Abstract
Larger keratinocyte carcinoma (KC) lesions are associated with higher morbidity. This study examined the association of potentially modifiable characteristics, including treatment delay, with KC defect size after Mohs micrographic surgery (MMS). A stratified random sample of patients treated for KC with MMS were selected for telephone interview. Two hundred and nineteen interviews were completed (refusal rate 24%). Regression models were used to examine the predictors to defect size and delay. Anatomic site, age, histology, and gender predicted defect size (R2 =0.39) and were used as control variables. Self-reported delay between initial physician examination and MMS predicted defect size (p =0.0004), with greater than 1 y delay being associated with a doubling of defect size (adjusted odds ratio (OR) 2.0; 95% confidence interval (CI) 1.3–3.1). Delays of this duration were associated with initial examination by a primary provider (unadjusted OR 3.9; 95% CI 1.7–8.8), misdiagnosis (unadjusted OR 6.8; 95% CI 2.5–18.7), being treated without biopsy (unadjusted OR 23.3; 95% CI 6.5–83.7), and multiple surgical removals (unadjusted OR 6.2; 95% CI 2.5–15.5). All but provider specialty were independent predictors of delay. Attention to processes of care delivery for KC may have a greater impact on morbidity than efforts are earlier detection by the public.
Keywords: cancer control, health services research, Mohs micrographic surgery, skin cancer
Abbreviations: BCC, basal cell carcinoma; CI, confidence interval; KC, keratinocyte carcinoma; MMS, Mohs micrographic surgery; OR, odds ratio; SCC, squamous cell carcinoma
Skin cancer is the most common cancer in the United States. The American Cancer Society estimates that more than one million cases of keratinocyte carcinoma (KC) (Weinstock et al, 2001), which includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), are diagnosed each year (Jemal et al, 2003). Mortality from KC is relatively low. They, however, usually arise in cosmetically sensitive areas, such as the face, and advanced lesions are associated with higher morbidity and more frequent recurrence (Miller, 1991; Smeets et al, 2004). The potentially modifiable factors that contribute to more advanced lesions at the diagnosis of the KC have not been established.
Skin cancer information campaigns and screening programs are based on the presumption that earlier disease diagnosis can minimize disease impact (Silfen et al, 2002). In the last several decades there have been some investigations of the association of delay, either by patient or provider, and the extent of tumor invasion, with the overwhelming majority of these studies focusing on melanoma (Levine et al, 1981; Temoshok et al, 1984, 1985; Cassileth et al, 1988; Rampen et al, 1989; Dunkley and Morris, 1991; Hennrikus et al, 1991; Blum et al, 1999; Oliveria et al, 1999; Richard et al, 1999, 2000a, Richard et al, b; Brochez et al, 2001; Osborne and Hutchinson, 2001; McKenna et al, 2002; Schmid-Wendtner et al, 2002; Silfen et al, 2002). The data regarding the relationship of treatment or diagnostic delay with KC size is very limited (Kirkup and De Berker, 1999).
We sought to determine the association of potentially modifiable characteristics, including delay in treatment, with size of defect at the time of KC removal by Mohs micrographic surgery (MMS). MMS is accepted as the most effective KC treatment modality for high-risk cancers because of its cure rate of approximately 97%–99% (Rowe et al, 1989; Randle, 1996). Because the technique involves careful histological examination of tumor margins, Mohs surgical defect size approximates tumor size (Zitelli et al, 1997). Therefore, we have chosen the final defect size prior to repair to be a proxy for KC size in our study. We hypothesized that patients with longer delay would have larger defects.
Results
No differences were found between interviewed and non-interviewed subjects regarding defect size (mean size: 4.3 vs 4.4 cm2, T test =0.16, p =0.9), anatomic location, histologic subtype, or gender (Table I). Interviewed participants were younger (mean age: 66 vs 72 y, T test =5.64, p<0.0001) than those not interviewed.
Table I.
Comparison of interviewed and non-interviewed subjects n =500 (%)
| Interviewed participants (%) | Non-interviewed (%) | Total (%) | |
|---|---|---|---|
| Anatomic sitea | |||
| Upper limb | 4 (1.8) | 1 (0.4) | 5 (1.0) |
| Back | 1 (0.5) | 1 (0.4) | 2 (0.4) |
| Chest | 3 (1.4) | 2 (0.7) | 5 (1.0) |
| Posterior ear | 2 (0.9) | 5 (1.8) | 7 (1.4) |
| Anterior ear | 18 (8.2) | 27 (9.6) | 45 (9.0) |
| Eye | 17 (7.8) | 23 (8.2) | 40 (8.0) |
| Neck | 6 (2.7) | 5 (1.8) | 11 (2.2) |
| Submandible | 1 (0.5) | 0 (—) | 1 (0.2) |
| Jawline | 1 (0.5) | 2 (0.7) | 3 (0.6) |
| Chin | 5 (2.3) | 3 (1.1) | 8 (1.6) |
| Vermillion lip | 6 (2.7) | 5 (1.8) | 11 (2.2) |
| Anterior lip | 7 (3.2) | 11 (3.9) | 18 (3.6) |
| Alar lip | 5 (2.3) | 14 (5.0) | 19 (3.8) |
| Nose | 39 (17.8) | 66 (23.5) | 105 (21.0) |
| Nasofacial fold | 20 (9.1) | 17 (6.1) | 37 (7.4) |
| Cheek | 26 (11.9) | 31 (11.0) | 57 (11.4) |
| Pre-auricular | 4 (1.8) | 6 (2.1) | 10 (2.0) |
| Post-auricular | 3 (1.4) | 3 (1.1) | 6 (1.2) |
| Forehead | 26 (11.9) | 20 (7.1) | 46 (9.2) |
| Scalp | 25 (11.4) | 39 (13.9) | 64 (12.8) |
| Age (y)b | |||
| <40 | 8 (4) | 3 (1) | 11 (2) |
| 41–50 | 29 (13) | 15 (5) | 44 (9) |
| 51–60 | 36 (16) | 47 (17) | 83 (17) |
| 61–70 | 43 (20) | 36 (13) | 79 (16) |
| 71–80 | 80 (37) | 87 (31) | 167 (33) |
| 81–90 | 22 (10) | 83 (30) | 105 (21) |
| >90 | 1 (0) | 10 (4) | 11 (2) |
| Histologic subtypec | |||
| Morpheaform BCC | 10 (4.6) | 13 (4.6) | 23 (4.6) |
| Infiltrating BCC | 22 (10.0) | 27 (9.6) | 49 (9.8) |
| Superficial BCC | 6 (2.7) | 6 (2.1) | 12 (2.4) |
| Basosquamous BCC | 4 (1.8) | 10 (3.6) | 14 (2.8) |
| Nodular BCC | 60 (27.4) | 77 (27.4) | 137 (27.4) |
| SCC in situ | 13 (5.9) | 17 (6.0) | 30 (6.0) |
| Moderately differentiated SCC | 5 (2.3) | 6 (2.1) | 11 (2.2) |
| Well-differentiated SCC | 18 (8.2) | 16 (5.7) | 34 (6.8) |
| Superficial SCC | 4 (1.8) | 5 (1.8) | 9 (1.8) |
| Other types of SCC | 8 (3.7) | 8 (2.8) | 16 (3.2) |
| No histological subtype available | 69 (31.5) | 96 (34.1) | 165 (33.0) |
| Genderd | |||
| Females | 94 (42.9) | 110 (39.1) | 204 (40.8) |
| Males | 125 (57.1) | 171 (60.9) | 296 (59.2) |
| Total subjects | 219 | 281 | 500 |
Logistic regression Wald χ2 =15.46, p =0.7.
Logistic regression Wald χ2 =28.55, p<0.0001.
Logistic regression Wald χ2 =3.149, p =1.0.
χ2 =0.726, p =0.4.
BCC, basal cell carcinoma; SCC, squamous cell carcinoma.
In the overall study population (Table II), a substantial portion of the variation in defect size was explained by histology, age, gender, and anatomic location (R2 =0.39, p<0.0001). No age by gender interaction was identified, and the quadratic age term was not associated with defect size. The majority of the variance was attributed to anatomic location (partial R2 =0.29). Larger surgical defects were associated with several anatomic sites, including pre- and post-auricular, chest, and the submandible. There was no association when BCC was compared with SCC (β=−0.16, 95% confidence interval (CI) −0.4 to 0.04, p =0.12). When more refined histologic subtype was entered into the model, superficial, morpheaform, and infiltrating BCC were associated with larger defects as were older age and male gender. Similar findings were also observed in the analysis of interviewed participants (R2 =0.47, anatomic site partial R2 =0.37).
Table II.
Predictors of log-surgical defect size (weighted multiple linear regression, N =500)
| Variable | Partial R2 | DF | F | p-value |
|---|---|---|---|---|
| Anatomic site | 0.29 | 19,480 | 10.1 | <0.0001 |
| Age (y) | 0.044 | 1479 | 31.6 | <0.0001 |
| Histologic subtype | 0.045 | 10,469 | 3.39 | 0.0003 |
| Gender (female =1) | 0.016 | 1468 | 11.9 | 0.0006 |
| Overall model | 0.39 | 31,468 | 10.4 | <0.0001 |
Controlling for anatomic location, histology, age and gender, all time-delay intervals were associated with defect size when examined individually (p-values from partial F test: discovery (p =0.034, median =194 d), suspicion (p =0.014, median =138 d), initial evaluation (p =0.004, median =107 d), and biopsy (p =0.025, median =90 d)). When an additional time-delay interval was, however, entered into a model already containing one of the time-delay intervals, significance for both time-delay intervals was lost, with one exception. Consistently, only delay from initial evaluation (the duration of time between initial provider evaluation until MMS) was associated with larger defects (partial R2 =0.018, p =0.004) whereas all other time-delay intervals failed to maintain a significant association with size. We evaluated the duration–response relationship between delay from initial evaluation and defect size (Table III). The size-delay association was seen only in the last quintile of time delay, i.e. greater than 1 year, which was associated with a doubling of defect size (size ratio (eβ) =2.0; 95% CI 1.3–3.1).
Table III.
Association of defect size with delay from initial evaluation
| Duration of delay from initial evaluation | Number of subjects | Coefficient β (SE) | Size ratio (CI)a | p-value |
|---|---|---|---|---|
| <1 mo | 41 | 0 | 1 | (Reference) |
| 1–3 mo | 35 | −0.06 (0.22) | 0.9 (0.6–1.4) | 0.8 |
| 3–6 mo | 41 | −0.10 (0.21) | 0.9 (0.6–1.4) | 0.6 |
| 6–12 mo | 39 | 0.06 (0.20) | 1.1 (0.7–1.6) | 0.8 |
| More than 1 y | 40 | 0.70 (0.22) | 2.0 (1.3–3.1) | 0.0016 |
| Unknown | 23 |
Coefficients are standardized with anatomic site, age, histologic subtype, and gender controlled in analysis. The dependent variable was the log of defect size (cm2). p-value is based on F test.
Size ratio is defined by using eβ, e.g. e0.70 =2.0, which clinically represents a doubling of defect size.
CI, confidence interval.
Controlling for anatomic location, gender, age, and histologic subtype as well as with or without delay (Table IV), other factors were then examined to further explain variation in defect size. Size variation was not explained by personal or family history of skin cancer or by specialty of initial care provider. No variation was attributable to marital status, education level, smoking, provider skin examination, personal history of previous cancer other than skin, number of provider visits in the 2 years prior to surgery, treatment by initial health provider, if skin cancer was diagnosed initially, the Whitley index, the attitudinal variables, medical costs, or insurance concerns.
Table IV.
Predictors of defect size in interviewed sample (n =219)
| Variable | Coefficient (β) | DF | SE | Partial R2 | p-value |
|---|---|---|---|---|---|
| Anatomic site | — | 19,199 | — | 0.37 | <0.0001 |
| Age (y) | 0.01 | 1198 | 0.005 | 0.019 | 0.014 |
| Histologic subtype | — | 10,188 | — | 0.044 | 0.16 |
| Gender (female =1) | −0.33 | 1187 | 0.15 | 0.027 | 0.0039 |
| Delay >1 y from initial exam until MMS | 0.73 | 1186 | 0.17 | 0.054 | <0.0001 |
| Final model | 32,186 | 0.52 | <0.0001 |
MMS, Mohs micrographic surgery.
Defect size (p-value based on partial F value) was associated with who discovered lesion (self, spouse, or a clinician; p =0.014), skin self-examination (SSE) (p =0.035), and the number of prior removals (p =0.03). All of these factors, however, failed to maintain the significance in controlling for anatomic location, histologic subtype, age, gender, and time interval greater than 1 years between provider evaluation and surgery. Out-of-pocket cost of diagnosis had a marginal association with larger lesions (β=0.005, p =0.049).
In separate logistic regression analyses, we examined factors associated with long delay (dependent variable: greater than 1 y from evaluation until MMS). The initial provider seen was a dermatologist for 68% of patients whereas most of the remaining patients saw a primary provider first (Table V). Patient who saw a primary care provider first, compared with those who saw a dermatologist, were more likely to have a long delay (unadjusted odds ratio (OR) 3.9; 95% CI 1.7–8.8). Delay was not associated with reason for initial office visit. The provider’s initial diagnostic impression of the lesion at the time of initial evaluation, however, differed in those with more than a year delay compared with those with shorter delay. If the skin cancer was misdiagnosed initially (unadjusted OR 6.8; 95% CI 2.5–18.7) or if the lesion was treated as opposed to biopsied on the initial evaluation (unadjusted OR 23.3; 95% CI 6.5–83.7), the patient was more likely to have delay. Patients who had one (unadjusted OR 6.2; 95% CI 2.5–15.5) or more prior surgeries (unadjusted OR 47.3; 95% CI 5.5–403.8) before MMS were also more likely to have a long delay. Using multivariate logistic regression, delay of more than 1 y from initial evaluation until MMS was associated with initial misdiagnosis, initial treatment, and having one or more removals prior to MMS (Table V). No other variables, including anatomic location, histologic subtype, age, and gender were significantly associated with delay.
Table V.
Health system factors that predict delay from initial evaluation until MMS (n =196)
| Delay >1 y (%) | Less delay (%) | Univariate OR (95% CI) | Multivariate OR (95% CI)a | |
|---|---|---|---|---|
| Specialty of first provider to evaluate lesion | ||||
| Dermatologist | 19 (48) | 115 (73) | 1.0 | — |
| Primary care provider | 14 (35) | 22 (14) | 3.9 (1.7–8.8) | — |
| Other/unknown | 7 (17.5) | 19 (12.2) | 2.2 (0.8–6.0) | — |
| Initial diagnosis of first evaluating provider | ||||
| Skin cancer | 10 (25) | 79 (51) | 1.0 | 1.0 |
| Other benign lesion | 12 (30) | 14 (9) | 6.8 (2.5–18.7) | 3.2 (1.2–8.5) |
| Other/unknown | 18 (45) | 63 (40.4) | 2.3 (1.0–5.2) | 1.3 (0.5–3.2) |
| Procedure performed by first evaluating provider | ||||
| Biopsy | 13 (32.5) | 109 (70) | 1.0 | 1.0 |
| Treated | 11 (27.5) | 4 (3) | 23.3 (6.5–83.7) | 9.3 (2.2–38.4) |
| No procedure | 15 (37.5) | 43 (27.6) | 3.2 (1.4–7.3) | 2.8 (1.1–6.9) |
| Other/unknown | 1 (2.5) | — | ||
| Number of lesion removals prior to MMS | ||||
| 0 | 21 (52.5) | 142 (91) | 1.0 | 1.0 |
| 1 | 12 (30) | 13 (8.3) | 6.2 (2.5–15.5) | 4.1 (1.3–12.6) |
| >1 | 7 (17.5) | 1 (1) | 47.3 (5.5–403.8) | 42.4 (4.5–395.8) |
Multivariate model with initial diagnosis of first evaluating provider, procedure performed by first provider, and number of lesion removals prior to MMS included.
MMS, Mohs micrographic surgery; OR, odds ratio; CI, confidence interval.
Discussion
This study suggests that there are several factors that are associated with the size of MMS defects in the treatment of KC. Delay of more than 1 y between initial provider evaluation of a KC and its surgical removal with MMS was associated with surgical defects that were twice as large as those where delay was shorter. We note that important predictors of this delay include initial misdiagnosis, initial procedure performed, and the number of surgical removals prior to MMS. We also found important effects of anatomic site, age, histologic subtype, and gender on defect size, with the largest portion of the variation in size explained by anatomic site. This is consistent with previous findings in which both anatomic location and histologic subtype have been noted to be associated with the extent of infiltration and tissue invasion in KC (Batra and Kelley, 2002). In our study, a woman’s surgical defect size was approximately half the size of a man’s defect.
There are several limitations of our study. One limitation is the absence of data from referring providers and the uncertainty of detailed explanations for the long delays (greater than 1 y) between initial evaluation and MMS. We do not have detailed information on prior medical care, including prior treatment with electrodessication and curettage, a relatively common treatment method for KC (Silverman et al, 1991). This study did not examine adequacy of pathology specimens (Rampen et al, 1989) or result follow-up (Cassileth et al, 1988). We do not know if there were difficulties surrounding the reason for the referral of suspected skin lesions (Dunkley and Morris, 1991) or misunderstandings of the urgency of scheduling the referral evaluations (Dunkley and Morris, 1991; Brochez et al, 2001). Furthermore, most of our data was collected by self-report, including the information relating to dates and progression through the medical system prior to MMS. Imprecision in the assessment of delay is possible because of inaccurate or non-specific recall of dates. To minimize potential error introduced by recall bias, the time intervals used the date of MMS, a date available in the medical record.
Another limitation of this study is that some pathology reports were incomplete or missing. Histologic-subtype identification and verification were not available for all tumors. Ideally this information would have been present for all individuals. Furthermore, we have noted an association with diagnostic suspicion of lesion on first evaluation, initial care plan and number of prior surgical removals with delay. There may, however, be other sources of delay that we did not adequately assess, including severe unrelated illness in the interval between initial evaluation and MMS, patient’s personal priorities, problems with referring or scheduling appointments, or additional visits related to ‘‘following’’/observing lesion.
A strength of this study is that it examines factors associated with defect size itself, a measure of KC morbidity. The use of surgical defect margins from MMS cases allows a precise estimate of tumor size. This study demonstrates evidence of the association between treatment delay and KC outcomes. All cases were obtained from the practice of a single Mohs surgeon. At the time of this study, a majority of all MMS cases in the state of Rhode Island were treated by this dermatologic surgeon. The use of a single surgical provider reduces provider-associated variability and cases are felt to be representative of Rhode Island KC patients. It is unclear if these results are generalizable outside of this population, including patients not referred for Mohs surgery.
A review of patient care delay for cancer symptoms shows that cancer patients with co-paid fee-for-service coverage waited longer to seek healthcare than patients enrolled in health maintenance organizations (Love, 1991). Although almost all study participants had medical coverage, co-payments and fear of non-covered expenses could conceivably have contributed to patient delay in this study population. Participants were, however, asked specifically if the cost of seeing a health provider was a barrier to care, and we found no significant association between response to this question and defect size. The meaning of the observed borderline association found between out of pocket expense and surgical defect size is unclear.
There has been limited investigation of the relevance of treatment delay and KC size (Kirkup and De Berker, 1999; Bandaranayake, 2002). Kirkup and De Berker (1999) examined 50 patients with BCC on the face who were identified as suitable for elective excision. Major and minor diameters (i.e. horizontal and vertical dimensions of lesion) were measured at presentation and immediately before excision. Delay time ranged from 3 wk to 6 mo (mean of 10 wk), and no correlation was found with change in size and time to surgery. The authors did note, that because of the poor correlation between growth and time, that it was difficult to predict the effect of longer delays. Furthermore, it should be noted that if our study had used the same delay period of 6 mo or less, we too would have found no association between delay and size.
A recent doctoral dissertation examined the relationship of delay to KC size (Bandaranayake, 2002). Although this Australian case–control study included people with smaller lesions and shorter duration of delay, her results were consistent with our findings. Bandaranayake noted longer delay between the final physician evaluation and definitive treatment in those with larger lesions (cases: greater than 1–2.25 cm2). Controlling for sex, age, and histology, patients with longer delay (more than 61 d) between consultation with final physician until definitive treatment were more likely to have larger lesions than those treated within 2 wk (OR 1.96; 95% CI 1.42–2.71). She, however, found evidence that the delay was attributable to the more complex procedures required for treatment of the larger lesions.
Patient and tumor factors that relate to subclinical extension of non-melanoma skin cancer were examined in a study of 1131 MMS patients using a dependent variable of 3 or more MMS layers during surgery. The authors noted an interaction between anatomic location of the skin cancer, histologic classification, and sex that was predictive of the extent of subclinical invasion. Tumors located on the eyelids, temple, or ear helix; basosquamous, morpheaform, nodular, and recurrent BCC on the nose; and morpheaform BCC on the cheek were found to be important predictors of extension. Pre-operative size greater than 10 mm and recurrent malignancies or those on the neck in men also were predictive of infiltration (Batra and Kelley, 2002). Although our study differed substantially in methods, the results were broadly consistent.
Previous studies have focused on the examination of factors contributing to health-care delay and thickness of melanoma. The association between delay and size has been inconsistent (Cassileth et al, 1988; Krige et al, 1991; Schmid-Wendtner et al, 2002). Temoshok et al 1985 found that the most significant variable in a hierarchical multiple regression analysis of melanoma thickness was delay (p<0.0005), defined as time from patient first becoming suspicious of lesion until evaluation by a physician specifically for that reason. Other than skin type, no significance was found for other variables including previous knowledge, understanding of treatment, age, faith, and personality.
Richard et al (1999, 2000a, b) investigated delay and melanoma thickness using five dates, similar to those used in this study: when the lesion was first noticed, first suspicion of lesion, first examination by a provider, first proposed removal, and melanoma resection. Her findings, suggested that in some groups, patient and provider delay may be associated with melanoma depth (Richard et al, 1999), although the relationships, when present, were complex.
Some prior studies examining melanoma thickness have found an association between initial examining physician specialty and melanoma thickness (Richard et al, 2000a, b) whereas others have failed to find an association (Brochez et al, 2001). In our data, it does not appear that the specialty of the initial provider (primary care provider or a dermatologist) was associated with size or delay, after controlling for other factors. We also did not find the number of prior surgical removals to be associated with the lesion size. This is consistent with a study by Brochez et al 2001 that found no association of prior treatment with melanoma thickness. Unlike Brochez, we, however, found an association with prior removals and delay.
We were able to explore the relationship between diagnostic and treatment delay and KC size in greater depth than other studies. Although the results from this study do not prove that delay causes larger surgical defects, it does suggest an important association. Besides the number of prior removals, we note several aspects of the initial provider evaluation that relate to this delay, including the initial diagnostic impression and type of procedures performed at this evaluation. Some have speculated about the role of medical providers in prolonged delay (Cassileth et al, 1988; Dunkley and Morris, 1991; Blum et al, 1999; MacKie, 1999; Richard et al, 1999). Many of the clinicians that treat BCC and SCC in their practices have little formal training in dermatology (only 60% of skin cancer treatments are provided by dermatologists) (Joseph et al, 2001). In our study, almost half of the participants with more than 1 y delay were first evaluated by a dermatologist.
On the basis of these results, we suggest that for KC, delay after presentation to a health-care provider may be more important than delay before initially presenting for care. This should be evaluated in other settings and locations. Further study to investigate mediating variables in health system delay in the diagnosis and treatment of KC is also necessary. This inquiry should include issues of misdiagnosis, inadequate follow-up efforts, inadequate initial treatment, interim events during the course of medical care, and cost-effectiveness of available therapeutic options. KC causes substantial morbidity, and optimal management of this public health problem requires both primary prevention and efficient medical management.
Methods
Subjects were selected from all cases of KC treated with MMS at an academic dermatological surgery practice between September 12, 2000 and September 12, 2001 (n =1123 surgical cases). These MMS cases represented 892 different individuals. Of the surgical patients who had more than one procedure, the surgical case with the largest final defect size was selected. Immunologically compromised patients were excluded. There were 20 surgical cases for which it was not possible to calculate final defect size and 12 surgical cases in which the diagnoses were not KC; all of which were excluded. Hence, 860 unique MMS patients were eligible for inclusion. Cases were then stratified by size of defect into quartiles. A random sample of 100 cases was selected using computer-generated random numbers (Microsoft Foxpro Version 2.6, Redmond, Washington) from each quartile except the largest, from which 200 cases were selected. (Patients with large defect size were over-sampled because factors contributing to those with more morbidity were of special interest, and we wished to ensure statistical stability for analysis related to these large lesions.) Approximate quartile parameters were: <1, 1–2, 2–4 cm2, and greater than 4 cm2 (maximum size: 43 cm2). Eighty percent of each quartile (n =400) were chosen for interview because of resource constraints.
Of these 400 subjects, 28 individuals could not be reached for interview. An additional subgroup was ineligible to participate because of communication barriers, including not speaking English (n=32), other communication issues (e.g. hearing loss, dementia) (n =43), or death (n =5). Of those remaining, 71 people (24%) refused participation, and two completed only partial interviews. Hence, after giving verbal consent, 219 people completed the telephone interview.
Surgical records were used to obtain information recorded at time of MMS. The remaining variables were obtained through computer-assisted telephone interviews, which were conducted between January 2, 2002 and October 2, 2002 and were approximately 25 min in duration.
Analysis utilized SAS System software, release 8.2, Enterprise Guide Version 2.0 (SAS Institute, Cary, North Carolina). After weighting the quartiles 1, 2, and 3 by a factor of 2 to reflect the sampling method, size was transformed, using a log transformation, to graphically approximate a normal distribution.
Weighted linear regression was used to model the transformed-dependent variable, log final size of surgical defect. Residuals were checked using a Q–Q plot, and co-linearity was assessed using variance inflation factors. The following variables were evaluated using linear regression to establish the base model in the overall sample of 500 subjects: age, gender, histologic subtype, anatomic location, an age × gender interaction, and age-squared. Controlling for the base model variables, remaining variables were then entered into the model individually. In separate analyses, predictors of delay were examined using logistic regression and χ2 test (or Fisher’s exact test, if appropriate).
Twenty different anatomic locations were defined a priori using information recorded at the time of surgery. These anatomic areas were obtained from the chart (Table I). Comparison was to the location of the nose, the most common location of lesions. BCC and SCC histologic subtypes were obtained from records. Because of referral sources, 33% of charts did not have pathology reports with information on histologic subtype (32% of the interviewed and 34% of non-interviewed subjects). Review of the medical charts of the interviewed participants reduced the number of missing subtypes to 20%.
Four time intervals, considered potential sources of treatment delay, were evaluated (Fig 1). These intervals were measured and analyzed in days (continuous variable), then by the number of the quintile of delay (ordinal variable), and finally with indicator variables to represent each of the individual quintiles (dummy variables; the first quintile was the comparison) to examine any possible gradient. Quintile intervals for delay from initial evaluation approximated <1, 1–3, 3–6, 6 mo to 1 y, and greater than 1 y between reference date and date of MMS. All dates were available for 196 patients (89.5%).
Figure 1. Definition of Delay Intervals.
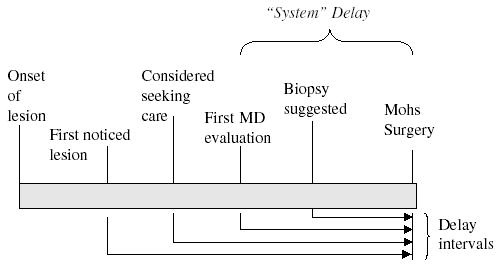
Time line illustration and definitions of time points and delay intervals (all intervals were measure until the date of Mohs surgery, which was obtained from the medical record).
We hypothesized that demographic, socio-economic, health-care resources, and psychosocial variables may be related to delay, and hence defect size. Other factors analyzed were: marital status, living alone, education level completed (a 1–6 scale, ranging from less than ninth grade to graduate school degree), smoking status, provider skin examination (both as dichotomous variable representing having had examination that year and as a variable with a 1–5 scale, ranging from never to monthly examination), SSE (both as dichotomous variable representing having had examination that year and as a variable with a 1–5 scale, ranging from never to monthly exam), previous history of skin cancer, family history of skin cancer, personal history of any cancer other than skin cancer, who discovered lesion (self, spouse, provider), specialty of first provider to evaluate, number of visits to a primary provider or dermatologist in 2 y prior to surgery, clinician diagnostic and therapeutic management on first evaluation, and number of treatments of lesion with the intention of cure prior to surgery. The Whitley index (Pilowsky, 1967) (scale: 0–14) was assessed, followed by assessment of three of the subcomponents of the scale: bodily pre-occupation (scale: 0–3), disease phobia (scale: 0–4), and hypochondriasis (scale: 0–3). A summary variable was also created that utilized five-point Likert responses to a series of questions on excuses for not seeking care (e.g. hoping spot would clear on its own). Attitudinal Likert variables assessing confidence in detecting skin cancer, being too busy to seek medical care, ease of obtaining an appointment, faith in health care, cost of health care as a problem, and tendency to wait and hope problems will go away on their own were also examined. Out-of-pocket cost of diagnosis (in dollars), out-of-pocket cost of surgery (in dollars), and self-report of concerns of insurance status resulting in delay in seeking health care were also assessed. The racial and ethnic background of the study population was homogeneous, 99% white by self-report, and 99% also reported having insurance at time of both diagnosis and surgery. Hence, these variables were not analyzed. Declaration of Helsinki guidelines were adhered to, and the study was approved by the Committees on Human Subjects for Lifespan Hospitals and for the Providence VA Medical Center (Providence, Rhode Island).
Footnotes
The study was supported by the Agency for Healthcare Research & Quality (AHRQ), Institutional National Research Service Award (NRSA) HS 00011-16 (Dr Eide) and by grants CSP 402 from the Department of Veterans Affairs, Office of Research and Development and CA 78800 from the National Cancer Institute (NCI) (Dr Weinstock). The methods used in this research were derived in part from those developed by Dilhani Bandaranayake and Afaf Girgis of the University of Newcastle, under the supervision of one of the authors (Dr Armstrong). Findings were presented at the Society for Investigative Dermatology Annual Meeting, Providence, Rhode Island, May 1, 2004.
References
- Bandaranayake DM. Doctor of philosophy dissertation. University of Newcastle; Newcastle: 2002. Why do some non-melanocytic skin cancers reach an advanced stage before they are treated? The effect of delay and predictors of delay in presenation, referral and treatment of NMSC. [Google Scholar]
- Batra RS, Kelley LC. Predictors of extensive subclinical spread in nonmelanoma skin cancer treated with Mohs micrographic surgery. Arch Dermatol. 2002;138:1043–1051. doi: 10.1001/archderm.138.8.1043. [DOI] [PubMed] [Google Scholar]
- Blum A, Brand CU, Ellwanger U, Schlagenhauff B, Stroebel W, Rassner G, Garbe C. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141:783–787. doi: 10.1046/j.1365-2133.1999.03196.x. [DOI] [PubMed] [Google Scholar]
- Brochez L, Verhaeghe E, Bleyen L, Naeyert J-M. Time delays and related factors in the diagnosis of cutaneous melanoma. Eur J Cancer. 2001;37:843–848. doi: 10.1016/s0959-8049(00)00418-4. [DOI] [PubMed] [Google Scholar]
- Cassileth BR, Temoshok L, Frederick BE, et al. Patient and physician delay in melanoma diagnosis. J Am Acad Dermatol. 1988;18:591–598. doi: 10.1016/s0190-9622(88)70081-x. [DOI] [PubMed] [Google Scholar]
- Dunkley MP, Morris AM. Cutaneous malignant melanoma: Audit of the diagnostic process. Ann R Coll Surg Engl. 1991;73:248–252. [PMC free article] [PubMed] [Google Scholar]
- Hennrikus D, Girgis A, Redman S, Sanson-Fisher RW. A community study of delay in presenting with signs of melanoma to medical practitioners. Arch Dermatol. 1991;127:356–361. [PubMed] [Google Scholar]
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer Statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- Joseph AK, Mark TL, Mueller C. The period prevalence and costs of treating nonmelanoma skin cancers in patients over 65 years of age covered by medicare. Dermatol Surg. 2001;27:955–959. doi: 10.1046/j.1524-4725.2001.01106.x. [DOI] [PubMed] [Google Scholar]
- Kirkup ME, De Berker DAR. Clinical measurement of dimensions of basal cell carcinoma: Effect of waiting for elective surgery. Br J Dermatol. 1999;141:876–879. doi: 10.1046/j.1365-2133.1999.03111.x. [DOI] [PubMed] [Google Scholar]
- Krige JEJ, Isaacs S, Hudson DA, King HS, Strover RM, Johnson CA. Delay in the diagnosis of cutaneous malignant melanoma. Cancer. 1991;68:2064–2068. doi: 10.1002/1097-0142(19911101)68:9<2064::aid-cncr2820680937>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Levine J, Kopf AW, Rigel DS, Bart RS, Hennessey P, Friedman RJ, Mintzis MM. Correlation of thicknesses of superficial spreading malignant melanomas and ages of patients. J Dermatol Surg Oncol. 1981;7:311–316. doi: 10.1111/j.1524-4725.1981.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Love N. Why patients delay seeking care for cancer symptoms. Postgrad Med. 1991;89:155–158. doi: 10.1080/00325481.1991.11700868. [DOI] [PubMed] [Google Scholar]
- MacKie RM. Thickness and delay in diagnosis of melanoma: How far can we go? Arch Dermatol. 1999;135:339–340. doi: 10.1001/archderm.135.3.339. [DOI] [PubMed] [Google Scholar]
- McKenna DB, Lee RJ, Prescott RJ, Doherty VR. The time from diagnostic excision biopsy to wide local excision for primary cutaneous malignant melanoma may not affect patient survival. Br J Dermatol. 2002;147:48–54. doi: 10.1046/j.1365-2133.2002.04815.x. [DOI] [PubMed] [Google Scholar]
- Miller SJ. Biology of basal cell carcinoma (part i) J Am Acad Dermatol. 1991;24:1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Christos PJ, Halpern AC, Fine JA, Barnhill RL, Berwick M. Patient knowledge, awareness, and delay in seeking medical attention for malignant melanoma. J Clin Epidemiol. 1999;52:1111–1116. doi: 10.1016/s0895-4356(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Osborne JE, Hutchinson PE. Clinical correlates of breslow thickness of malignant melanoma. Br J Dermatol. 2001;144:476–483. doi: 10.1046/j.1365-2133.2001.04071.x. [DOI] [PubMed] [Google Scholar]
- Pilowsky I. Dimensions of hypochondriasis. Br J Psychiatry. 1967;113:89–93. doi: 10.1192/bjp.113.494.89. [DOI] [PubMed] [Google Scholar]
- Rampen FHJ, Rumke P, Hart AAM. Patients’ and doctors’ delay in the diagnosis and treatment of cutaneous melanoma. Eur J Surg Oncol. 1989;15:143–148. [PubMed] [Google Scholar]
- Randle HW. Basal cell carcinoma. Identification and treatment of the high-risk patient. Dermatol Surg. 1996;22:255–261. doi: 10.1111/j.1524-4725.1996.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Richard MA, Grob JJ, Avril MF, et al. Melanoma and tumor thickness: Challenges of early diagnosis. Arch Dermatol. 1999;135:269–274. doi: 10.1001/archderm.135.3.269. [DOI] [PubMed] [Google Scholar]
- Richard MA, Grob JJ, Avril MF, et al. Delays in diagnosis and melanoma prognosis (i): The role of patients. Int J Cancer. 2000a;89:271–279. [PubMed] [Google Scholar]
- Richard MA, Grob JJ, Avril MF, et al. Delays in diagnosis and melanoma prognosis (ii): The role of doctors. Int J Cancer. 2000b;89:280–285. doi: 10.1002/1097-0215(20000520)89:3<280::aid-ijc11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rowe DE, Carroll RJ, Day CL. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: Implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:424–431. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Wendtner MH, Baumert J, Stange J, Vokenandt M. Delay in the diagnosis of cutaneous melanoma: An analysis of 233 patients. Melanoma Res. 2002;12:389–394. doi: 10.1097/00008390-200208000-00012. [DOI] [PubMed] [Google Scholar]
- Silfen R, Amir A, Regev D, Hauben DJ. Role of physicians and patients in the diagnostic delay of cutaneous malignant melanoma. Ann Plast Surg. 2002;49:439–442. doi: 10.1097/00000637-200210000-00019. [DOI] [PubMed] [Google Scholar]
- Silverman MK, Kopf AW, Grin CM, Bart RS, Levenstein MJ. Recurrence rates of treated basal cell carcinomas. J Dermatol Surg Oncol. 1991;17:713–718. doi: 10.1111/j.1524-4725.1991.tb03424.x. [DOI] [PubMed] [Google Scholar]
- Smeets NW, Kuijpers DI, Nelemans P, Ostertag JU, Verhaegh ME, Krekels GA, Neumann HA. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face—results of a retrospective study and review of the literature. Br J Dermatol. 2004;151:141–147. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- Temoshok L, DiClemente RJ, Sweet DM, Blois MS, Sagebiel RW. Prognostic and psychosocial factors related to delay behavior in patients with cutaneous malignant melanoma. Prog Clin Biol Res. 1984;156:168–179. [PubMed] [Google Scholar]
- Temoshok L, Heller BW, Sagebiel RW, Blois MS, Sweet DM, DiClemente RJ, Gold ML. The relationship of psychosocial factors to prognostic indicators in cutaneous malignant melanoma. J Psychosom Res. 1985;29:139–153. doi: 10.1016/0022-3999(85)90035-2. [DOI] [PubMed] [Google Scholar]
- Weinstock MA, Bingham SF, Cole GW, et al. Reliability of counting actinic keratoses before and after brief consensus discussion. Arch Dermatol. 2001;137:1055–1058. [PubMed] [Google Scholar]
- Zitelli JA, Brown CD, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422–429. doi: 10.1016/s0190-9622(97)70144-0. [DOI] [PubMed] [Google Scholar]


