Abstract
Studies have linked optimism to poorer immunity during difficult stressors. In the present report, when first-year law students (N = 46) relocated to attend law school, reducing conflict among curricular and extracurricular goals, optimism predicted larger delayed type hypersensitivity responses, indicating more robust in vivo cellular immunity. However, when students did not relocate, increasing goal conflict, optimism predicted smaller responses. Although this effect has been attributed to negative affect when difficult stressors violate optimistic expectancies, distress did not mediate optimism’s effects on immunity. Alternative affective mediators related to engagement – engaged affect and fatigue – likewise failed to mediate optimism’s effects, although all three types of affect independently influenced in vivo immunity. Alternative pathways include effort or self-regulatory depletion.
Keywords: optimism, goals, immune, engagement, distress
Dispositional optimism, defined as generalized positive outcome expectancies, has been associated with positive health consequences in a number of studies. More optimistic men recovered better from coronary artery bypass surgery (Fitzgerald, Tennen, Affleck, & Pransky, 1993; Scheier et al. 1999), more optimistic men and women had lower blood pressure during daily life (Räikkönen, Matthews, Flory, Owens, & Gump, 1999), and more optimistic women infected with both HIV and human papilloma virus had better immunity (Byrnes et al., 1998). These positive effects on physical health mirror optimism’s consistently beneficial effects on mental health.
However, four reports of optimism’s effects on the immune system – two experimental and two naturalistic – have also identified circumstances under which optimism is related to lower immune parameters that might have negative health consequences. First, an experimental study manipulated the degree of control that the participant had over a loud noise stressor (Sieber et al., 1992). Optimism’s effects differed depending on the condition, such that more optimism was associated with higher natural killer cell cytotoxicity (NKCC) when control was present but with lower NKCC when it was not. A second experimental study assigned some participants to perform a mental arithmetic task that became more difficult with better performance so that mastery was never attained; others did not perform the task (Segerstrom, Castaneda, & Spencer, 2003). Among those who did not perform the task, higher optimism associated with larger delayed-type hypersensitivity (DTH) responses, which measure in vivo cellular immunity; among those who did perform the task, higher optimism associated with smaller responses. Third, a prospective, correlational study examined the effects of optimism and naturalistic stressor duration (F. Cohen et al., 1999). Higher optimism associated with higher T cell counts (CD8+CD11b+) when stressors lasted less than one week, but with lower T cell counts (CD8+CD11b−) and NKCC when stressors lasted longer than one week. Finally, two prospective correlational studies associated higher optimism with higher T cell counts (CD4+ lymphocytes) and larger DTH responses among students experiencing low conflict between social and academic goals, but with lower T cell counts and smaller DTH responses among students with high conflict (Segerstrom, 2001). The pattern of results across these four reports suggests that optimism associates with higher immune parameters when stressors are straightforward (time-limited, controllable, responsive to coping efforts, not conflicting) but lower immune parameters when stressors are difficult (prolonged, uncontrollable, unresponsive to coping efforts, conflicting).
Affect was cited as a likely mediator for these effects. F. Cohen et al. (1999) and Sieber et al. (1992) posited that although optimists are typically less distressed than pessimists, optimists who encounter difficult stressors might be more disappointed, distressed, and demoralized than their pessimistic counterparts because such stressors violate their positive expectancies (c.f., Tennen & Affleck, 1987). In this disappointment model, optimism leads to negative affect that subsequently decreases immune parameters.
In contrast, the engagement or persistence model states that difficult stressors elicit different responses from optimists and pessimists: optimists reengage, putting forth more effort to overcome the stressors, whereas pessimists disengage (Carver & Scheier, 1998; Segerstrom, 2001; Segerstrom et al., 2003). Affect is a potential mediator in the engagement model as well, but the engagement model involves more narrowly specified categories of potential affective mediators, as opposed to the diffuse negative affect of the disappointment model. First, engaged affect (e.g., interested, determined) is associated with larger cardiovascular and immune responses to laboratory stressors, suggesting that, although positively valenced, engaged affect might have negative physiological consequences (S. Cohen et al., 2000; Maier, Waldstein, & Synowski, 2003). Second, fatigue could reflect the prolonged self-regulatory efforts of optimists facing difficult stressors. Studies of “ego depletion” or “self-control depletion” find that difficult self-regulatory tasks decrease persistence at subsequent problem-solving tasks and sometimes increase perceived fatigue but not other kinds of affect (Baumeister, Bratslavsky, Muraven, & Tice, 1998).
The present study examines the effects of optimism on cellular immunity under conditions of high and low goal conflict in first-year law students. The lack of time to pursue both academic and social goals (e.g., spending time with family and friends) is a major difficulty during the first year of law school (Segerstrom, 1996). Furthermore, students who do not geographically relocate are more likely than those who do to have goals relating to maintaining and especially finding time for existing relationships, suggesting that they experience this difficulty more acutely as a function of remaining in their usual social context (Segerstrom, 2005). Optimism was predicted to have a positive relationship with immunity among students experiencing little conflict (i.e., students who relocated) but a negative relationship with immunity among students experiencing more conflict (i.e., students who did not relocate).
Support for affective mediation consistent with the engagement model was predicted to be better than that for the disappointment model because (1) negative affect and negative affectivity have not accounted for the effects of optimism on immunity in some previous investigations (F. Cohen et al., 1999; Segerstrom, 2001); (2) potentially disappointing events such as health relapse have not increased distress for people with higher premorbid optimism, suggesting that optimists are not at risk for increased negative affect following expectancy violation (Litt, Tennen, Affleck, & Klock, 1992; Helgeson, 2003; Stanton & Snider, 1993); and (3) in the mental arithmetic study, the effects of conscientiousness, a personality factor related to goal engagement, paralleled those of optimism, where as neuroticism, a personality factor related to distress, did not (Segerstrom et al., 2003).
The present study used the DTH test to measure the cellular immune response in the skin, one of the sites in the body where pathogens are most commonly encountered. In the major steps of the DTH response, antigen-presenting cells in the skin stimulate T cells to produce cytokines, which recruit and activate effector cells. The result of the migration of these effector cells into the skin is redness and swelling or induration in the skin that can be measured reliably as an index of how robust the immune response is. Larger indurations, therefore, indicate more robust cellular immune responses (Janeway & Travers, 1997).
Method
Participants
Participants were 46 first-year law students. The sample was 61% female and 96% White, with a mean age of 23 years (SD = 1.9). Academic credentials were representative of the law school as a whole, with a median Law School Admission Test (LSAT) score (159) at the student body median (159). Slightly more than half (61%) had relocated to attend law school. There were no significant differences among students who did or did not relocate on any demographic, psychological, or immune measures.
Procedure
Participants received recruitment packets during the summer before starting law school. Interested law students returned a signed informed consent, contact information, and a form that was used to screen for mental health (e.g, psychotropic medication, self-reported history of impairment of function for 2 weeks or more), physical health (e.g., immunologically mediated disease, contraindications for skin testing, immunotropic medication), and substance use (e.g., more than two drinks of alcohol every day). Eligible participants were contacted and scheduled for the first visit. Participants completed questionnaires and had DTH skin tests at five time points: at the beginning of the first semester, midway through the first semester, during exams at the end of the first semester, at the beginning of the second semester, and one month into the second semester. Each assessment period lasted 48 hours. On Day 1, the DTH skin test was administered in the morning. All DTH tests were administered between 7 and 9 am to control for diurnal changes in immunity. Questionnaires were completed over Day 1 and Day 2 and returned when skin tests were read on the morning of Day 3, 48 hours after administration.
Measures
Demographic and background information
Students reported on their demographic characteristics, their LSAT scores, and whether or not they had relocated to the area to attend law school.
Dispositional optimism
Dispositional optimism was measured with the Life Orientation Test – Revised (LOT-R; Scheier, Carver, & Bridges, 1994), a measure of generalized positive and negative outcome expectancies. Three items are phrased positively, three negatively, and four are filler items that do not contribute to the total optimism score. In the validation sample, internal consistency was .78 over the six optimism items and test-retest correlations over 4 to 28 months ranged from .56 to .79. The LOT-R was administered at the first time point.
Affect
Affect was measured with the Expanded Positive and Negative Affect Schedule (PANAS - X; Watson & Clark, 1994). Participants completed the affect scale three times: once on the morning when the skin test was administered for affect on the day before (i.e., Day -1), on the evening of Day 1 for that day, and on the evening of Day 2 for that day. Skin tests were read on the following morning. Mean scores for each time point were taken across the three administrations. Distress was operationalized as the sum of 5 items (distressed, scared, upset, nervous, and afraid), which corresponds to the negative affect subscale of the PANAS (Watson, Clark & Tellegen, 1988). Mean internal reliability for these items over the 5 assessments was .90. Engaged affect was operationalized as the sum of 5 items (determined, enthusiastic, excited, inspired, and interested), which correspond to Waldstein and colleagues’s measure of engaged affect (Waldstein, Bachen, & Manuck, 1997; Maier et al., 2003). Mean internal reliability for these items was .90. Fatigue was operationalized as the mean of 4 items (sluggish, tired, sleepy, and drowsy), which correspond mainly to global fatigue items on the Multidimensional Fatigue Symptom Inventory (Stein, Martin, Hann, & Jacobsen, 1998). Mean internal reliability for these items was .92. The PANAS-X was administered at all five time points.
Expectations and class rank
At the first three time points, participants reported their hoped-for class rank, their expected class rank, and the lowest class rank they would find satisfactory. Note that class rank, rather than GPA, is the basis for several important outcomes, including membership on the law review and competitiveness for summer clerkships. At the fifth time point, participants reported their actual class rank. Predictions significantly decreased over the course of the fall semester (all p < .01), so those made at the third wave, which occurred during the second week of a 2-week final exam period, were used as being most proximal to the beginning of grade posting and most conservative with regard to potential for disappointment.
Cellular immunity
Cellular immunity was measured by delayed-type hypersensitivity responses to the Mumps Skin Test Antigen (MSTA; Aventis Pasteur), a preparation of heat-killed mumps virus. A nurse injected 0.1 ml of MSTA intradermally in the participant’s nondominant arm. Induration was measured 48 hours later using the ballpoint pen method (Longfield et al., 1984).
Data analysis
Data were analyzed using multilevel modeling (Singer & Willett, 2003). The multilevel model specifies a first-level, within-subjects model of change over time, in which the outcome for each person at each time point is a function of their own intercept, the effect of time (slope), and an error term. In the second-level, between-subjects model, the intercept and slope of the first-level model are random factors that can be predicted by between-subjects characteristics (e.g., optimism). The first-level submodel tested effects of and variability in the first-level parameters (i.e., mean differences as reflected in the intercept and differences in change as reflected in the slope). The second-level submodel then added dispositional optimism (centered around 0), relocation (coded 1 = relocated and 0 = did not relocate), and their interaction before and after including each of the affect categories (centered around 0) as a time-varying covariate. MSTA batch was also included to control for potential differences in potency. The models were tested using SAS PROC MIXED (Singer, 2002). Results are reported as γ weights with their associated t tests. γ is analogous to an unstandardized beta weight; because these parameters vary as a function of scaling, the standard effect size η is also reported. η can be interpreted on the same scale as r: .10 effects are considered small; .30, medium; and .50, large (J. Cohen, 1987; Rosenthal & Rosnow, 1984).
Results
Effects of optimism and stress on cellular immunity
In a multilevel model including only antigen lot and time, there was a significant effect of time on DTH responses, so that responses became smaller as the first year of law school progressed, γ = −2.27 (SE = .35), t (165) = −6.44, p < .0001, η = .45. There were not significant individual differences in the effects of time (i.e., slope, Z = 0.12, p < .46), so time was treated as a fixed effect in analyses of the effects of optimism and relocation. There were significant individual differences in the intercept (Z = 2.44, p < .01). When optimism, relocation, and their interaction were entered as predictors of this variance, only the expected interaction between dispositional optimism and relocation emerged as significant, γ = −5.46 (SE = 2.40), t (42) = −2.26, p < .03, η = .33 (see Figure 1). Among students who relocated to go to law school, dispositional optimism was associated with larger DTH responses, γ = 3.17 (SE = 2.58); among students who did not relocate, dispositional optimism was associated with smaller DTH responses, γ = −3.98 (SE = 1.91). The three-way interaction with time was not statistically significant (p < .06, η = .15) but suggested that the negative relationship between optimism and immune function among students who did not relocate was greater at earlier time points. As in previous studies, then, optimism had a positive relationship with cellular immunity when the stressor was more straightforward, but a negative relationship when the stressor was more complex or difficult.
Figure 1.
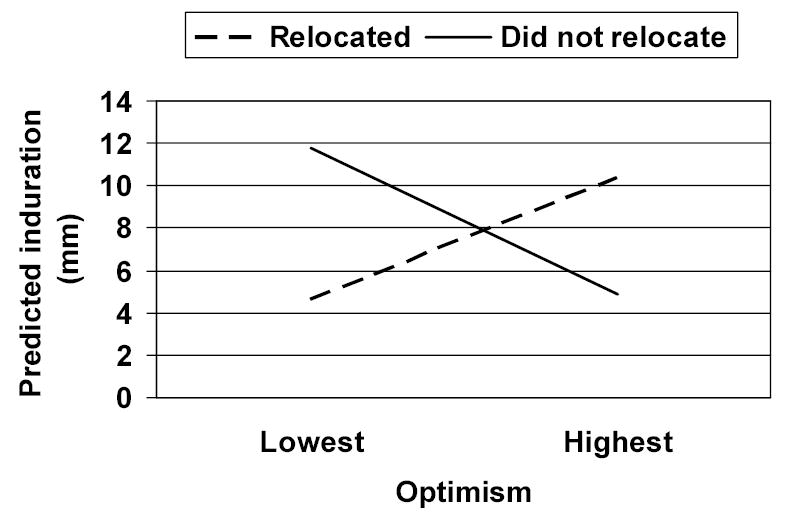
Effects of optimism and relocation on delayed-type hypersensitivity responses
Note. Predicted values from the multi-level model shown across the obtained range of optimism scores from lowest (mean item score = 2.50) to highest (mean item score = 4.83).
The role of affect
The same multilevel model was used to predict each affective category – distress, engaged affect, and fatigue. In particular, if the interaction between relocation and optimism predicting DTH response was due to their joint effects on affect, then the interaction should also predict affect, affect should also predict DTH responses, and the interaction effect should diminish after controlling for affect. The only significant effects were a decrease in engaged affect over time, γ = −.70 (SE = .20), t (166) = −3.50, p < .001, η = .22, and a three-way interaction between time, optimism, and relocation on fatigue, γ = 1.05 (SE = .39), t (166) = 2.71, p < .01, η = .21. In this interaction, less optimistic students who relocated had the largest increase in fatigue over time, illustrated by predicted values rising from 5.8 at baseline to 24.5 during finals week. More optimistic students who relocated had the largest decrease over time, illustrated by predicted values falling from 10.7 at baseline to −2.8 at finals week. (Note that predicted values can fall slightly outside of the actual scale range.)
Each affective category was then added to the DTH model. There was evidence that affect was related to DTH responses. When distress was added to the model, it tended to predict lower cellular immunity, γ = −.24 (SD = .14), t (158) = −1.69, p < .09, η = .13. When engaged affect was added to the model, it significantly predicted higher cellular immunity, γ = .24 (SD = .11), t (158) = 2.25, p < .03, η = .18. Finally, when fatigue was added to the model, it significantly predicted lower cellular immunity, γ = −.31 (SD = .16), t (158) = −1.98, p < .05, η = .16. However, because the interaction between optimism and relocation was generally unrelated to affect, its effect on DTH continued unchanged after including affect, η = .30 − .33. Likewise, the near-significant interaction among optimism, relocation, and time not only failed to decrease but increased slightly after including affect, η = .16 – .19 . Therefore, although affect predicted cellular immune responses, all categories of affect failed to account for the differences in cellular immunity arising from the interaction between optimism and relocation.
Effects of disappointment: First-semester grades
Although the disappointment model posits that optimists are distressed by any stressor quality that prevents their easy realization of positive outcomes (e.g., uncontrollability), the first semester of law school allows for the examination of an explicit and concrete disappointment in the release of first-semester grades at the beginning of the second semester. The sample held high expectations for their performance: During finals week, 95% of the sample hoped to be in the top half of the class; 83% thought they would be in the top half of the class; and 73% would be satisfied with ranking no lower than the top half of the class. However, their actual performance was consistent with their representativeness: 25% ranked in the top quartile of the class, 44% in the top half, and 80% in the top 3 quartiles. Dispositional optimism was associated with higher expectations (r = −.32, p < .05 for hoped-for rank, r = −.36, p < .05 for predicted rank, and −.20, p < .22 for lowest satisfactory rank), but not with the difference between predicted rank and actual rank (r = .02), so that optimists performed below their expectations no more or less often than pessimists. If optimists were more vulnerable than pessimists to underperformance and expectancy violation, one would expect to see particular evidence of this relationship at the fourth and fifth time points. However, optimism did not significantly interact with time, F < 1, η = .04, suggesting that optimistic expectancies did not increase vulnerability to immunosuppression after disappointing outcomes.
Discussion
Although optimism was originally conceptualized as a protective factor with regard to health (Scheier & Carver, 1985), evidence suggests that it is not always protective for the immune system. During stressors that offer few difficulties – that is, they are controllable, brief, responsive, or not conflicting – more optimism predicts higher immune parameters. During more difficult stressors, however, more optimism predicts lower immune parameters. The present study provided yet another example of this interaction. Among law students experiencing low academic-social goal conflict, more optimistic students had higher cellular immunity than their less optimistic counterparts. However, among students experiencing high conflict, this relationship reversed.
The interaction between optimism and stressor difficulty occurs consistently, but explanations of why this effect occurs are less consistent. The disappointment model states that optimists experience distress when their positive expectancies are disconfirmed by difficult stressors and that this distress suppresses immunity (F. Cohen et al., 1999; Sieber et al., 1992; Tennen & Affleck, 1987), whereas the engagement model states that because optimists tend to engage difficult stressors, they may experience greater effort, fatigue, and physiological stress than their more pessimistic counterparts (Baumeister et al., 1998; Peters et al., 1999; Segerstrom, 2001; Solberg Nes, Segerstrom, & Sephton, 2005). The present study suggests that affective differences between these models – distress in the disappointment model and engaged affect or fatigue in the engagement model – are not sufficient to explain the interaction.
The failure of affect to account for the effects of optimism and stressor difficulty poses a problem for the disappointment model, which relies heavily on negative affect as a mediator. One possibility is that the affect measure was insufficiently specific with regard to the kinds of affect that optimists experience on encountering a difficult stressor. Although the disappointment model suggests the experience of broadly defined negative affect, failure to make progress toward goals can specifically elicit frustration and annoyance, which were not measured in this study but are alternative candidates for affective mediation (Carver, 2004). On the other hand, the disappointment model failed an explicit test: Any negative effect of optimism on immunity was not more pronounced after the release of first-semester grades. As in previous work (Litt et al., 1992; Helgeson, 2003; Stanton & Snider, 1993), expectancy violation does not appear to be a major source of vulnerability for optimists.
The engagement model relies less highly on affect as a mediator and therefore remains a plausible model for optimism’s effects during difficult stressors. Motivational states, such as engagement, or their consequences, such as effort, are not always well reflected by affect. For example, experimental depletion of self-regulatory or “ego” strength causes a noticeable decrease in subsequent self-regulatory behavior, such as persistence on problem-solving tasks. Although depletion can result in fatigue, marked depletion has been found in the absence of fatigue or any other affective change (Schmeichel, Vohs, & Baumeister, 2003). Slightly different operationalization of fatigue (e.g., worn out, exhausted vs. sleepy, tired) might have yielded somewhat more promising results for fatigued affect. Nonetheless, further indication that optimism’s effects during difficult stressors are not due to affect comes from the mental arithmetic study, in which conscientiousness, which is more strongly related to motivation and behavior than to affect, could replicate the interaction between optimism and stressor difficulty (Segerstrom et al., 2003). These findings converge to suggest that affect, although a potential influence on immunity, is not the only such influence. Future research should explore other potential mediators such as self-regulatory strength and energy expenditure; in addition, relocation was used as a proxy for goal conflict in this study, but future research could measure aspects of stressor difficulty, such as conflict, explicitly.
Distress and fatigue were related to lower cellular immunity and engaged affect was related to higher cellular immunity. The failure of these affective dimensions to account for the optimism and stressor difficulty effect was not, therefore, because they were inert. These findings add to other research that indicates that positive and negative affect are related to immunity, with positive affect having generally enhancing effects on immune function and negative affect having generally suppressing effects. Stone and his colleagues (1987) found that daily mood affected the humoral branch of the immune system, which primarily provides protection against bacterial and parasitic infections. The present findings suggest that the same is true of the cellular branch, which primarily provides protection against viral and some kinds of bacterial infections.
The effects of optimism on immunity are clearly sensitive to the effects of stressor qualities and context. Under most circumstances, it seems that optimism will have the predicted relationship with higher immune parameters. However, a number of studies have demonstrated that difficult stressors have more potentially detrimental effects on the immune systems of more optimistic people. With regard to cellular immunity, it is apparently not always adaptive to expect the best, but neither is it always adaptive to expect the worst. This conclusion, however, should not be generalized to the entire organism: Research in ecological immunology demonstrates that directing resources away from the immune system is adaptive when those resources can serve more important functions (e.g., reproduction or protection of offspring; Barnard & Behnke, 2001). If optimists are more successful in fulfilling difficult but important goals, the short-term physiological costs may even be to their long-term benefit.
Acknowledgments
This study was supported by the National Institute of Mental Health (MH-061531). The author thanks Bann Kang for medical consultation and supervision and Mary Hundley, Jennifer Snedeker, Lise Solberg Nes, and Theresa Spencer for their assistance.
References
- Barnard, C.J., & Behnke, J.M. (2001). From psychoneuroimmunology to ecological immunology: Life history strategies and immunity trade-offs. In R. Ader, D. Felten, & N. Cohen (Eds)., Psychoneuroimmunology (2nd Ed., Vol. 2) (pp. 35–47). San Diego: Academic Press.
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Byrnes DM, Antoni MH, Goodkin K, Efantis-Potter J, Asthana D, Simon T, Manajj J, Ironson G, Fletcher MA. Stressful events, pessimism, natural killer cell cytotoxicity, and cytotoxic/suppressor T cells in HIV+ Black women at risk for cervical cancer. Psychosomatic Medicine. 1998;60:714–722. doi: 10.1097/00006842-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver, C.S., & Scheier, M.F. (1998). On the self-regulation of behavior New York: Cambridge University Press.
- Cohen F, Kearney KA, Zegans LS, Kemeny ME, Neuhaus JM, Stites DP. Differential immune system changes with acute and persistent stress for optimists vs. pessimists. Brain, Behavior, and Immunity. 1999;13:155–174. doi: 10.1006/brbi.1998.0531. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1987). Statistical power analysis for the behavioral sciences: Revised edition Hillsdale, NJ: Lawrence Erlbaum Associates.
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Annals of Behavioral Medicine. 2000;22:171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Fitzgerald TE, Tennen H, Affleck G, Pransky GS. The relative importance of dispositional optimism and control appraisals in quality of life after coronary artery bypass surgery. Journal of Behavioral Medicine. 1993;16:25–43. doi: 10.1007/BF00844753. [DOI] [PubMed] [Google Scholar]
- Helgeson VS. Cognitive adaptation, psychological adjustment, and disease progression among angioplasty patients: 4 years later. Health Psychology. 2003;22:30–38. doi: 10.1037//0278-6133.22.1.30. [DOI] [PubMed] [Google Scholar]
- Janeway, C.A., & Travers, P. (1997). Immunobiology: The immune system in health and disease (3rd ed.). New York: Garland Publishing.
- Litt MD, Tennen H, Affleck G, Klock S. Coping and cognitive factors in adaptation to in vitro fertilization failure. Journal of Behavioral Medicine. 1992;15:171–187. doi: 10.1007/BF00848324. [DOI] [PubMed] [Google Scholar]
- Longfield JN, Margileth AM, Golden SM, Lazoritz S, Bohan JS, Cruess DF. Interobserver and method variability in tuberculin skin testing. Pediatric Infectious Disease. 1984;3:323–326. doi: 10.1097/00006454-198407000-00010. [DOI] [PubMed] [Google Scholar]
- Maier KJ, Waldstein SR, Synowski SJ. Relation of cognitive appraisal to cardiovascular reactivity, affect, and task engagement. Annals of Behavioral Medicine. 2003;26:32–41. doi: 10.1207/S15324796ABM2601_05. [DOI] [PubMed] [Google Scholar]
- Peters ML, Godaert GLR, Ballieux RE, Brosschot JF, Sweep FCGJ, Swinkels LMJW, van Vliet M, Heijnen CJ. Immune responses to experimental stress: Effects of mental effort and uncontrollability. Psychosomatic Medicine. 1999;61:513–524. doi: 10.1097/00006842-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Raikkonen D, Matthews KA, Flory JD, Owens JF, Gump BB. Effects of optimism, pessimism, and trait anxiety on ambulatory blood pressure and mood during everyday life. Journal of Personality and Social Psychology. 1999;76:104–113. doi: 10.1037//0022-3514.76.1.104. [DOI] [PubMed] [Google Scholar]
- Rosenthal, R., & Rosnow, R.L. (1984). Essentials of behavioral research: Methods and data analysis. New York: McGraw-Hill.
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Matthews KA, Owens JF, Schulz R, Bridges MW, Magovern GJ, Carver CS. Optimism and rehospitalization after coronary artery bypass graft surgery. Archives of Internal Medicine. 1999;159:829–835. doi: 10.1001/archinte.159.8.829. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Vohs KD, Baumeister RF. Intellectual performance and ego depletion: Role of the self in logical reasoning and other information processing. Journal of Personality and Social Psychology. 2003;85:33–46. doi: 10.1037/0022-3514.85.1.33. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Perceptions of stress and control in the first semester of law school. Willamette Law Review. 1996;32:593–608. [Google Scholar]
- Segerstrom SC. Optimism, goal conflict, and stressor-related immune change. Journal of Behavioral Medicine. 2001;24:441–467. doi: 10.1023/a:1012271410485. [DOI] [PubMed] [Google Scholar]
- Segerstrom, S.C. (2005). [Likelihood of social but not other extracurricular goals depends on relocation status of law students.] Unpublished raw data.
- Segerstrom SC, Castaneda JO, Spencer TE. Optimism effects on cellular immunity: Testing the affective and persistence models. Personality and Individual Differences. 2003;35:1615–1624. [Google Scholar]
- Sieber WJ, Rodin J, Larson L, Ortega S, Cummings N, Levy S, Whiteside T, Herberman R. Modulation of human natural killer cell activity by exposure to uncontrollable stress. Brain, Behavior, and Immunity. 1992;6:141–156. doi: 10.1016/0889-1591(92)90014-f. [DOI] [PubMed] [Google Scholar]
- Singer, J.D. (2002). Fitting individual growth models using SAS PROC MIXED. In D.S. Moskowitz & S.L. Hershberger (Eds.), Modeling intraindividual variability with repeated measures data: Methods and applications (pp. 135–170). Mahwah, NJ: Lawrence Erlbaum Publishers.
- Singer, J.D., & Willett, J.B. (2003). Applied longitudinal data analysis: Modeling change and event occurrence New York: Oxford University Press.
- Solberg Nes L, Segerstrom SC, Sephton SE. Engagement and arousal: Optimism’s effects during a brief stressor. Personality and Social Psychology Bulletin. 2005;31:111–120. doi: 10.1177/0146167204271319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Snider PR. Coping with a breast cancer diagnosis: A prospective study. Health Psychology. 1993;12:16–23. doi: 10.1037//0278-6133.12.1.16. [DOI] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Stone AA, Cox DS, Valdimarsdottir H, Jandorf L, Neale JM. Evidence that secretory IgA antibody is associated with daily mood. Journal of Personality and Social Psychology. 1987;52:988–993. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- Tennen H, Affleck G. The costs and benefits of optimistic explanations and dispositional optimism. Journal of Personality. 1987;55:377–393. doi: 10.1111/j.1467-6494.1987.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Bachen EA, Manuck SB. Active coping and cardiovascular reactivity: A multiplicity of influences. Psychosomatic Medicine. 1997;59:620–625. doi: 10.1097/00006842-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Watson, D., & Clark, L.A. (1994). The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. Unpublished manuscript, University of Iowa, Iowa City.
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]


