Abstract
Body dysmorphic disorder (BDD) is a relatively common and impairing disorder. However, little is known about non-BDD symptoms and well-being in patients with this disorder. Seventy-five outpatients with DSM-IV BDD completed the Symptom Questionnaire, a validated self-report measure with four scales: depression, anxiety, somatic/somatization, and anger-hostility. Scores were compared to published norms for normal subjects and psychiatric outpatients. Participants in an open-label fluvoxamine trial completed the Symptom Questionnaire at baseline and endpoint. Compared to normal controls, BDD subjects had markedly elevated scores on all four scales, indicating severe distress and psychopathology. Compared to psychiatric patients, BDD subjects had higher scores on the depression, anxiety, and anger/hostility scales but not on the somatic/somatization scale. Scores on all scales significantly decreased with fluvoxamine. In conclusion, patients with BDD have markedly high levels of distress, are highly symptomatic, and have poor well-being in the domains of depression, anxiety, somatic symptoms, and anger-hostility. All of these symptoms significantly improved with fluvoxamine.
Keywords: body dysmorphic disorder, depression, anxiety, hostility, somatic symptoms
Body dysmorphic disorder (BDD), also known as dysmorphophobia, is a distressing and relatively common disorder (1–4). BDD frequently causes impaired functioning, such as social isolation and difficulties in occupational and academic functioning (1,2). Patients with BDD also have notably poor mental health-related quality of life (5) and high lifetime rates of psychiatric hospitalization (48%), suicidal ideation (45%–82%), and suicide attempts (22%–24%) (1,6). However, relatively little is known about non-BDD symptoms and well-being in patients with BDD.
Regarding non-BDD symptoms in patients with this disorder, BDD pharmacotherapy studies have reported mild to moderate depressive symptoms on the Hamilton Depression Rating Scale (HAM-D) (7,8) and the MADRS (8). A report of patients who were treated with medication, intensive cognitive behavioral therapy, and rehabilitation in a residential treatment setting found that BDD patients had higher pre-treatment scores on the HAM-D and Hamilton Anxiety (HAM-A) scale than patients with obsessive compulsive disorder (OCD) treated in the same setting (9); BDD patients’ HAM-A scores were in the moderate to severe range. In a small open-label BDD fluvoxamine study (n = 12) by Perugi and colleagues, on the self-report Hopkins Symptom Checklist-90, subjects reported that they were distressed by depression “moderately” to “quite a bit” and by anxiety “a little bit” to “moderately” (10). BDD patients also have elevated levels of perceived stress, with scores that are notably higher than those in a large national probability sample and in most normal, medical, and psychiatric samples (11).
In this study, we assessed symptoms and well-being in the domains of depression, anxiety, somatic/somatization, and anger-hostility using the Symptom Questionnaire, a widely used, reliable, and valid measure (12). To our knowledge, only one small (n = 12) study has assessed anger or hostility in patients with BDD, finding that subjects were distressed by anger-hostility “a little bit” to “moderately.” Case reports suggest that at least some patients have high levels of anger that may even culminate in violence—for example, toward physicians who provide treatment (e.g., surgery) with which the patient is dissatisfied (13,14). In addition, to our knowledge, somatic/somatization symptoms have been investigated in a sample ascertained for BDD only in the previously noted Perugi study, in which subjects were distressed by somatization symptoms “a little bit.” It seems important to further study somatization in patients with BDD, given that BDD is classified in DSM-IV and ICD-10 as a somatoform disorder.
In light of the above findings and our clinical experience, we hypothesized that BDD patients would have relatively high levels of depressive and anxiety symptoms. We also predicted that BDD patients would have elevated levels of somatic symptoms compared to normal controls but not compared to psychiatric controls, even though BDD is classified as a somatoform disorder. This hypothesis was based primarily on a previous study of patients ascertained for major depression (atypical subtype) which found that levels of somatic symptoms in depressed patients who also had BDD were similar to those of depressed patients without BDD (15). We hypothesized that BDD patients would also have relatively high anger-hostility scores, based on Perugi’s finding as well as a study in a larger sample (n = 100) that used the NEO-Five Factor Inventory, which found that BDD patients scored in the low-average range for the personality trait of agreeableness (16). In addition, we hypothesized that subjects with more severe BDD symptoms and those who were more delusional would have higher Symptom Questionnaire scores, indicating more severe distress and psychopathology. Finally, we hypothesized that Symptom Questionnaire scores would decrease in patients treated with fluvoxamine, consistent with studies reporting treatment-related improvement on this scale in patients with other psychiatric disorders and symptoms (12,17). In Perugi’s study, fluvoxamine treatment was associated with significant improvement in distress due to depression; distress due to anxiety, anger-hostility, and somatization also improved although not to a statistically significant degree, perhaps because of the very small sample size.
METHODS
Subjects
The study sample consisted of 75 consecutive outpatients with DSM-IV BDD, a distressing or impairing preoccupation with an imagined or slight defect in appearance. Thirty seven (49.3%) subjects were female, and 38 (50.7%) were male. The mean age was 31.7 ± 10.9 years. Subjects with appearance preoccupations that were delusional (delusional disorder, somatic type) were included in the study because available data indicate that BDD’s delusional and nondelusional forms are variants of the same disorder (18). In addition, according to DSM-IV, delusional individuals may receive diagnoses of both delusional disorder and BDD. The most common current comorbid diagnoses were major depression (61.3%, n = 46), social phobia (25.3%, n = 19), and OCD (18.7%, n = 14). An institutional review board approved the study, and all subjects provided written informed consent.
Forty eight (64.0%) of the subjects participated in an ongoing interview (phenomenology) study of BDD’s clinical features, for which the only inclusion/exclusion criterion was the presence of current DSM-IV BDD; this study has been described in greater detail elsewhere (1,18). Twenty-seven (36.0%) subjects participated in a 16-week open-label fluvoxamine treatment study in BDD (8). The fluvoxamine study inclusion and exclusion criteria were standard for a pharmacotherapy efficacy trial and are reported in detail elsewhere (8). In brief, inclusion criteria included the presence of DSM-IV BDD or its delusional disorder variant for at least 6 months, age 18–65, and a minimum score of 5 on the first three items of the BDD-YBOCS (19; see below). Exclusion criteria included unstable medical illness, recent clinically significant suicidality, initiation of psychotherapy or behavior therapy within 3 months before baseline, current or recent substance abuse or dependence, and lifetime history of bipolar disorder type I, schizophrenia, or dementia. During the study, subjects did not begin psychotherapy or receive any other psychotropic medication except chloral hydrate 0.5–2.0 gm/day if needed for insomnia. Fluvoxamine was begun at 50 mg/day and titrated to a maximum dose of 300 mg/day.
Assessments
Symptom Questionnaire
All subjects completed the Symptom Questionnaire (12), a 92-item yes/no and true/false self-report questionnaire consisting of state scales for the domains of depression, anxiety, somatic symptoms/somatization, and anger-hostility. Each of these four scales has two subscales: one sub-scale evaluates symptoms, and the other evaluates well-being. The sub-scales are the following: 1) Depression: depressive symptoms and contented; 2) Anxiety: anxiety symptoms and relaxed; 3) Somatic/ somatization: somatic symptoms and somatic well-being; and 4) Anger-hostility: anger-hostility symptoms and friendly. Higher scores on the symptom subscales indicate greater symptom severity, whereas higher scores on the well-being subscales indicate greater well-being. Higher total scores for each domain indicate greater distress in that domain (12). Published normative data for domain scores, as well as for symptom and well-being subscales, are available for normal controls (i.e., randomly chosen employees) and for nonpsychotic psychiatric outpatients (12,20). Domain scores between one and two standard deviations above the mean for normal control subjects suggest the presence of moderate distress; scores above two standard deviations suggest the presence of substantial or severe distress or psychopathology (12).
The Symptom Questionnaire has been widely used and extensively validated (12). It is reliable and highly sensitive in discriminating distress levels in various groups of patients and in psychiatric patients versus normals (12). It is also sensitive to change with treatment; for example, it discriminates between active drug and placebo in clinical trials (12).
Other Measures
Current severity of BDD symptoms was assessed with the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) (19). This scale is a reliable and valid 12-item semistructured clinician-administered measure. It assesses obsessional preoccupation with the perceived appearance defect, associated repetitive behaviors, delusionality, and avoidance. Higher scores indicate more severe BDD symptoms. The current delusionality of appearance-related beliefs (i.e., how convinced subjects are that their appearance is abnormal and how fixed their belief is) was assessed with the BDD-YBOCS delusionality item. A higher score on this item indicates the presence of greater delusionality (i.e., greater conviction and fixity). All subjects were evaluated with the Structured Clinical Interview for DSM-III-R (SCID-P) (21) to assess comorbidity. BDD was diagnosed with a reliable semistructured SCID-like diagnostic instrument based on DSM-IV criteria for BDD (22).
Study Procedures
Phenomenology study participants were assessed with the above instruments on one occasion. Fluvoxamine study participants were assessed with all instruments before receiving medication, and were reassessed with the Symptom Questionnaire and the BDD-YBOCS at study termination.
Statistical Analysis
Symptom Questionnaire mean ± standard deviation scores were calculated (total scores, symptom subscale scores, and well-being subscale scores). These scores were compared to published norms for a normal control sample (n = 50) and a psychiatric outpatient sample (n = 44) using a standardized difference score (d score). Multivariate analysis of variance (MANOVA) with the Hottlelling trace was used to analyze the relationship between Symptom Questionnaire scores and gender, which was followed by univariate post-hoc tests if indicated. The relationship between Symptom Questionnaire scores, BDD severity, and degree of delusionality was examined using the Pearson product-moment correlation. For subjects who participated in the fluvoxamine study, baseline and termination scores on the Symptom Questionnaire were compared using paired samples t-tests (n = 18). All tests were two tailed; an alpha level of .05 was used to determine statistical significance.
RESULTS
As predicted, BDD subjects’ scores on all four Symptom Questionnaire scales were notably higher than scores for normal controls (see Table 1). Scores on all symptom subscales were higher than for normal controls, whereas scores on all well-being subscales were lower than for normal controls. Effect sizes (d scores) were very large for all comparisons.
TABLE 1.
Symptom Questionnaire Scores in BDD Subjects and Normative Samples
| Symptom Questionnaire scales | BDD subjects(n = 75) | Normal subjects(n = 50) | d scores: BDD vs. normal subjectsa | Psychiatric outpatients (n = 44) | d scores: BDD vs. psychiatric outpatientsa |
|---|---|---|---|---|---|
| Depression | 16.57 ± 6.42 | 2.56 ± 2.87 | 2.64 | 13.90 ± 7.56 | 0.39 |
| Depression symptoms | 12.41 ± 4.92 | 1.77 ± 2.16 | 2.63 | 10.60 ± 5.90 | 0.34 |
| Contented | 1.84 ± 2.05 | 5.21 ± 1.11 | 1.94 | 2.70 ± 2.16 | 0.41 |
| Anxiety | 16.53 ± 5.21 | 3.84 ± 3.87 | 2.70 | 14.81 ± 7.00 | 0.29 |
| Anxiety symptoms | 11.81 ± 4.33 | 2.54 ± 2.85 | 2.43 | 11.24 ± 5.52 | 0.12 |
| Relaxed | 1.28 ± 1.77 | 4.70 ± 1.47 | 2.06 | 2.53 ± 1.95 | 0.68 |
| Somatic | 11.00 ± 6.18 | 4.49 ± 4.14 | 1.19 | 11.20 ± 6.92 | 0.03 |
| Somatic symptoms | 6.88 ± 4.94 | 2.82 ± 2.91 | .96 | 7.56 ± 5.47 | 0.13 |
| Somatic well-being | 1.88 ± 1.91 | 3.88 ± 1.76 | 1.08 | 2.27 ± 1.97 | 0.20 |
| Anger-hostility | 13.31 ± 6.61 | 3.90 ± 3.79 | 1.66 | 9.63 ± 6.91 | 0.55 |
| Hostility symptoms | 10.00 ± 5.42 | 3.43 ± 3.32 | 1.40 | 8.00 ± 5.88 | 0.36 |
| Friendly | 2.69 ± 2.22 | 5.53 ± 1.06 | 1.54 | 3.37 ± 1.67 | 0.33 |
A d score of .2 is considered small, .5 is medium, and .8 is large.
The pattern of results was generally similar for BDD patients versus psychiatric outpatient norms on the depression, anxiety, and anger-hostility scales (see Table 1). However, the effect sizes were generally small to medium, and were smaller than for BDD patients versus normal controls. On the somatic scale, in contrast, BDD patients’ scores were similar to those of psychiatric outpatient norms.
Scores on all four Symptom Questionnaire scales were significantly positively correlated with BDD severity: depression: r = .60, p < .001; anxiety: r = .32, p = .006; somatic: r = .27, p = .023; and anger-hostility: r = .41, p < .001. Greater delusionality was significantly positively correlated with depression (r = .49, p < .001), anxiety (r = .24, p = .047), and anger-hostility (r = .34, p = .004). MANOVA revealed a gender effect (F (4, 70) = 2.70, p = .037), with males scoring higher than females on all scales except the somatic scale, although post-hoc univariate tests did not show a significant gender difference on any individual scale.
As previously reported, fluvoxamine treatment led to significant improvement in BDD symptoms as assessed by the BDD-YBOCS (t = 6.7, df = 29, p < .001) (8). Also as previously reported, delusional patients were as likely as nondelusional patients to have response of BDD symptoms to fluvoxamine, and delusionality significantly improved in both delusional and nondelusional subjects (23). Regarding the Symptom Questionnaire, all scores on this scale significantly decreased with fluvoxamine treatment: depression (t = 4.3, df = 17, p < .001), anxiety (t = 6.2, df = 17, p < .001), somatic/somatization (t = 4.6, df = 17, p < .001), and anger-hostility (t = 4.3, df = 17, p < .001) (See Figure 1).
FIGURE 1.
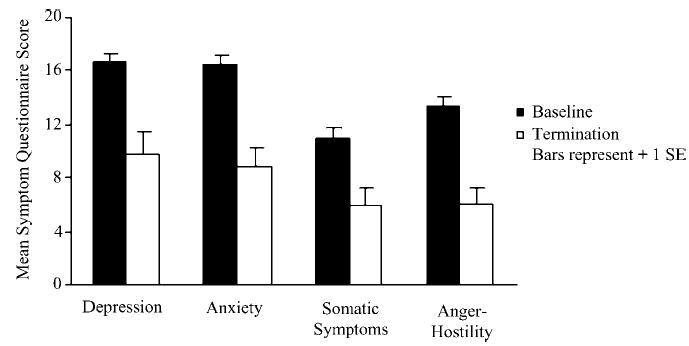
Change in Symptom Questionnaire scores with fluvoxamine treatment.
DISCUSSION
This study found, as predicted, that patients with BDD have high levels of distress, are highly symptomatic, and have poor well-being as assessed by the Symptom Questionnaire. Total scores for depression, anxiety, and anger-hostility were more than two standard deviations above the means for normal controls, suggesting substantial/severe distress and psychopathology. The total score for the somatic/somatization domain was one to two standard deviations above the mean for normal subjects, suggesting moderate distress and psychopathology. Total domain scores for depression, anxiety, and anger-hostility were also higher than those reported for psychiatric outpatient controls. Of note, depression scores (as well as scores in the other domains) were generally comparable to those reported for outpatients with major depression in a depression research program (17).
The somatic/somatization score, while elevated compared to that of normal controls, was similar to psychiatric outpatient norms (12) and even slightly lower than scores reported for depressed patients in a depression research program (17), even though BDD is classified as a somatoform disorder. This result is consistent with a previous report that depressed patients with and without BDD have similar scores on the somatic/somatization scale (15). In addition, individuals with BDD have relatively low rates of comorbid somatoform disorders as assessed by the SCID-P (24). In fact, the question has been raised of whether BDD might be more closely related to other disorders, such as OCD, than to the other somatoform disorders with which it is classified (25,26).
As hypothesized, patients with more severe BDD symptoms and a greater degree of delusionality had higher Symptom Questionnaire scores on all scales, with the exception that greater delusionality was not significantly associated with the somatic/somatization score. Thus, more severely ill and delusional BDD patients experience more severe distress and psychopathology in multiple domains. This result is consistent with findings that greater BDD severity and greater delusionality are associated with higher levels of perceived stress (11), greater functional impairment (18), and poorer mental-health related quality of life (5).
Fluvoxamine treatment led to significant improvement in all four domains, although post-treatment scores remained elevated compared to normal controls. The finding that depression, as assessed by self-report on the Symptom Questionnaire, improved with treatment is consistent with previously reported findings from the fluvoxamine study that depression as assessed by the clinician-rated HAM-D and MADRS improved with treatment (8). While improvement in depression and anxiety—and perhaps somatic concern—might be expected with fluvoxamine treatment, it is interesting that anger-hostility scores improved as well. The anger-hostility scale consists of items such as “feeling angry,” “feeling hostile,” and “not feeling kind to people.” Our finding that anger-hostility improved is consistent with data indicating that anger-hostility improves in depressed patients treated with amitriptyline or fluoxetine (17,27,28). It is also consistent with reports of the efficacy of serotonin-reuptake inhibitors for aggression (29) and with evidence of dysregulation of serotonergic neurotransmission in aggression (30).
This study has a number of limitations, including the lack of a control group from the study setting. In addition, subjects were selected from a specialty BDD program, which makes the generalizability of the results to other settings unclear. It is possible that individuals with BDD in the community who do not come to clinical attention might have lower Symptom Questionnaire scores than our study subjects. On the other hand, 36% of our study subjects participated in a medication study, which excluded highly suicidal patients or individuals who could not cooperate with the study protocol; these biases may have resulted in somewhat lower depression and anger-hostility scores than other treatment-seeking individuals with BDD. Another limitation is that the fluvoxamine study used an open-label design and had a small number of subjects, so the treatment results should be considered preliminary. Nonetheless, they are consistent with improvement in Symptom Questionnaire scores following treatment in other psychiatric disorders (12,17). The study also had a number of strengths, including a well-characterized sample, the use of measures with strong psychometric properties, and assessment of clinically important constructs, including several (somatic/somatization symptoms and anger-hostility) that have not previously been assessed in a sample ascertained for BDD.
In conclusion, this study, the first to use the Symptom Questionnaire in patients ascertained for BDD, found that patients with this disorder have severe distress and psychopathology, and that non-BDD symptoms improved with fluvoxamine treatment. Additional studies are needed to investigate a range of symptoms in patients with BDD, as well as change in symptoms and well-being with treatment.
Acknowledgments
This study was supported in part by an unrestricted educational grant from Solvay Pharmaceuticals, Inc., and Pharmacia & Upjohn.
Contributor Information
Katharine A. Phillips, Department of Psychiatry and Human Behavior, Brown Medical School, Butler Hospital, Providence, RI..
Jason M. Siniscalchi, Butler Hospital, Providence, RI.
Susan L. McElroy, University of Cincinnati School of Medicine, Cincinnati, OH.
References
- 1.Phillips KA, McElroy SL, Keck PE, Jr, et al. Body dysmorphic disorder: 30 cases of imagined ugliness. American Journal of Psychiatry. 1993;150:302–308. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- 2.Perugi G, Giannotti D, Frare F, et al. Prevalence, phenomenology, and comorbidity of body dysmorphic disorder (dysmorphophobia) in a clinical population. International Journal of Psychiatry and Clinical Practice. 1997;1:77–82. doi: 10.3109/13651509709024707. [DOI] [PubMed] [Google Scholar]
- 3.Grant JE, Kim SW, Crow SJ. Prevalence and clinical features of body dysmorphic disorder in adolescent and adult psychiatric inpatients. Journal of Clinical Psychiatry. 2001;62:517–522. doi: 10.4088/jcp.v62n07a03. [DOI] [PubMed] [Google Scholar]
- 4.Bienvenu OJ, Samuels JF, Riddle MA, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: Results from a family study. Biological Psychiatry. 2000;48:287–293. doi: 10.1016/s0006-3223(00)00831-3. [DOI] [PubMed] [Google Scholar]
- 5.Phillips KA. Quality of life for patients with body dysmorphic disorder. Journal of Nervous and Mental Disease. 2000;188:170–175. doi: 10.1097/00005053-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Veale D, Boocock A, Gournay K, et al. Body dysmorphic disorder: A survey of fifty cases. British Journal of Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- 7.Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Archives of General Psychiatry. 2002;59:381–388. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- 8.Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. Journal of Clinical Psychiatry. 1998;59:165–171. doi: 10.4088/jcp.v59n0404. [DOI] [PubMed] [Google Scholar]
- 9.Saxena S, Winograd A, Dunkin JJ. A retrospective review of clinical characteristics and treatment response in body dysmorphic disorder versus obsessive compulsive disorder. Journal of Clinical Psychiatry. 2001;62:67–72. doi: 10.4088/jcp.v62n0114b. [DOI] [PubMed] [Google Scholar]
- 10.Perugi G, Giannotti D, Di Vaio F, et al. Fluvoxamine in the treatment of body dysmorphic disorder (dysmorphophobia) International Clinical Psychopharmacology. 1996;11:247–254. doi: 10.1097/00004850-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 11.DeMarco L, Li L, Phillips KA, McElroy S. Perceived stress in body dysmorphic disorder. Journal of Nervous and Mental Disease. 1998;186:724–726. doi: 10.1097/00005053-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kellner R. A symptom questionnaire. Journal of Clinical Psychiatry. 1987;8:268–274. [PubMed] [Google Scholar]
- 13.Lucas P. Violence may be serious in men with body dysmorphic disorder (letter) British Medical Journal. 2002;324:678. doi: 10.1136/bmj.324.7338.678/b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips KA, McElroy SL, Lion JR. Body dysmorphic disorder in cosmetic surgery patients (letter) Plastic and Reconstructive Surgery. 1992;90:333–334. [PubMed] [Google Scholar]
- 15.Phillips KA, Nierenberg AA, Brendel G. Prevalence and clinical features of body dysmorphic disorder in atypical major depression. Journal of Nervous and Mental Disease. 1996;184:125–129. doi: 10.1097/00005053-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KA, McElroy SL. Personality disorders and traits in patients with body dysmorphic disorder. Comprehensive Psychiatry. 2000;41:229–236. doi: 10.1053/comp.2000.7429. [DOI] [PubMed] [Google Scholar]
- 17.Fava M, Rosenbaum JF, Pava JA, et al. Anger attacks in unipolar depression, part 1: Clinical correlates and response to fluoxetine treatment. American Journal of Psychiatry. 1993;150:1158–1163. doi: 10.1176/ajp.150.8.1158. [DOI] [PubMed] [Google Scholar]
- 18.Phillips KA, McElroy SL, Keck PE, Jr, et al. A comparison of delusional and nondelu-sional body dysmorphic disorder in 100 cases. Psychopharmacology Bulletin. 1994;30:179–186. [PubMed] [Google Scholar]
- 19.Phillips KA, Hollander E, Rasmussen SA, et al. A severity rating scale for body dysmorphic disorder: Development, reliability, and validity of a modified version of the Yale-Brown Obsessive-Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- 20.Kellner R, Abbott P, Pathak D, et al. Hypochondriacal beliefs and attitudes in family practice and psychiatric patients. International Journal of Psychiatry and Medicine. 198384;13:127–139. doi: 10.2190/9jpt-a66k-72bh-c3my. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer RL, Williams JBW, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID): I. History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 22.Phillips KA, Atala KD, Pope HG. American Psychiatric Association 148th Annual Meeting. Miami, FL: American Psychiatric Association; 1995. Diagnostic instruments for body dysmorphic disorder, in New Research Program and Abstracts; p. 157. [Google Scholar]
- 23.Phillips KA, McElroy SL, Dwight MM, et al. Delusionality and response to open-label fluvoxamine in body dysmorphic disorder. Journal of Clinical Psychiatry. 2001;62:87–91. doi: 10.4088/jcp.v62n0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Comprehensive Psychiatry. 2003;44:270–276. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollander E. Introduction, in Obsessive-Compulsive Related Disorders. In: Hollander E, editor. Washington, DC: American Psychiatric Press; 1993. [Google Scholar]
- 26.Cohen LJ, Stein DJ, Simeon D, et al. Obsessive-compulsive spectrum disorders, in Obsessive-Compulsive Disorders. In: Hollander E, Stein DJ, editors. New York: Marcel Dekker; 1997. pp. 47–74. [Google Scholar]
- 27.Fava GA, Kellner R, Lisansky J, et al. Hostility and recovery from melancholia. Journal of Nervous and Mental Disease. 1986;174:414–417. doi: 10.1097/00005053-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Fava M, Davidson K, Alpert JE, et al. Hostility changes following antidepressant treatment: Relationship to stress and negative thinking. Journal of Psychiatric Research. 1996;30:459–467. doi: 10.1016/s0022-3956(96)00034-9. [DOI] [PubMed] [Google Scholar]
- 29.Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Archives of General Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 30.Lee R, Coccaro E. The neuropsychopharmacology of criminality and aggression. Canadian Journal of Psychiatry. 2001;46:35–44. doi: 10.1177/070674370104600106. [DOI] [PubMed] [Google Scholar]


