Abstract
The monoterpene d-limonene exhibits chemotherapeutic and chemopreventive potential in breast cancer patients. D-limonene and its related compounds, perillyl alcohol and perillyl aldehyde, were chosen as candidate drugs for application in a screen for nontoxic inhibitors of cell migration. Using the nontumorigenic human breast cell line MCF-10A, we delineated the toxicity as greatest for the perillyl aldehyde, intermediate for perillyl alcohol, and least for limonene. A noncytotoxic concentration of 0.5 mmol/L perillyl alcohol inhibited the migration, while the same concentration of limonene failed to do so. Adhesion of the MCF-10A cell line and the human breast cancer cell line MDA-MB 435 to fibronectin was unaffected by 1.5 mmol/L perillyl alcohol. 0.4 mmol/L perillyl alcohol inhibited the growth of MDA-MB 435 cells. All migration-inhibiting concentrations of perillyl alcohol for MDA-MB 435 cells proved to be toxic. These results suggest that subtoxic doses of perillyl alcohol may have prophylactic potential in the treatment of breast cancer.
INTRODUCTION
Cancer remains the second leading cause of death in the US, American Cancer Society, http://www.cancer.org. Tumor metastasis is the most deadly characteristic of cancer. Unlike primary tumors that can be surgically removed and treated with adjuvant chemotherapy and/or radiotherapy, metastases are difficult to treat and usually prove fatal [1].
The development of secondary tumors is a sequential process, commonly referred to as a “metastatic cascade,” and failure to complete any one step prevents metastasis [1]. During the metastatic cascade, primary tumor cells digest their surrounding extracellular matrix, migrate through interstitial spaces, and enter blood or lymphatic vessels where they are carried to distant organs. Once lodged in the target organs, these cells migrate into the interstitial spaces and continue to grow to develop a secondary tumor, or metastasis [2]. Thus, the migration and invasion of cancer cells provides many potential targets for therapeutic intervention.
Most anticancer drugs target the hyperproliferation of metastatic cells. While many of these drugs are efficacious in treating the beginning stages of cancer, none are curative for metastatic disease. Any delay in the diagnosis also renders many of these drugs ineffective [3]. In addition, antiproliferation compounds cause many adverse side effects, including nausea, vomiting, suppressed immune system, and hair loss. It is, therefore, paramount that alternative therapies be developed to treat a greater scope of the disease with less disturbance to the wellbeing of the patient.
Even though tumor cell migration is a hallmark feature of metastasis, since 1978, fewer than 100 compounds that have some capacity to inhibit tumor cell migration have been reported; however, thousands of compounds have yet to be tested. To address the need for a more comprehensive screen of possible antimigration compounds, we recently, developed an automated high-throughput cytotoxicity/cell migration assay suitable for screening large numbers of samples [4].
During the development of our automated assay, we applied cell proliferation and migration assays to screen drugs, already known to have anticancer effects, for noncytotoxic antimigration properties. This report focuses on the effects of d-limonene and its derivatives. The chemotherapeutic and chemopreventative effects of d-limonene, a monoterpene found in the essential oils of citrus fruits, spices, and herbs, have been studied extensively in spontaneous and chemically induced rodent tumors [5]. Limonene serves as a precursor to other oxygenated monocyclic monoterpenes such as carveol, carvone, menthol, perillyl alcohol (POH), and perillyl aldehyde [6]. Due to success in tumor regression in various rodent cancer models, clinical testing of the cancer chemotherapeutic activity of these compounds is in progress, including Phase I clinical trials of POH in breast cancer patients [7, 8].
D-limonene and its derivatives disrupt isoprenylation of members of the Ras family of G proteins by geranylgeranyl transferases in some instances [9], though the effects of this disruption on downstream cellular behaviors are only now being elucidated. Thus much of the focus far has been on the inhibition of the cell cycle machinery and the induction of apoptosis [10]. The effects of these compounds on migration, especially in breast cells, are unreported to date.
We report here that POH inhibits migration of both malignant and nonmalignant human breast cells, and that migration inhibiting doses also prove cytotoxic in malignant cells. These results suggest that subtoxic doses of POH may act as a preventative treatment for breast cancer.
MATERIALS AND METHODS
Materials
MCF-10A and MDA-MB 435 cells were purchased from American Type Culture Collection. Fibronectin, epidermal growth factor, cholera toxin, and insulin were obtained from Calbiochem (La Jolla, Calif). Hydrocortisone was purchased from Sigma-Aldrich Chemicals (St. Louis, Mo). Horse serum was purchased from Irvine Scientific (Santa Ana, Calif). Calcein-AM was purchased from Molecular Probes (Eugene, Ore). Transwell migration plates were purchased from Costar (Cambridge, Mass). Cell culture flasks and 96-well plates were purchased from VWR (Plainfield, NJ). Limonene, POH, and perillyl aldehyde were purchased from Aldrich Chemical Co. (Milwaukee, Wis).
Cell culture
Cells were grown in 75 cm2 Falcon tissue culture flasks obtained from VWR (Plainfield, NJ). Cells were maintained at 37°C and 5% CO2 in humidified chambers. MCF-10A cells were maintained in AM media—1:1 mixture of Ham's F-12 medium and Dulbecco's Modified Eagle's Medium High Glucose with 2 mmol/L L-glutamine from Irvine Scientific (Santa Ana, Calif, USA) supplemented with the following: epidermal growth factor (20 ng/mL), cholera toxin (100 ng/mL), insulin (0.01 mg/mL), hydrocortisone (500 ng/mL), and 5% horse serum. MDA-MB 435 cells were maintained in RPMI Medium 1640 supplemented with 10% fetal bovine serum and 1% L-glutamine (29.2 mg/mL), penicillin G (10 000 units/mL) and streptomycin sulfate (10 000 μg/mL) from Irvine Scientific (Santa Ana, Calif). Cells were routinely passaged using trypsin/EDTA from Irvine Scientific (Santa Ana, Calif).
Proliferation assay
In 96-well plates, 30 × 103 cells were seeded in the appropriate serum-containing media and incubated for 2 hours at 37°C and 5% CO2 in humidified chambers. Cells were then washed twice with PBS and grown in migration media (MCF-10A: DMEM and 0.1% BSA; MDA-MB 435: RPMI 1640 and 0.1% BSA) with indicated concentrations of limonene, perillyl alcohol, or perillyl aldehyde for 24 hours. Cells were then washed twice with PBS, fixed in 3.7% formaldehyde for 15 minutes, stained with crystal violet and lysed with 1% sodium docecyl sulfate (SDS). Absorbance was read at 595 nm using a plate reader. Growth was measured as compared to a standard curve of cells grown in serum-containing media.
Adhesion assay
Cell adhesion assays were performed as previously described in Costar 96-well Cell Culture Cluster plates [11]. Tissue culture plates were coated with purified fibronectin (20 μg/mL in 50 mmol/L carbonate buffer, pH 9.3) for 1 hour at room temperature. Wells were then washed with phosphate-buffered saline with 0.2% Tween-20 (PBST), blocked with 5% blotto (PBST with 5% skim milk) for 1 hour at 25°C. Wells were then washed twice with PBST prior to assay. Cells were seeded at a concentration of 1.0 × 106 per well in cell culture plates with and without migration media (MCF-10A: DMEM and 0.1% BSA; MDA-MB 435: RPMI 1640 and 0.1% BSA) and perillyl alcohol and were allowed to attach for 30 minutes at 37°C in a humidified incubator containing 5% CO2. Unbound cells were dislodged by inverting, submerging and rocking the plate in PBS for 15 minutes. Cells were then fixed in 3.7% formaldehyde, stained with crystal violet, and lysed with 1% SDS. Absorbance was measured at 595 nm in a plate reader.
Migration assay
Cell migration assays were performed as previously described in Costar transwell filter plates [6]. Filters were coated with purified fibronectin at a concentration of 20 μg/mL in carbonate buffer (50 mmol/L, pH 9.3) for 1 hour at room temperature. Filters were then aspirated and blocked in blotto (phosphate-buffered saline with 0.2% Tween-20 (PBST) and 5% skim milk) for 1 hour at room temperature. Next, filters were washed in PBST prior to the assay. Thirty minutes prior to starting the assay, cells were pre-incubated at 37°C in migration media and the indicated concentrations of POH. Cells were seeded at a concentration of 1.2 × 106/well on transwell filters with and without fibronectin in the presence or absence of soluble growth factors (serum-enriched media) and varying concentrations of POH and allowed to migrate for 18 hours at 37°C in 5% CO2. Thirty minutes before measuring the migration, 5 μmol/L of calcein-AM (Molecular Probes; Eugene, Ore) was added to the migration wells. To quantify the migration, cells were removed from the top of the filter with cotton swabs, washed in phosphate-buffered saline and the fluorescence measured from the bottom of the filter with a plate reader for the incorporation of calcein-AM [7]. Relative fluorescence values for each experimental condition were expressed relative to control untreated samples.
RESULTS AND DISCUSSION
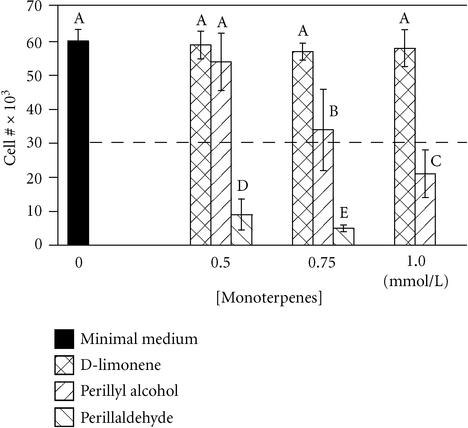
D-limonene and its derivatives, POH and perillyl aldehyde, exhibited different degrees of toxicity in a 24-hour growth assay. D-limonene did not inhibit growth of MCF-10A cells at concentrations up to 1.0 mmol/L (Figure 1) or greater (not shown), while 0.5 mmol/L perillyl aldehyde was completely toxic. In contrast, 0.5 mmol/L POH was not cytotoxic, but concentrations of 0.75 mmol/L and 1.0 mmol/L were cytostatic and cytotoxic, respectively. The relatively high toxicity of perillyl aldehyde may be due to a proportionally high affinity for the enzymes farnesyl transferase (FT) and geranylgeranyl transferase (GGT) as has been shown for perillic acid methyl ester, a minor metabolite of limonene, and POH [12]. These enzymes are involved in post-translational modification of small G-proteins that are involved in a myriad of cell activities such as growth and migration. Inhibition of FT and GGT is suspected to be the basis of the antitumor effects of POH and limonene [12]. However, in accord with our data, an equal concentration of limonene is not as effective as POH in blocking cell proliferation in other cell types [12, 13].
Figure 1.
Monoterpenes exhibit varying degrees of inhibition of breast cell growth. MCF-10A cells were plated at 30 × 103 cells/well (dashed line) in serum-free minimal medium containing the indicated concentrations of monoterpenes, and counted 24 hours later. Bars represent mean ± standard deviation (n = 10). A student-Numan-Keuls test identified five groups (letters A-E; P ≤ .05) which were statistically different from one another (ANOVA F:[9, 98] = 121.63, P < .0001).
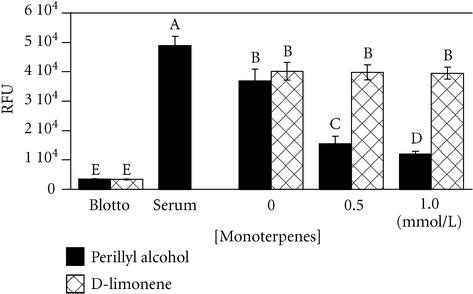
Migration assays established that, a subtoxic dose (0.5 mmol/L) of POH inhibited haptotactic migration of MCF-10A cells on fibronectin (Figure 2). Again, this difference is most likely attributable to POH, having a greater potency than limonene in the inhibition of small G-protein isoprenylation [13]. A probable mediator of this inhibited migration is the small G-protein RhoA, a member of the Rho family of small GTPases, that is involved in signaling a pathway for cell migration [13, 14].
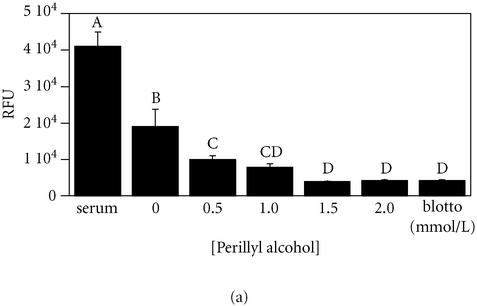
Figure 2.
Perillyl alcohol, but not d-limonene, inhibits breast cell migration. MCF-10A cells were allowed to migrate towards the bottom side of an 8- μm pore filter coated with fibronectin for 18 hours in serum-free minimal medium containing the indicated concentrations of monoterpenes, or serum as a control. Migration towards nonfat dried milk (blotto) served as a negative control for random migration. Migration was quantitated by fluorescently labeling cells on the bottom of the filters and reading the fluorescence with a plate reader. Results expressed as mean of Relative Fluorescence Units, ± standard deviation (n = 3). A student-Numan-Keuls test identified five groups (letters A-E; P ≤ .05) which were statistically different from one another (ANOVA F:[7, 23] = 231.76, P < .0001).
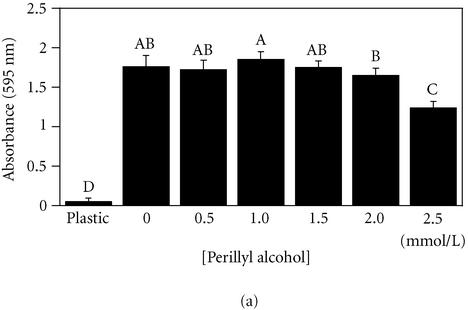
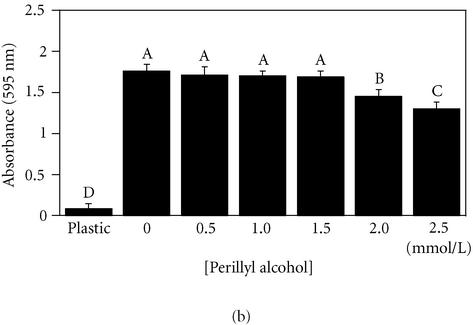
To test the effect of POH on adhesion to fibronectin, both noncancerous (MCF-10A) cells and cancerous (MDA-MB 435) cells were exposed for 18 hours to increasing concentrations of POH prior to a 30-minute adhesion assay. Both MCF-10A and MDA-MB 435 cell lines tolerated up to 1.5 mmol/L POH with no statistically significant variation compared to 0 mmol/L (Figure 3). The treated cells exhibited rounding and a loss of cytoskeletal organization, but remained viable as determined by Trypan Blue exclusion and MTT reduction assays (Wagner et al, unpublished data, 2000). These results, independently, confirm the low toxicity of POH, and suggest that POH may inhibit the migration by disrupting the cytoskeletal machinery required to exert the necessary force for lamellar extension. Integrin mediated adhesion, however, remained unaffected.
Figure 3.
Perillyl alcohol (≤ 1.5 mmol/L) does not interfere with breast cell adhesion to fibronectin. MCF-10A cells (a) or MDA-MB-435 cells (b) were plated on fibronectin for 30 minutes in the presence of the indicated concentrations of perillyl alcohol. Adherent cells were quantitated by staining with crystal violet, solubilizing adherent dye, and measuring absorbance at 595 nm. Results expressed as mean ± standard deviation (n = 8). A student-Numan-Keuls test identified in (a) two partly overlapping groups (letters A and B) and two independent groups (letters C and D); and in (b), four independent groups (letters A-D), P ≤ .05. All groups are statistically different from one another (panel (a), ANOVA F:[6,55] = 144.88, P < .0001; panel (b), ANOVA F:[6, 41] = 252.45, P < .0001).
To, directly, assess the dose response to POH treatment, growth and migration assays were performed in parallel for both cell lines. In MCF-10A cells, 0.5 mmol/L POH inhibited the migration by over 75% and allowed the cell growth, although at a lower rate (Figure 4). In contrast, in MDA-MB 435 cells, this dose inhibited the migration by over 85% but also reduced the cell number by nearly 20%. In fact, all concentrations of POH that inhibited migration were cytotoxic in this cell line (Figure 5). This may be because this cell line expressing a higher level of RhoA, as has been reported in other tumor cells as compared to nontumorigenic cells [15]. An increased invasive phenotype is also characteristic of some liver and breast tumor cells in response to RhoA activation [15, 16]. Additionally, POH treatment significantly increases apoptosis in pancreatic cancer cells relative to nonmalignant pancreas cells [17]. Thus, POH may exert its effects through separate mechanisms in MCF-10 and MDA-MB 435 cells.
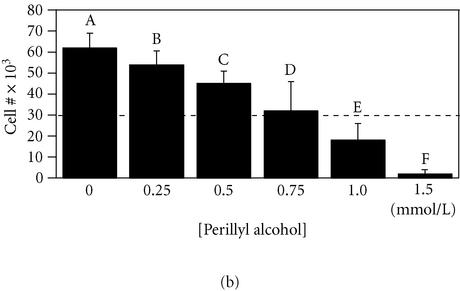
Figure 4.
0.5 mmol/L perillyl alcohol is a noncytotoxic inhibitor of MCF-10A cell migration on fibronectin. Parallel measurements of cell migration (panel (a)) and cell growth (panel (b)) were performed as described for Figures 1 and 2 using MCF-10A cells exposed to the indicated concentrations of perillyl alcohol in a serum-free minimal medium. For panel A, a student-Numan-Keuls test identified four groups, two of which overlapped (letters A–D; P ≤ .05). For panel (b), a student-Numan-Keuls test identified six groups (letters A–F; P ≤ .05). All groups in each panel were statistically different from one another (panel (a), ANOVA F:[6, 20] = 33.80, P < .0001; panel (b), ANOVA F:[7, 79] = 253.58, P < .0001).
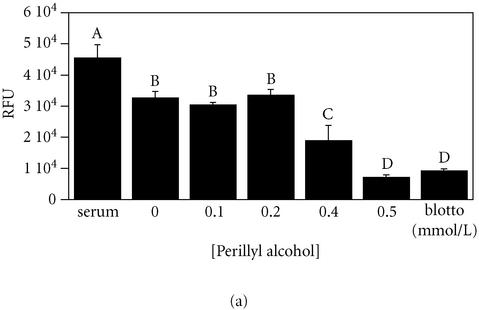
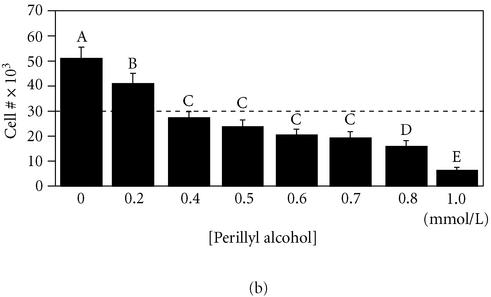
Figure 5.
Perillyl alcohol is a cytotoxic inhibitor of MDA-MB-435 cell migration on fibronectin. Parallel measurements of cell migration (panel (a)) and cell growth (panel (b)) were performed as described for Figures 1 and 2 using MDA-MB-435 cells exposed to the indicated concentrations of perillyl alcohol in serum-free minimal medium. For panel (a), a student-Numan-Keuls test identified four groups (letters A–D; P ≤ .05). For panel (b), a student-Numan-Keuls test identified five groups (letters A–E; P ≤ .05). All groups in each panel were statistically different from one another (panel (a), ANOVA F:[6, 23] = 33.80, P < .0001; panel (b), ANOVA F:[7, 80] = 56.08, P < .0001).
Given that a noncytotoxic dose of POH can inhibit the migration of noncancerous breast cells, and the same dose can kill cancerous breast cells, this suggests the possibility that POH may be used as a prophylactic treatment for patients at risk of developing breast cancer. Such a strategy is currently being used with the anti-estrogen drug, tamoxifen [18]. Further studies are under way in our laboratory to explore the effects of low doses of POH on tumor progression.
Acknowledgments
ACKNOWLEDGMENTS
We wish to thank J. Steven de Belle for assistance with statistical analysis. This work was supported by a career development grant DAMD17-98-1-8325 to GEP from the United States Army Breast Cancer Research Program.
References
- Alison M, Sarraf C. Cambridge University Press; London: 1997. Understanding Cancer. [Google Scholar]
- Liotta L A. Tumor invasion and metastases—role of the extracellular matrix: Rhoads memorial award lecture. Cancer Res. 1986;46(1):1–7. [PubMed] [Google Scholar]
- Kohn E C, Liotta L A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55(9):1856–1862. [PubMed] [Google Scholar]
- Rust W L, Huff J L, Plopper G E. Screening assay for promigratory/antimigratory compounds. Anal Biochem. 2000;280(1):11–19. doi: 10.1006/abio.2000.4510. [DOI] [PubMed] [Google Scholar]
- Crowell P L, Gould M N. Chemoprevention and therapy of cancer by d-limonene. Crit Rev Oncog. 1994;5(1):1–22. doi: 10.1615/critrevoncog.v5.i1.10. [DOI] [PubMed] [Google Scholar]
- Crowell P L. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129(3):775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- Vigushin D M, Poon G K, Boddy A, et al. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Chemother Pharmacol. 1998;42(2):111–117. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- Phillips L R, Malspeis L, Supko J G. Pharmacokinetics of active drug metabolites after oral administration of perillyl alcohol, an investigational antineoplastic agent, to the dog. Drug Metab Dispos. 1995;23(7):676–680. [PubMed] [Google Scholar]
- Ren Z, Elson C E, Gould M N. Inhibition of type I and type II geranylgeranyl-protein transferases by the monoterpene perillyl alcohol in NIH3T3 cells. Biochem Pharmacol. 1997;54(1):113–120. doi: 10.1016/s0006-2952(97)00151-2. [DOI] [PubMed] [Google Scholar]
- Clark S S, Perman S M, Sahin M B, Jenkins G J, Elegbede J A. Antileukemia activity of perillyl alcohol (POH): uncoupling apoptosis from G0/G1 arrest suggests that the primary effect of POH on Bcr/Abl-transformed cells is to induce growth arrest. Leukemia. 2002;16(2):213–222. doi: 10.1038/sj.leu.2402369. [DOI] [PubMed] [Google Scholar]
- Plopper G E, Domanico S Z, Cirulli V, Kiosses W B, Quaranta V. Migration of breast epithelial cells on Laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res Treat. 1998;51(1):57–69. doi: 10.1023/a:1006086218174. [DOI] [PubMed] [Google Scholar]
- Gelb M H, Tamanoi F, Yokoyama K, Ghomashchi F, Esson K, Gould M N. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 1995;91(2):169–175. [Google Scholar]
- Crowell P L, Ren Z, Lin S, Vedejs E, Gould M N. Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol. 1994;47(8):1405–1415. doi: 10.1016/0006-2952(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Kjoller L, Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999;253(1):166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999;59(8):2004–2010. [PubMed] [Google Scholar]
- Bourguignon L Y, Zhu H, Shao L, Zhu D, Chen Y W. Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43(4):269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stayrook K R, McKinzie J H, Burke Y D, Burke Y A, Crowell P L. Induction of the apoptosis-promoting protein Bak by perillyl alcohol in pancreatic ductal adenocarcinoma relative to untransformed ductal epithelial cells. Carcinogenesis. 1997;18(8):1655–1658. doi: 10.1093/carcin/18.8.1655. [DOI] [PubMed] [Google Scholar]
- Duffy S W, Nixon R M. Estimates of the likely prophylactic effect of tamoxifen in women with high risk BRCA1 and BRCA2 mutations. Br J Cancer. 2002;86(2):218–221. doi: 10.1038/sj.bjc.6600064. [DOI] [PMC free article] [PubMed] [Google Scholar]