membrane of PnII Lamellar Bodies
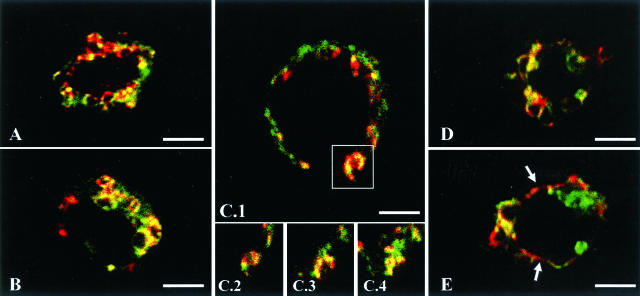
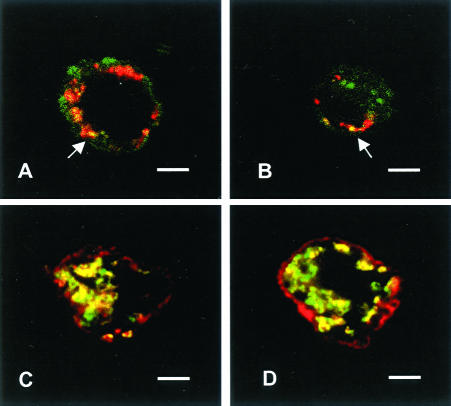
In human maturing DCs, DC-LAMP has been shown to accumulate at the limiting membrane of the lysosomal compartment devoted to the intracellular storage of MHC class II molecules. To determine the subcellular localization of the protein in PnIIs, DC-LAMP+ cells enriched from total mouse lung single-cell suspensions were analyzed by CLM (Figure 3). CD45-depleted cell preparations were immobilized onto poly-l-lysine slides and stained with anti-DC-LAMP and anti-lbm180 antibodies. Anti-lbm180 mAb recognizes lbm180/ABCA3, an ABC transporter specifically present on the limiting membrane of PnII LBs.14,15 A partial co-localization of both molecules was observed in intracellular ring-like structures, identified as LBs according to the presence of specific marker lbm180. Two studies14,22 have reported a patchy surface expression of lbm180 that corresponds to recent sites of LB fusion with the plasma membrane, leading to the release of surfactant into the alveolar space. Figure 3, C1 to C4, illustrates such an exocytosis event, where LB co-expressing DC-LAMP and lbm180 has fused with the cell surface membrane. Both molecules are also observed to form adjacent patches at the cell surface (Figure 3E, arrows).
Figure 3.
Subcellular localization of DC-LAMP in PnIIs. Double staining with lbm180 (green) and DC-LAMP (red) was performed on CD45-depleted mouse lung cells immobilized on poly-l-lysine-coated slides. CLM analysis showed co-localization of both proteins in intracellular ring-like organelles (lamellar bodies, LB) (A, B, D). A LB fusing with the plasma membrane is represented in C1 (square), and shown at different levels of the cell (C2 to C4). Cell surface patches of DC-LAMP adjacent to lbm180 were also detected (E, arrows). Original magnifications, ×1000. Scale bars, 3 μm.
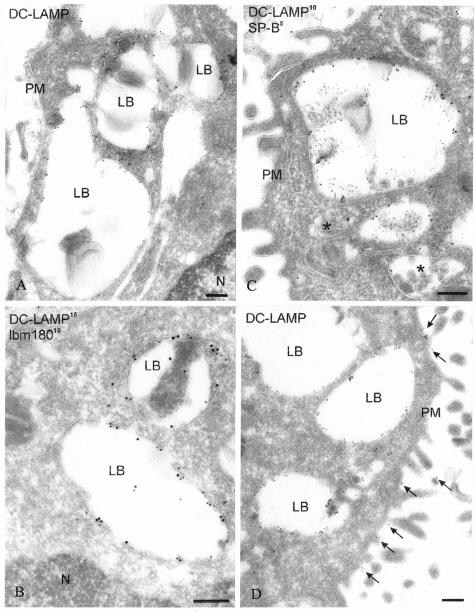
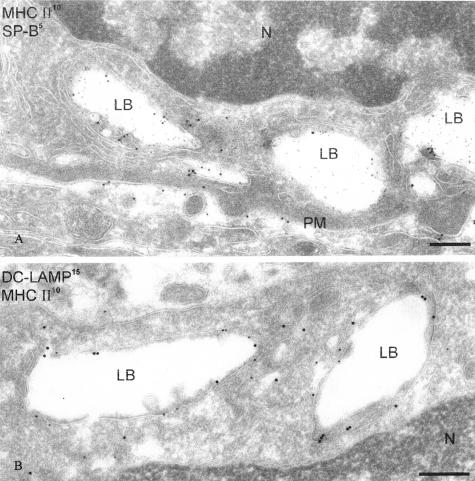
The subcellular localization of DC-LAMP was further illustrated by IEM. In agreement with the CLM data, DC-LAMP was primarily detected at the limiting membrane of LB (Figure 4A), where it co-localized with lbm180 (Figure 4B). The nature of these organelles expressing DC-LAMP at their limiting membrane was confirmed by double labeling with surfactant protein B (SP-B) (Figure 4C). Furthermore, a low density of DC-LAMP signal was detected in endosomal multivesicular bodies (Figure 4C) and at the cell surface membrane (Figure 4D).
Figure 4.
DC-LAMP accumulates in lamellar bodies and is also expressed at the cell surface of PnIIs. A: Ultrathin cryosections of enriched mouse PnII were immunolabeled for DC-LAMP and analyzed by IEM, showing abundant immunogold labeling in LB. B: Double immunolabeling for DC-LAMP (15-nm gold particles) and lbm180 (10-nm gold particles) demonstrated that both proteins are present at the limiting membrane of the LB. C: Double immunolabeling for DC-LAMP (10-nm gold particles) and surfactant protein B (SP-B, 5-nm gold particles) showed that DC-LAMP and SP-B co-localize in LB. Gold particles for DC-LAMP were also present in multivesicular bodies (asterisk). D: DC-LAMP is also detected on the cell surface membrane (arrows). N, nucleus; PM, plasma membrane. Original magnifications, ×35,000. Scale bars, 200 nm.
Mouse DC-LAMP Co-Localizes with MHC Class II Molecule in PnII LB
Immunohistological and flow cytometry analysis showed expression of both DC-LAMP and MHC class II molecules by PnIIs. Considering the tight association of both molecules within the MIIC of human maturing DCs,4 their relative localizations in PnIIs were compared (Figures 5 and 6). CLM analysis revealed a heterogeneous staining, with both single- and double-positive cells. However, in cells expressing both molecules, MHC class II molecules were present not only at the cell surface (Figure 5; A to D), but occasionally co-localized with DC-LAMP in intracellular compartments (Figure 5, A and B) that also contained lbm180 (Figure 5, C and D) and correspond therefore to LB. Of note, MHC class II molecules appear to co-localize more extensively with ABCA3 than DC-LAMP. This suggests that the vesicular membranes and the Golgi cis-ternae stained with anti-lbm180 mAb14 also contain MHC class II molecules, but not detectable amounts of DC-LAMP.
Figure 5.
Localization of DC-LAMP and MHC class II molecules in PnIIs. Relative localization of DC-LAMP and MHC class II molecules was analyzed by CLM on CD45-depleted mouse lung cells immobilized on poly-l-lysine-coated slides. Double staining with MHC class II (green) and DC-LAMP (red) showed occasional co-localization of both proteins in intracellular vesicles (A and B, arrows). Within PnIIs, MHC class II molecules (red) co-localize also with lbm180 (green) in intracellular ring-like structures (LB) (C, D). Original magnifications, ×1000. Scale bars, 3 μm.
Figure 6.
PnIIs express MHC class II molecules at their surface and at the limiting membrane of lamellar bodies. A: Double-immunogold labeling of an isolated mouse lung cell ultrathin cryosection showed the presence of MHC class II on the basolateral plasma membrane (PM) and in lamellar bodies (LB) together with SP-B. B: Double-immunogold staining for MHC class II (10 nm) and DC-LAMP (15 nm) demonstrated co-localization of both proteins at the LB limiting membrane. Original magnifications, ×35,000. Scale bars, 200 nm.
The subcellular distribution of both DC-LAMP and MHC class II molecules was further explored by IEM (Figure 6). Double labeling showed that MHC class II molecules localized in the limiting membranes of organelles filled with SP-B, namely the LB (Figure 6A). In agreement with CLM data, DC-LAMP also was found to co-localize with MHC class II molecules in these membranes (Figure 6B). Interestingly, surface expression of MHC class II molecules was predominant at the basolateral membrane of PnIIs (Figure 6A), as described earlier.23 These results further establish that both mouse DC-LAMP and MHC class II molecules are present at the limiting membrane of LB in PnIIs, and that, in contrast to human DCs,5 PnIIs express DC-LAMP at their surface membrane too.
DC-LAMP Is Expressed in Normal and Transformed Human PnIIs
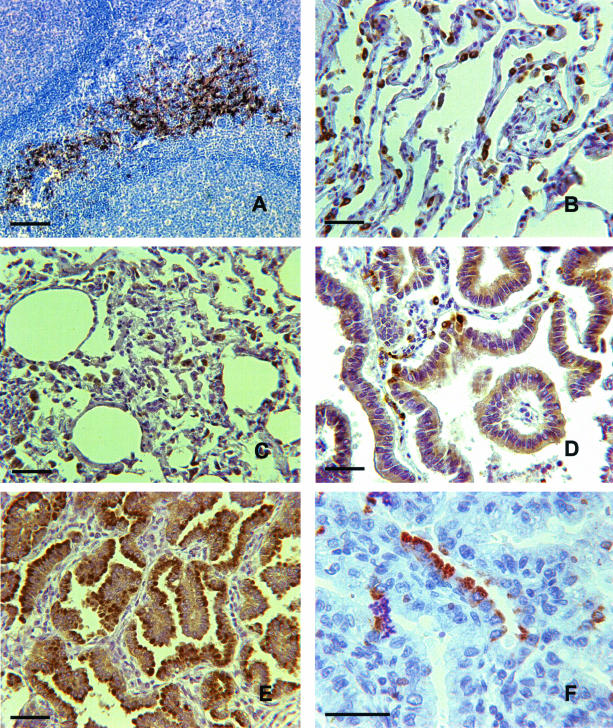
Several of the mAbs raised against mouse DC-LAMP were found to specifically label cells transfected with human DC-LAMP (not shown). One of those was selected for its ability to label mature DCs on paraffin-embedded tonsil sections (Figure 7A). Interestingly, when tested on lung tissue sections, this antibody also labeled human (Figure 7B), sheep (Figure 7C), and rat (not shown) PnIIs. PnIIs are known for their capacity to restore the alveolar surface integrity after lung injury. They can indeed re-enter into cell cycle and repopulate the wounded alveolae. However, alveolar invasion by uncontrolled proliferation of transformed PnIIs leads to a distinct type of peripheral lung carcinoma identified as bronchioloalveolar carcinoma (BAC). To determine whether transformed PnIIs would still express DC-LAMP, tissue sections from three histologically confirmed BACs were stained with the specific mAb. The three tumors showed a homogeneous signal at the apical side of most cancer cells (Figure 7D), therefore demonstrating that DC-LAMP expression is maintained in PnIIs after malignant transformation. This conclusion was corroborated by a strong apical labeling of transformed PnIIs into lesions of JSRV-induced ovine pulmonary adenocarcinoma24 (Figure 7E). In contrast, DC-LAMP was detected in only 12 of 50 lung adenocarcinomas other than BACs, and when detected, it was only expressed in scarce cells (Figure 7F) (N Freymond et al, manuscript in preparation).
Figure 7.
DC-LAMP expression by transformed PnIIs. Paraffin-embedded human and sheep tissue sections were stained with the 1010E1 rat anti-mouse DC-LAMP mAb, and labeling was revealed with DAB substrate (except F: AEC) and counterstained with hematoxylin. A: Human tonsil interdigitating DCs clustered with T cells are intensely stained. On human (B) and sheep (C) normal lung tissue sections, cells protruding in the lumen of the alveolae and identified as PnIIs were uniformly labeled. D: Human BAC tissue section showed a discrete but homogeneous and mostly apical labeling of transformed cells. E: A similar although more intense labeling was observed on ovine pulmonary adenocarcinoma induced by JSRV. F: The luminal side of some clustered human adenocarcinoma cells was occasionally strongly labeled. Original magnifications: ×250 (A–E); ×400 (F). Scale bars, 40 μm.
Discussion
The present study establishes that the lysosome-associated membrane protein DC-LAMP, that has been regarded so far as a reliable marker of human DC maturation is also constitutively expressed by a population of lung peripheral cells, in mouse, in human, in sheep, in dog, and in rat (our unpublished data). The detection of DC-LAMP in PnIIs from the five species examined so far, including rat and mice that do not express this molecule in their mature DCs raises the question of what cell type (PnIIs or DCs) first expressed this molecule during evolution. Although the mechanism that regulates DC-LAMP mRNA and protein expression in PnIIs remains unknown, it is clearly different from the activation-driven control observed in human DCs.4 Indeed, PnIIs express this molecule constitutively in normal lung. In a search for cell lines expressing DC-LAMP, human PnII-like A549 cells were labeled with specific antibodies. DC-LAMP expression was very weak and variable (unpublished data), but whether this cell line indeed presents PnII characteristic features remains to be clearly established.9
The DC-LAMP glycoprotein cytoplasmic portion contains a conserved lysosome-targeting motif that can be recognized by adaptor complexes AP1, AP2, and AP3 found at the trans-Golgi network, the plasma membrane and the trans-Golgi network-endosomal system, respectively.25 It is therefore not surprising to find DC-LAMP co-localizing in PnIIs with lbm180 on the limiting membrane of LB know to bear other lysosomal membrane proteins, including LAMP-1 and CD63.13,26 Furthermore, LBs contain lysosomal proteases such as cathepsins that are enabled to process surfactant proproteins by the low intravesicular pH.27,28 A sorting role has also been ascribed for LBs,29 where surfactant endocytosed via endosomes and multivesicular bodies would be segregated either toward lysosomes for degradation or back to the secretory pathway for recycling. Indeed, pulmonary surfactant is stored, before secretion, in LBs that consist of densely packed lamellae of surfactant surrounded by a limiting membrane that can fuse with the apical cell membrane to release their content into the alveolar space.30,31 Altogether, DC-LAMP appears to be expressed in specialized PnII secretory lysosomes32 involved in the trafficking of lung surfactant.
In this context, a notable difference between PnIIs and human DCs is the detection of DC-LAMP at the plasma membrane of the former cells. Cell surface patches of lbm180 molecules were previously reported14,22 and interpreted as remnants of recent LB exocytosis. Using lbm180 as a tracer, these residual LB membranes were shown to be retrieved back to existing LBs via an active endocytic pathway involving endosomes and multivesicular bodies. This would provide a mechanism for surfactant recycling.33 Such a scenario is compatible with the occasional detection of DC-LAMP in endosomal multivesicular bodies, the known precursors of LBs.13,26 Other LAMP family members can transit through the cell surface, where they might perform specialized functions: CD68 behaves as a receptor for oxidized low-density lipoprotein that is rapidly internalized after surface expression by macrophages,34 whereas cell surface-expressed LAMP-1 has been linked to tumor cell migration35 and Th1-specific polarization by activated macrophages.36 Whether DC-LAMPs exert some specific function(s) when displayed at the PnII cell surface remains however to be determined.
Nevertheless, DC-LAMP surface expression provides an efficient tool for the purification and the enumeration of PnIIs by fluorescence-activated cell sorting. Morphometric analyses have shown that PnIIs represent between 10 and 15% of all lung cells,37,38 but their proportion among total lung single-cell preparations depends on the protocol for tissue digestion. In our hands, without enzymatic instillation before recovering the lungs, we obtained lower proportion of DC-LAMP+ cells (mean value, ∼4%). It is difficult to compare directly our fluorescence-activated cell sorting data with results obtained by (immuno)-cytochemistry on cells recovered following different protocols of digestion. Although all lamellar body-containing cells were stained by DC-LAMP mAb on electron microscopy (Figures 4 and 6, and data not shown), we cannot formally exclude that some PnIIs do not express DC-LAMP, as suggested by the detection of few lbm180+/DC-LAMP− mAb cells in the lung (Figure 2A).
Based on the coincidence of DC-LAMP appearance on the limiting membrane of MIIC and MHC class II molecule translocation to the cell surface, a role for DC-LAMP in the reorganization and transfer of MIIC to the plasma membrane in human activated DCs was proposed.4 Alternatively, the luminal portion of DC-LAMP may modulate the processing of antigens by lysosomal cathepsins that are up-regulated on DC maturation, and also protect the lysosomal membrane against proteolysis, as suggested for other LAMP family members.39 In the absence of functional data regarding DC-LAMP neither in DCs nor in PnIIs, the role of this molecule remains unclear. However, morphological as well as functional parallel between the MIIC compartment of DCs and the LB of PnIIs suggest a regulatory role for DC-LAMP in the purpose of both specialized secretory lysosomes. The striking difference in DC-LAMP expression by those two cell types is also compatible with a regulatory function: the quick induction of DC-LAMP in maturing DCs may be linked to the sharp increase of lysosomal proteolytic activity associated with antigen processing and to the rapid translocation of loaded MHC class II molecules to the cell surface, whereas the constitutive expression of DC-LAMP in PnIIs may be related to the continuous processing and release of surfactant by those cells.
Our data suggest that exocytosis of LB, which results in surfactant secretion, also brings MHC class II molecules to the cell surface together with lbm180 and DC-LAMP. This may link DC-LAMP to the controversial role of PnIIs in MHC class II-restricted antigen presentation. PnIIs express constitutively MHC class II molecules,18 and this expression is increased by IFN-γ in rat23 or in some pathological conditions in human.40,41 PnIIs also express cathepsins,42 CD54,20 and co-stimulatory molecules such as CD80 and CD86.43 Therefore, these epithelial cells, strategically localized in the lung at the interface with the outside environment, express many of the molecules required for efficient antigen presentation to CD4+ T cells. Indeed, PnIIs can activate T cells in vitro19 and induce IL-2 production by Jurkat cells.43 It remains however unclear whether PnIIs play a role as lung antigen-resenting cells in vivo, and whether DC-LAMP is involved.
Whatever functions DC-LAMP might fulfill, its maintained expression in transformed PnIIs may provide a welcome tool for studying bronchioloalveolar adenocarcinomas (BACs). BAC is the rarest subtype of lung adenocarcinomas and is characterized by the colonization, without destruction or invasion, of the alveolae by transformed PnIIs.44 Given the better prognosis of BAC and its lower association with tobacco smoking, when compared with other lung adenocarcinomas,45 accurate diagnosis is important both for the treatment and for the understanding of the disease. However, despite continuous progress in identifying the lung carcinoma subsets,46 BAC histological diagnosis is notoriously difficult, given the lack of markers specific for normal and transformed PnIIs,47,48 and that the mechanisms of transformation are primarily unknown.44 Recently, differential expression of cytokeratin 7 and 20 subtypes had been proposed to facilitate the distinction between BAC and the other lung adenocarcinomas as well as lung metastasis of other adenocarcinomas,49,50 but these results were not confirmed. The present finding that, in contrast with other lung adenocarcinomas, the alveolae in BACs are infiltrated by transformed PnIIs homogeneously stained with DC-LAMP mAb suggests that this marker may facilitate the diagnosis of this infrequent cancer. Further study will obviously be needed to establish the value of DC-LAMP as a diagnostic marker for BAC and to determine whether it plays a role in the oversecretion of surfactant that characterizes clinically many BAC patients. In this regard, detection of DC-LAMP in JSRV-induced ovine pulmonary adenocarcinoma, which closely resembles human BAC,44 may help to understand the development of this type of lung cancer. Last but not least, the irregular expression of DC-LAMP in nonbronchioloalveolar lung adenocarcinomas may provide new insights regarding the cellular origin and diversity of those cancers. Interestingly, Ozaki and colleagues6 have reported the expression of TSC403 mRNA (that is identical to DC-LAMP) in human lung, but also in cancers of various origins, including esophagus, colon, ovary, breast, fallopian tube, and liver. The cells expressing DC-LAMP mRNA in non-lung cancers are most likely mature DCs that frequently surround or infiltrate primary tumors.51–52 In lung adenocarcinomas, both transformed PnIIs and mature DCs can express DC-LAMP, but those cell types are easily distinguished on the basis of their morphology and their localization (N Freymond et al, manuscript in preparation). Furthermore, the homogeneous expression of DC-LAMP by PnIIs is of lower intensity in human BAC tumor cells than in normal PnIIs or DCs (Figure 7). This may also explain the apparent down-regulation of TSC403 mRNA observed when gene expression profiles of three BAC samples were compared to the corresponding normal lung tissue using oligonucleotide microarray.53
In conclusion, DC-LAMP appears to be constitutively expressed in normal and transformed PnIIs, where its subcellular localization suggests a role in the processing and/or trafficking of surfactant and MHC class II molecules. Although the exact function of DC-LAMP in PnII-specialized secretory lysosomes remains to be established, this marker may become a useful tool for facilitating the study and the diagnosis of the PnII-derived tumors. To notify these results, we suggest DC-LAMP to be referred as CD208 from now on.10
Acknowledgments
We thank Patrice Douillard for his support with fluorescence studies, Alain Vicari for his helpful expertise with mice, Rene Scriwanek and Mark van Peski for quality photographical work, and Jean-François Mornex and Giorgio Trinchieri for critical reading of this manuscript and helpful comments.
Footnotes
Address reprint requests to Serge Lebecque, Schering Plough, LIR, 27 ch. des Peupliers, 69571 Dardilly, France. E-mail: serge.lebecque@spcorp.com.
Supported by the Région Rhone Alpes; the Fondation Marcel Mérieux, Lyon, France (to B.S.); and the Netherlands Organization for Scientific Research (grant 805-48-014 to M.K.).
References
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Furuta K, Yang XL, Chen JS, Hamilton SR, August JT. Differential expression of the lysosome-associated membrane proteins in normal human tissues. Arch Biochem Biophys. 1999;365:75–82. doi: 10.1006/abbi.1999.1147. [DOI] [PubMed] [Google Scholar]
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Aït-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- Barois N, de Saint-Vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Nagata M, Suzuki M, Fujiwara T, Ueda K, Miyoshi Y, Takahashi E, Nakamura Y. Isolation and characterization of a novel human lung-specific gene homologous to lysosomal membrane glycoproteins 1 and 2: significantly increased expression in cancers of various tissues. Cancer Res. 1998;58:3499–3503. [PubMed] [Google Scholar]
- Fuller CL, Choi YK, Fallert BA, Capuano S, III, Rajakumar P, Murphey-Corb M, Reinhart TA. Restricted SIV replication in rhesus macaque lung tissues during the acute phase of infection. Am J Pathol. 2002;161:969–978. doi: 10.1016/S0002-9440(10)64257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun B, de Saint-Vis B, Clair-Moninot V, Pin JJ, Barthelemy-Dubois C, Kissenpfennig A, Peronne C, Bates E, Mattei MG, Lebecque S. Cloning and characterization of the mouse homologue of the human dendritic cell maturation marker CD208/DC-LAMP. Eur J Immunol. 2003;33:2619–2629. doi: 10.1002/eji.200324175. [DOI] [PubMed] [Google Scholar]
- Mason RJ, Williams MC. Type II alveolar cell. Defender of the alveolus. Am Rev Respir Dis. 1977;115:81–91. doi: 10.1164/arrd.1977.115.S.81. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, Pin JJ, Mattéi M-G, Ait-Yahia S, Bates E, Kissenpfennig A, Malissen B, Koch F, Fossiez F, Romani N, Lebecque S, Saeland S. Identification of mouse Langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- Kleijmeer MJ, Posthuma G, Geuze HJ. Immunoelectron microscopy of antigen processing in dendritic cells. Robinson SP, Stagg AJ, editors. Totowa: Humana Press,; Methods in Molecular MedicineDendritic Cell Protocols. 2001:pp 387–411. doi: 10.1385/1-59259-150-7:387. [DOI] [PubMed] [Google Scholar]
- Voorhout WF, Veenendaal T, Haagsman HP, Weaver TE, Whitsett JA, van Golde LM, Geuze HJ. Intracellular processing of pulmonary surfactant protein B in an endosomal/lysosomal compartment. Am J Physiol. 1992;263:L479–L486. doi: 10.1152/ajplung.1992.263.4.L479. [DOI] [PubMed] [Google Scholar]
- Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am J Physiol. 1998;275:L172–L183. doi: 10.1152/ajplung.1998.275.1.L172. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- Kasper M, Gunthert U, Dall P, Kayser K, Schuh D, Haroske G, Muller M. Distinct expression patterns of CD44 isoforms during human lung development and in pulmonary fibrosis. Am J Respir Cell Mol Biol. 1995;13:648–656. doi: 10.1165/ajrcmb.13.6.7576702. [DOI] [PubMed] [Google Scholar]
- Steiniger B, Sickel E. Class II MHC molecules and monocytes/macrophages in the respiratory system of conventional, germ-free and interferon-gamma-treated rats. Immunobiology. 1992;184:295–310. doi: 10.1016/S0171-2985(11)80588-7. [DOI] [PubMed] [Google Scholar]
- Cunningham AC, Milne DS, Wilkes J, Dark JH, Tetley TD, Kirby JA. Constitutive expression of MHC and adhesion molecules by alveolar epithelial cells (type II pneumocytes) isolated from human lung and comparison with immunocytochemical findings. J Cell Sci. 1994;107:443–449. doi: 10.1242/jcs.107.2.443. [DOI] [PubMed] [Google Scholar]
- Christensen PJ, Armstrong LR, Fak JJ, Chen GH, McDonald RA, Toews GB, Paine R., III Regulation of rat pulmonary dendritic cell immunostimulatory activity by alveolar epithelial cell-derived granulocyte macrophage colony-stimulating factor. Am J Respir Cell Mol Biol. 1995;13:426–433. doi: 10.1165/ajrcmb.13.4.7546772. [DOI] [PubMed] [Google Scholar]
- Guzman J, Izumi T, Nagai S, Costabel U. ICAM-1 and integrin expression on isolated human alveolar type II pneumocytes. Eur Respir J. 1994;7:736–739. doi: 10.1183/09031936.94.07040736. [DOI] [PubMed] [Google Scholar]
- Fine A, Anderson NL, Rothstein TL, Williams MC, Gochuico BR. Fas expression in pulmonary alveolar type II cells. Am J Physiol. 1997;273:L64–L71. doi: 10.1152/ajplung.1997.273.1.L64. [DOI] [PubMed] [Google Scholar]
- Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, Morohoshi T, Ogawa J, Shioda S, Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, DeFerrari M, Skoskiewicz MJ, Russell PS, Colvin RB. Induction of MHC-determined antigens in the lung by interferon-gamma. Lab Invest. 1986;55:138–144. [PubMed] [Google Scholar]
- Platt JA, Kraipowich N, Villafane F, DeMartini JC. Alveolar type II cells expressing jaagsiekte sheep retrovirus capsid protein and surfactant proteins are the predominant neoplastic cell type in ovine pulmonary adenocarcinoma. Vet Pathol. 2002;39:341–352. doi: 10.1354/vp.39-3-341. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Wasano K, Hirakawa Y. Lamellar bodies of rat alveolar type 2 cells have late endosomal marker proteins on their limiting membranes. Histochemistry. 1994;102:329–335. doi: 10.1007/BF00268903. [DOI] [PubMed] [Google Scholar]
- Beers MF. Inhibition of cellular processing of surfactant protein C by drugs affecting intracellular pH gradients. J Biol Chem. 1996;271:14361–14370. doi: 10.1074/jbc.271.24.14361. [DOI] [PubMed] [Google Scholar]
- Yayoi Y, Ohsawa Y, Koike M, Zhang G, Kominami E, Uchiyama Y. Specific localization of lysosomal aminopeptidases in type II alveolar epithelial cells of the rat lung. Arch Histol Cytol. 2001;64:89–97. doi: 10.1679/aohc.64.89. [DOI] [PubMed] [Google Scholar]
- Gibson KF, Widnell CC. The relationship between lamellar bodies and lysosomes in type II pneumocytes. Am J Respir Cell Mol Biol. 1991;4:504–513. doi: 10.1165/ajrcmb/4.6.504. [DOI] [PubMed] [Google Scholar]
- Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. EMBO J. 1994;8:957–967. doi: 10.1096/fasebj.8.12.8088461. [DOI] [PubMed] [Google Scholar]
- Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:233–243. doi: 10.1016/s1095-6433(01)00320-8. [DOI] [PubMed] [Google Scholar]
- Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Schaller-Bals S, Bates SR, Notarfrancesco K, Tao JQ, Fisher AB, Shuman H. Surface-expressed lamellar body membrane is recycled to lamellar bodies. Am J Physiol. 2000;279:L631–L640. doi: 10.1152/ajplung.2000.279.4.L631. [DOI] [PubMed] [Google Scholar]
- Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh O, Wang WC, Lotan R, Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992;267:5700–5711. [PubMed] [Google Scholar]
- Prasad DV, Parekh VV, Joshi BN, Banerjee PP, Parab PB, Chattopadhyay S, Kumar A, Mishra GC. The Th1-specific costimulatory molecule, m150, is a posttranslational isoform of lysosome-associated membrane protein-1. J Immunol. 2002;169:1801–1809. doi: 10.4049/jimmunol.169.4.1801. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Barry BE, O’Neil JJ, Raub JA, Pratt PC, Crapo JD. Morphologic changes in the lung during the lifespan of Fischer 344 rats. Am J Anat. 1982;164:155–174. doi: 10.1002/aja.1001640206. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- Winchester BG. Lysosomal membrane proteins. Eur J Paediatr Neurol. 2001;5:11–19. doi: 10.1053/ejpn.2000.0428. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Yamamoto M, Shimokata K, Nagura H. Phenotypic characterization of alveolar capillary endothelial cells, alveolar epithelial cells and alveolar macrophages in patients with pulmonary fibrosis, with special reference to MHC class II antigens. Virchows Arch A Pathol Anat Histopathol. 1989;415:79–90. doi: 10.1007/BF00718607. [DOI] [PubMed] [Google Scholar]
- Arbustini E, Morbini P, Diegoli M, Grasso M, Fasani R, Vitulo P, Fiocca R, Cremaschi P, Volpato G, Martinelli L. Coexpression of aspartic proteinases and human leukocyte antigen-DR in human transplanted lung. Am J Pathol. 1994;145:310–321. [PMC free article] [PubMed] [Google Scholar]
- Kos J, Sekirnik A, Kopitar G, Cimerman N, Kayser K, Stremmer A, Fiehn W, Werle B. Cathepsin S in tumours, regional lymph nodes and sera of patients with lung cancer: relation to prognosis. Br J Cancer. 2001;85:1193–1200. doi: 10.1054/bjoc.2001.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissel G, Ernst M, Rabe K, Papadopoulos T, Magnussen H, Schlaak M, Muller-Quernheim J. Human alveolar epithelial cells type II are capable of regulating T-cell activity. J Investig Med. 2000;48:66–75. [PubMed] [Google Scholar]
- Mornex JF, Thivolet F, De las Heras M, Leroux C. Pathology of human bronchioloalveolar carcinoma and its relationship to the ovine disease. Curr Top Microbiol Immunol. 2003;275:225–248. doi: 10.1007/978-3-642-55638-8_9. [DOI] [PubMed] [Google Scholar]
- Barsky SH, Cameron R, Osann KE, Tomita D, Holmes EC. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer. 1994;73:1163–1170. doi: 10.1002/1097-0142(19940215)73:4<1163::aid-cncr2820730407>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- Sorensen JB, Hirsch FR, Gazdar A, Olsen JE. Interobserver variability in histopathologic subtyping and grading of pulmonary adenocarcinoma. Cancer. 1993;71:2971–2976. doi: 10.1002/1097-0142(19930515)71:10<2971::aid-cncr2820711014>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Saleh HA, Haapaniemi J, Khatib G, Sakr W. Bronchioloalveolar carcinoma: diagnostic pitfalls and immunocytochemical contribution. Diagn Cytopathol. 1998;18:301–306. doi: 10.1002/(sici)1097-0339(199804)18:4<301::aid-dc11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Shah RN, Badve S, Papreddy K, Schindler S, Laskin WB, Yeldandi AV. Expression of cytokeratin 20 in mucinous bronchioloalveolar carcinoma. Hum Pathol. 2002;33:915–920. doi: 10.1053/hupa.2002.126876. [DOI] [PubMed] [Google Scholar]
- Lau SK, Desrochers MJ, Luthringer DJ. Expression of thyroid transcription factor-1, cytokeratin 7, and cytokeratin 20 in bronchioloalveolar carcinomas: an immunohistochemical evaluation of 67 cases. Mod Pathol. 2002;15:538–542. doi: 10.1038/modpathol.3880560. [DOI] [PubMed] [Google Scholar]
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. 2002;196:37–43. doi: 10.1002/path.1018. [DOI] [PubMed] [Google Scholar]
- Goodwin LO, Mason JM, Hajdu SI. Gene expression patterns of paired bronchioloalveolar carcinoma and benign lung tissue. Ann Clin Lab Sci. 2001;31:369–375. [PubMed] [Google Scholar]