Abstract
Background
Although research on body dysmorphic disorder (BDD) is increasing, no follow-up studies of this disorder’s course of illness have been published.
Methods
The status of 95 outpatients with BDD treated in a clinical practice was assessed by chart review. Standard scales were used to rate subjects at baseline and the most recent clinic visit (mean duration of follow-up, 1.7 ± 1.1; range, 0.5–6.4 years). Ratings were also done at 6-month intervals over the first 4 years of follow-up.
Results
Allowing for censoring, life table analysis estimated that the proportion of subjects who achieved full remission from BDD at the 6-month and/or 12-month assessment was 24.7%; the proportion who attained partial or full remission at 6 months and/or 12 months was 57.8%. After 4 years of follow-up, 58.2% had experienced full remission, and 83.8% had experienced partial or full remission, at one or more 6-month assessment points. Of those subjects who attained partial or full remission at one or more assessment points, 28.6% subsequently relapsed. Between baseline and the most recent assessment, BDD severity and functioning significantly improved: at the most recent assessment, 16.7% of subjects were in full remission, 37.8% were in partial remission, and 45.6% met full criteria for BDD. Greater severity of BDD symptoms and the presence of major depression or social phobia at baseline were associated with more severe BDD symptoms at study end point. All subjects received at least one medication trial, and 34.3% received some type of therapy during the follow-up period.
Conclusions
A majority of treated patients with BDD improved, although improvement was usually partial. Prospective longitudinal studies are needed to further elucidate the course of BDD.
1. Introduction
Body dysmorphic disorder (BDD), a distressing or impairing preoccupation with an imagined or slight defect in appearance, is a relatively common disorder [1,2]. It is associated with high rates of functional impairment and suicide attempts [3,4], high levels of perceived stress [5], and markedly poor quality of life [6,7]. Although research on BDD is rapidly increasing, this disorder’s course of illness has received virtually no investigation. Available data are from case reports [8], which generally report a chronic course, and 2 studies (n = 188 and n = 200) that asked subjects to retrospectively describe the past course of their BDD symptoms [9,10]. These 2 studies reported a similar mean duration of BDD: 15.7 ± 11.9 years (range, 1–69 years) in one study [9] and 15.8 ± 12.3 years (range, 1–51 years) [10] in the other. Both studies also retrospectively reported a chronic course of BDD symptoms (ie, less than 1 month of remission since onset) in a majority of cases (82% [9] and 81% [10]) as well as a generally worsening course over time (in 61% [9] and 53% [10] of patients). In these studies, information on illness course was limited to these few questions, standard measures were not used to assess course, and all information on course of illness was obtained retrospectively.
Although these data suggest that BDD is usually chronic, results from short-term treatment studies (up to 16 weeks) indicate that a majority of patients improve with cognitive-behavioral therapy (CBT) [11–14] or a serotonin-reuptake inhibitor (SRI) [15–18]. Many of these studies, however, found that treatment typically results in only partial remission, with most patients having remaining symptoms; this is particularly the case for studies with more severely ill patients [13–17]. To our knowledge, no continuation or maintenance treatment studies have been done in BDD except for a small study (n = 10) which found that intensive exposure and response prevention followed by relapse prevention maintained improvement at 2 years [19].
This paper reports on a chart-review study of 95 outpatients with BDD who were treated in a specialty BDD clinical setting for up to more than 6 years. To our knowledge, there are no published follow-up studies on the course of BDD. We hypothesized that although available retrospective data suggest that BDD is usually chronic, a majority of this treated cohort would improve (consistent with results from short-term treatment studies) or further improve after short-term treatment, but that improvement would usually be partial. We also hypothesized that delusional patients and those with more severe BDD symptoms would have a worse course of illness, as these patients tend to be more functionally impaired [20] and have higher levels of perceived stress [5] and poorer quality of life [6,7]. We also predicted that patients with a personality disorder would have a worse course of illness, consistent with the literature on other Axis I disorders [21].
2. Methods
2.1. Subjects and treatment
The sample consisted of 95 outpatients with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) BDD referred to a BDD specialty program for treatment of BDD. Patients were selected for this study from the larger number of patients who received a BDD consultation or treatment based on the following: (1) they were treated in the BDD program clinical practice “naturalistically” (ie, treatment was not protocol-based) for at least 6 months (mean duration, 1.7 ± 1.1 years; range, 0.5–6.4 years); patients were accepted for treatment depending on whether they were interested in receiving treatment and whether there were openings in the practice; (2) patients had to live within driving distance of the clinic, which was in the Northeast; (3) because treatment occurred in a private hospital setting, patients were either insured (in the majority of cases) or paid for treatment themselves; and (4) 54.7% (n = 52) of subjects had previously participated in a descriptive study of BDD’s clinical characteristics (which had no exclusion criteria) [3], and 45.3% (n = 43) had previously participated in an open-label [15] or placebo-controlled [17] BDD pharmacotherapy study. Pharmacotherapy study participants were included in the present study only if they were subsequently treated in the program’s clinical practice for at least 6 months after completion of the clinical trial. Clinical trial data are not included in the present study’s analyses.
All patients received pharmacotherapy in the specialty BDD setting. However, in nearly all cases in which psychotherapy was received, it was provided outside the BDD specialty setting by other clinicians at the hospital where the BDD program is located or by community therapists. Some patients who sought treatment in the BDD program were already receiving ongoing therapy, whereas others were referred for therapy by the first author after she began treating them. Patients were referred for psychotherapy for a variety of reasons: if patients requested it (although therapy was sometimes not received because of insurance/financial restrictions), if the first author thought that their BDD symptoms might benefit from CBT, or if it appeared that problems other than BDD (eg, psychosocial stressors) would potentially benefit from psychodynamic and/or supportive psychotherapy.
The sample’s mean age was 30.6 ± 11.7 years (range, 6–65 years); 80.0% (n = 76) were adults, 18.9% (n = 18) were adolescents, and 1.0% (n = 1) was a child; 54.7% (n = 52) were female; 62.4% (n = 58) were single, 26.9% (n = 25) were married, and 10.8% (n = 10) were divorced. The mean age of BDD onset was 17.2 ± 8.1 years (range, 5–44 years). The most common current comorbid disorders were major depression (52.8%, n = 47), social phobia (29.2%, n = 26), and obsessive-compulsive disorder (OCD; 26.9%, n = 24).
2.2. Assessments
2.2.1. Baseline assessments
The Psychiatric Status Rating Scale for Body Dysmorphic Disorder (BDD-PSR) evaluated whether subjects met full criteria for BDD or were in partial or full remission. PSRs are disorder-specific, reliable, and valid global ratings of disorder severity used in numerous longitudinal studies to track course of illness [22–24]. A PSR for BDD, based on DSM-IV criteria, was adapted from PSRs for mood and anxiety disorders. The BDD-PSR has good interrater and test-retest reliability (intraclass correlation [ICC] = 0.95 and 0.81, respectively) as well as good convergent validity (KAP, unpublished data). The BDD-PSR is a 7-point scale that reflects whether BDD symptoms currently meet full DSM-IV criteria for BDD, are in partial remission, or are in full remission. A score of 1 or 2 indicates full remission (1 = no symptoms of BDD; 2 = some appearance concerns but no distress or impairment in functioning due to BDD); a score of 3 or 4 indicates partial remission (3 = some appearance concerns with either mild distress or mildly impaired functioning; 4 = appearance concerns with both mild distress and mild impairment in functioning); and a score of 5, 6, or 7 indicates full criteria for BDD (5 = appearance concerns present for at least 1 hour per day, and either moderate distress or moderate functional impairment; 6 = appearance preoccupations cause significant distress and significant functional impairment; 7 = appearance concerns cause severe or extreme distress and functional impairment). The Yale-Brown Obsessive Compulsive Scale Modified for Body Dysmorphic Disorder (BDD-YBOCS) is a reliable and valid 12-item semistructured clinician-administered instrument that evaluates current BDD severity [25]. It assesses BDD-related preoccupations, repetitive behaviors, insight, and avoidance. The mean baseline BDD-YBOCS score was 29.3 ± 6.6, reflecting moderately severe BDD.
The Clinical Global Impressions (CGI) Scale, a 7-point scale, was used to evaluate global severity of illness and improvement or worsening of symptoms [26]. Severity ranges from “normal” to “among the most extremely ill patients,” and improvement ratings range from “very much worse” to “very much improved.” Much or very much improvement (score of 1 or 2) on the CGI Scale was defined as improvement in BDD, OCD, or depression. Subjects were also evaluated with the Global Assessment of Functioning (GAF), a global measure of symptom severity and psychological, social, and occupational functioning [27]; scores range from 0 to 90, with more severe illness corresponding to lower scores. The Social and Occupational Functioning Assessment Scale (SOFAS) was added later during the study to assess functioning; it is a 100-point global measure of overall functioning, with scores of 81 to 100 reflecting good or excellent functioning, and scores below 50 reflecting serious or major impairment in functioning [28]. The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R) (SCID-P) [29,30] was used to obtain comorbidity data, and subjects who had previously participated in a pharmacotherapy study were evaluated with the Structured Clinical Interview for DSM-III-R Personality Disorders [31,32] (n = 43).
Because the SCID-P does not include BDD, this disorder was diagnosed with a reliable semistructured SCID-like diagnostic instrument based on DSM-IV criteria [33]. A semistructured instrument (KAP, unpublished data) used in other BDD research [3,9,17] was used to obtain data on demographics and BDD’s clinical characteristics, such as age of BDD onset. Subjects who were evaluated after the Brown Assessment of Beliefs Scale was developed (n = 52) were assessed with this reliable and valid clinician-administered measure to determine the current delusionality of appearance-related beliefs (ie, how convinced they were that their appearance was abnormal) [34]. At baseline, the mean score was 17.4 ± 4.9 (poor insight–delusional range), and 42.3% (n = 22) of subjects were classified as delusional.
2.2.2. Follow-up assessments
Clinical records of treatment in the first author’s BDD program were reviewed to obtain follow-up ratings. Ratings were done for the most recent clinic visit and also at 6-month (±2 months) intervals over the first 4 years of follow-up. Ratings were available for 67 subjects after 1 year, 28 subjects after 2 years, 12 subjects after 3 years, and 3 subjects after 4 years. Six-month interval ratings were not done beyond 4 years because data were available for too few subjects to permit meaningful statistical analyses. Assessments done at 6-month intervals and the most recent clinic visit were (1) BDD-PSR; (2) the first 3 items of the BDD-YBOCS (only the first 3 items were used because this rating could be obtained from charts; these 3 items correspond to DSM-IV diagnostic criteria for BDD and assess preoccupation, interference in functioning, and distress due to appearance concerns on a scale of 0 [no symptoms] to 4 [extreme symptoms]); (3) CGI severity for BDD; (4) CGI improvement for BDD, depression (if major depression was present at baseline), and OCD (if OCD was present at baseline); (5) GAF; and (6) SOFAS.
Information was obtained on treatment received during the entire follow-up period. This included type of medication or therapy, maximum medication dosage, and number of clinic visits. Subjects were considered to have received CBT if treatment included exposure, response prevention, or cognitive techniques that focused on BDD symptoms. Differentiation of supportive from insight-oriented psychotherapy was not attempted, as many treatments consisted of a combination of the 2 approaches.
2.3. Statistical analysis
Kaplan-Meier survival curves [35] estimated the probability of recovery from BDD during up to 4 years of follow-up. Between-group differences were tested using χ2 analysis and Fisher exact test for categorical variables and independent-samples t tests for continuous variables. Within-group differences were measured using the dependent-samples t test. Correlations between selected variables were examined using the Pearson product moment correlation coefficient. Because data were obtained from clinical records, some data are missing; all missing data were excluded on a pairwise basis for analyses. All tests were 2-tailed; a P value of less than .05 was used to determine statistical significance.
3. Results
The life table analysis in Fig. 1 (allowing for censoring) shows the estimated partial remission rates and the partial or full remission rates achieved at one or more 6-month assessment points (as assessed by the BDD-PSR) during the 4-year follow-up period. For example, by 1 year, 24.7% of subjects had achieved full remission and 57.8% had achieved partial or full remission at the 6-month and/or 12-month assessment point. After 4 years, 58.2% of subjects had achieved full remission, and 83.8% had achieved partial or full remission, in at least one assessment point. Of those subjects who achieved partial or full remission at one or more assessment points, and who had at least one 6-month assessment subsequent to attaining remission, 71.4% (n = 25) remained in remission (either partial or full) at all subsequent 6-month follow-up assessments (ie, 28.6% subsequently relapsed at one or more 6-month assessment points). Twenty-nine (30.5%) patients discontinued treatment during the follow-up period; excluding the 7 patients who discontinued treatment because the first author moved her practice, the treatment dropout rate was 23.2% (n = 22). Patients dropped out of treatment for the following reasons: lost contact/unknown (n = 13), the patient moved (n = 2), poor insight and belief that treatment was not necessary (n = 2), financial (n = 2), the patient was doing well with no treatment (n = 1), the patient wanted a different provider (n = 1), and excessive driving distance (n = 1).
Fig. 1.
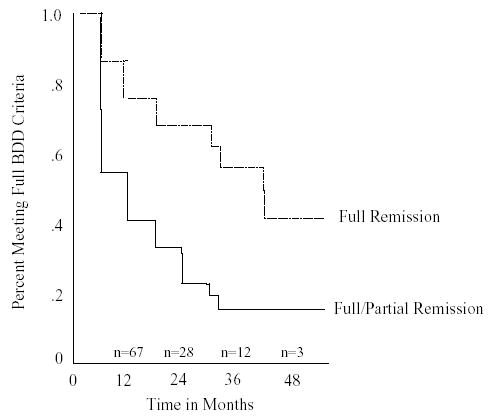
Time to full or partial remission of BDD. The life table analysis above (allowing for censoring) shows the estimated partial remission rates and the partial or full remission rates achieved at one or more 6-month assessment points (as assessed by the BDD-PSR) during the 4-year follow-up period. For example, by 1 year, 24.7% of subjects had achieved full remission and 57.8% had achieved partial or full remission at the 6-month and/or 12-month assessment point.
Table 1 shows ratings at baseline and the most recent clinic visit. Baseline BDD-PSR and BDD-YBOCS scores indicate that at baseline most subjects met full criteria for BDD, which was moderately severe. Body dysmorphic disorder symptom severity significantly decreased, as assessed by the BDD-PSR, BDD-YBOCS, and BDD-CGI severity scale. The mean BDD-PSR score decreased from 5.7 ± 1.0 at baseline to 4.1 ± 1.5 at the last assessment (t = 9.9, df = 89, P < .001). Patients who dropped out of treatment were significantly less ill at the last assessment than those who remained in treatment (end point BDD-PSR score of 3.7 for dropouts vs 4.4 for nondropouts; t = 2.04, df = 88, P = .045). On the BDD-CGI improvement scale, 59.1% of patients were improved at the most recent visit. (This CGI result is roughly comparable to the survival data because, as noted above, of the 83.8% of subjects who achieved partial or full remission at some point during follow-up, 28.6% subsequently relapsed, leaving 59.8% in remission at all remaining follow-up assessments.) Overall symptom severity and level of functioning as assessed by the GAF and SOFAS also significantly improved.
Table 1.
Symptom severity and functional impairment at baseline and the most recent assessment for patients with BDD
| Measure | Baseline (n = 90) | Most recent assessment (n = 90) | Test statistic | df | P |
|---|---|---|---|---|---|
| BDD-PSR | 11.1 | 4 | .026 | ||
| Full remission | 2 (2.2) | 15 (16.7) | |||
| Partial remission | 4 (4.4) | 34 (37.8) | |||
| Full criteria | 84 (93.3) | 41 (45.6) | |||
| BDD-YBOCS | |||||
| Preoccupation | 3.0 ± 0.8a | 2.0 ± 1.0a | 7.5 | 60 | <.001 |
| Interference in functioning | 2.5 ± 0.8b | 1.2 ± 1.2b | 7.8 | 55 | <.001 |
| Distress | 2.5 ± 0.8c | 1.7 ± 1.0c | 7.4 | 56 | <.001 |
| BDD-CGI severity | 4.5 ± 0.9d | 3.4 ± 1.3d | 5.9 | 52 | <.001 |
| BDD-CGI improvement | |||||
| Very much improved | – | 33 (39.8) | |||
| Much improved | – | 16 (19.3) | |||
| Minimally improved | – | 14 (16.9) | |||
| Unchanged | – | 20 (24.1) | |||
| GAF | 50.6 ± 11.9 | 65.0 ± 16.2 | −6.4 | 55 | <.001 |
| SOFAS | 58.0 ± 10.2 | 68.6 ± 19.9 | −4.1 | 27 | <.001 |
Values are expressed as mean ± SD or as n (%). BDD-CGI severity indicates CGI scale for BDD severity; BDD-CGI improvement, CGI scale for BDD improvement.
2 = 1 to 3 hours per day, 3 = >3 to 8 hours per day.
1 = mild interference, 2 = moderate interference, 3 = severe interference.
1 = mild distress, 2 = moderate distress, 3 = severe distress.
3 = mildly ill, 4 = moderately ill, 5 = markedly ill.
Regarding predictors of outcome, baseline BDD severity (BDD-YBOCS score), as hypothesized, was significantly positively correlated with BDD severity (BDD-PSR score) at the most recent assessment (r = 0.34, P = .003). Current major depression (r = 0.33, P = .002) and current social phobia (r = 0.24, P = .03) at baseline were also significantly correlated with BDD severity at the most recent assessment. However, there was no significant association between end point BDD severity and the presence of a personality disorder (r = 0.22, P = .19) or OCD (r = 0.21, P = .06) at baseline, baseline delusionality (r = −0.11, P = .44), or BDD duration (r = −0.04, P = .73).
Of those subjects whose BDD improved (on the BDD-CGI) between baseline and the most recent assessment, 13 of 15 with major depression at baseline also had improvement in depression, and 6 of 6 with OCD at baseline also had improvement in OCD. Conversely, of subjects with current OCD at baseline, 6 of 10 had improvement in OCD, all 6 of whom also had improvement in BDD. Of subjects with major depression at baseline, 18 (54.5%) of 33 had improvement in depression, 13 (76.5%) of whom also had improvement in BDD.
During the follow-up period, all subjects received psychotropic medication, with 2.8 ± 2.3 (range, 1–13) medication trials and 17.2 ± 24.2 (range, 2–164) medication visits per subject; on average, medication visits occurred every 5 weeks. All subjects received an SRI, 36.4% (n = 32) received buspirone, 26.1% (n = 23) an antipsychotic, 12.5% (n = 11) a benzodiazepine, 9.1% (n = 8) a non-SRI antidepressant, 8.0% (n = 7) a mood stabilizer, and 5.7% (n = 5) a stimulant. The mean maximum SRI doses were: fluoxetine, 68.3 ± 26.7 mg/d (range, 20–140 mg/d; n = 72 trials); clomipramine, 165.5 ± 76.0 mg/d (range, 50–250 mg/d; n = 21 trials); fluvoxamine, 229.4 ± 93.2 mg/d (range, 25–350 mg/d; n = 17 trials); sertraline, 164.7 ± 76.1 mg/d (range, 25–300 mg/d; n = 17 trials); and paroxetine, 41.5 ± 14.6 mg/d (range, 20–70; n = 13 trials). There was a trend for subjects meeting full BDD criteria at the most recent assessment to have received more medications than those who did not meet full criteria (3.5 ± 2.3 vs 2.5 ± 2.5, t = −1.8, df = 81, P = .08). A total of 48.6% (n = 36) of subjects received therapy: 34.3% (n = 24) received supportive or insight-oriented psychotherapy, and 21.7% (n = 20) received CBT. The mean number of therapy sessions was 20.9 ± 23.8 (range, 3–88). Subjects who met full BDD criteria at the most recent assessment were as likely to have received CBT as those who did not meet full criteria (57.9% [n = 11] vs 42.1% [n = 8], χ2 = .47, df = 1, P = .47); this was also the case for non-CBT psychotherapy (50.0% [n = 11] for both groups).
4. Discussion
Although BDD has been considered a chronic disorder [3,8,9], a majority of the treated patients in this study improved. Between the baseline and final assessments, BDD symptom severity significantly decreased, and level of functioning significantly improved. In addition, a majority of subjects attained remission at one or more 6-month assessment points. Because patients who dropped out of treatment were significantly less ill than those who remained in treatment (although the difference was only marginally significant), the course of treated patients may actually be somewhat more favorable than found in this study. Nonetheless, more than one quarter of subjects who remitted during the follow-up period subsequently relapsed. Also, at study end point, partial remission was more common than full remission. Thus, BDD symptoms tended to improve but continued to be present to some degree over time.
The remission rates found in this study are higher than those suggested by previous course data. This finding probably reflects the fact that earlier data indicating a more chronic course of illness corresponded to a period when BDD was less recognized by mental health professionals [36] and less was known about what constituted effective treatment [8]. It seems likely that a study of course of illness in untreated patients would find a more chronic and unremitting course than the present study, in which all patients were treated in a BDD program. Nonetheless, although it is likely that the current study’s remission rates are at least partly attributable to treatment, a causal relationship between treatment received and course of illness cannot be established in a naturalistic study such as this.
It is interesting that the proportion of subjects who were very much improved on the CGI between baseline and the last assessment (39.8%) was the same as in a small citalopram study (40%) [37] but higher than in 2 short-term BDD pharmacotherapy studies (12% in a placebo-controlled fluoxetine trial [17] and 30% in an open-label fluvoxamine trial) [15]. Thus, although in this study the relationship between treatment and outcome is unclear, these results nonetheless suggest that longer-term treatment may be associated with a more favorable outcome.
It is worth noting that there was a trend for subjects who met full BDD criteria at the last assessment to have received more medication trials than subjects who did not meet full criteria. This outcome is counterintuitive because if treatment is effective, subjects with worse outcomes would be expected to have received less medication treatment than those with better outcomes. This result is similar, however, to results of other naturalistic studies [38,39] and probably reflects continued provision of treatment to subjects who failed previous treatments and remained ill (ie, confounding by severity).
It is notable that only 21.7% of patients received CBT, although clinical series and studies using waiting list controls suggest that CBT is often effective for BDD [11–14,19]. Although we did not collect data on the reasons CBT was received by relatively few patients, our impression is that this was generally attributable to several factors: lack of adequate insurance coverage; limited availability of CBT-trained therapists; some patients’ reluctance to engage in a treatment perceived as requiring effort; and acceptable response to an initial pharmacotherapy trial. Our finding that subjects who received CBT did not have a more favorable course than those who did not receive CBT may in part reflect referral bias, in that patients who had an acceptable response to pharmacotherapy were not referred for CBT, whereas attempts were made to refer those with more refractory symptoms. Approximately one third of subjects received non-CBT psychotherapy, which very limited data suggest is generally ineffective for BDD [3]; some patients were already receiving this treatment before they received medication in the BDD specialty program, whereas in other cases the program referred them for this treatment to focus on symptoms and issues other than BDD (eg, psychosocial stressors). Because our study was a naturalistic follow-up study that investigated course of illness, we did not focus on outcomes of specific treatments; further efficacy and effectiveness studies are needed to examine this issue. Our clinical impression, however, is that BDD often improves with CBT, consistent with published efficacy trials [11–14], and that supportive and/or psychodynamic therapy may be helpful for other issues and problems, such as coping with psychosocial stressors.
Although this study had limited power to investigate predictors of outcome, more severe BDD symptoms at baseline predicted poorer outcome, as is often found with other Axis I disorders [38,40–42]. Our finding that patients with a personality disorder did not have a worse outcome is inconsistent with many, although not all, studies of other Axis I disorders [21] and with a previous report that patients with BDD who have a personality disorder tend to have more severe BDD symptoms [43]. Our hypothesis that more delusional subjects would have a poorer outcome was not supported. However, the number of subjects in these analyses was relatively small, and larger studies are needed to investigate predictors of outcome in BDD.
It is interesting that BDD always improved concurrently with comorbid OCD and often, but not always, improved concurrently with comorbid major depression. These results suggest that BDD may be closely related to OCD and depression, as has been hypothesized [44–46]. An alternative explanation is that the treatments patients received (SRIs) are effective for OCD and depression as well as BDD. It is worth noting, however, that although BDD and depression usually improved concurrently, 23.5% of individuals whose major depression improved did not have improvement in BDD, suggesting that BDD is not simply a symptom of depression. This finding is consistent with results from pharmacotherapy studies, in which improvement in BDD and depression has been significantly correlated but with correlation coefficients of only 0.40 [15] to 0.65 [17].
This study’s major limitation is that ratings were based on chart review, and follow-up ratings were done retrospectively. Some ratings could not be obtained; for example, the clinical records had inadequate information on the course of social phobia, which has been hypothesized to be related to BDD [8,47]. In addition, more detailed assessments of psychosocial functioning (other than the GAF, SOFAS, and item 2 of the BDD-YBOCS) were not done. Another limitation is that ratings were done at only 6-month intervals; some remissions and relapses likely occurred between rating points, so that remission and relapse rates may actually be somewhat higher than were captured by doing ratings only every 6 months. Analyses of predictors of outcome were limited by the relatively small number of subjects with personality disorder ratings. In addition, there are a number of factors that may limit the generalizability of the results. First, all subjects were treated in a BDD specialty program, which may have led to better outcomes than might occur in other settings. On the other hand, many patients were treated in an era before it was known what constituted efficacious treatment of BDD (and knowledge about this is still limited), so that even “specialty” treatment has limitations. Second, most patients were seen in a private psychiatric hospital setting in the Northeastern United States and were insured; thus, there is a need for follow-up studies in other settings. Third, it is possible that because patients were seen in a specialty setting they tended to be more severely ill than community patients. On the other hand, it is possible that they were less severely ill because nearly half were first treated in a pharmacotherapy study, which excluded certain patients (eg, those with clinically significant suicidality or a current substance-related disorder). Despite these limitations, the study also has a number of strengths, including the well-characterized nature of the sample, use of standard rating scales, and nonretrospective assessments at baseline.
In conclusion, this study—the first to investigate the course of BDD—suggests that a majority of patients treated in a clinical outpatient setting improve over time, although many improve only partially. Further course studies are needed in which larger and more diverse samples are assessed (eg, nonspecialty clinical samples, community samples), follow-up data are obtained prospectively, and ratings are done at more frequent intervals. Such studies will further elucidate the course of this relatively common and severe psychiatric disorder.
Footnotes
This study was supported by a grant from the Department of Psychiatry and Human Behavior, Brown Medical School (Providence, RI), and by NIMH (Bethesda, MD) grant K24-MH63975 to Doctor Phillips.
References
- 1.Bienvenu OJ, Samuels JF, Riddle MA, Hoehn-Saric R, Liang KY, Cullen BAM, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry. 2000;48:287–93. doi: 10.1016/s0006-3223(00)00831-3. [DOI] [PubMed] [Google Scholar]
- 2.Perugi G, Akiskal HS, Lattanzi L, Cecconi D, Mastrocinque C, Patronelli A, et al. The high prevalence of “soft” bipolar (II) features in atypical depression. Compr Psychiatry. 1998;39:63–71. doi: 10.1016/s0010-440x(98)90080-3. [DOI] [PubMed] [Google Scholar]
- 3.Phillips KA, McElroy SL, Keck PE, Jr, Pope HG, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry. 1993;150:302–8. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- 4.Veale D, Boocock A, Gournay K, Dryden W, Shah F, Wilson R, et al. Body dysmorphic disorder: a survey of fifty cases. Br J Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- 5.DeMarco LM, Li LC, Phillips KA, McElroy SL. Perceived stress in body dysmorphic disorder. J Nerv Ment Dis. 1998;186:724–6. doi: 10.1097/00005053-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Phillips KA. Quality of life for patients with body dysmorphic disorder. J Nerv Ment Dis. 2000;188:170–5. doi: 10.1097/00005053-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Phillips KA, Menard W, Fay C, Pagano M. Psychosocial functioning and quality of life in body dysmorphic disorder. Compr Psychiatry. 2005;46:254–60. doi: 10.1016/j.comppsych.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips KA. Body dysmorphic disorder: the distress of imagined ugliness. Am J Psychiatry. 1991;148:1138–49. doi: 10.1176/ajp.148.9.1138. [DOI] [PubMed] [Google Scholar]
- 9.Phillips KA, Diaz S. Gender differences in body dysmorphic disorder. J Nerv Ment Dis. 1997;185:570–7. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with BDD. Psychosomatics. doi: 10.1176/appi.psy.46.4.317. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veale D, Gournay K, Dryden W, Boocock A, Shah F, Willson R, et al. Body dysmorphic disorder: a cognitive behavioural model and pilot randomized controlled trial. Behav Res Ther. 1996;34:717–29. doi: 10.1016/0005-7967(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 12.Rosen JC, Reiter J, Orosan P. Cognitive-behavioral body image therapy for body dysmorphic disorder. J Consult Clin Psychol. 1995;63:263–9. doi: 10.1037//0022-006x.63.2.263. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm S, Otto MW, Lohr B, Deckersbach T. Cognitive behavior group therapy for body dysmorphic disorder: a case series. Behav Res Ther. 1999;37:71–5. doi: 10.1016/s0005-7967(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 14.Neziroglu F, Khemlani-Patel S. A review of cognitive and behavioral treatment for body dysmorphic disorder. CNS Spectr. 2002;7:464–71. doi: 10.1017/s1092852900017971. [DOI] [PubMed] [Google Scholar]
- 15.Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry. 1998;59:165–71. doi: 10.4088/jcp.v59n0404. [DOI] [PubMed] [Google Scholar]
- 16.Hollander E, Allen A, Kwon J, Aronowitz B, Schmeidler J, Wong C, et al. Clomipramine vs desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry. 1999;56:1033–9. doi: 10.1001/archpsyc.56.11.1033. [DOI] [PubMed] [Google Scholar]
- 17.Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry. 2002;59:381–8. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- 18.Phillips KA. Pharmacologic treatment of body dysmorphic disorder: review of the evidence and a recommended treatment approach. CNS Spectr. 2002;7:453–60. doi: 10.1017/s109285290001796x. [DOI] [PubMed] [Google Scholar]
- 19.McKay D. Two-year follow-up of behavioral treatment and maintenance for body dysmorphic disorder. Behav Modif. 1999;23:620–9. doi: 10.1177/0145445599234006. [DOI] [PubMed] [Google Scholar]
- 20.Phillips KA, McElroy SL, Keck PE, Jr, Hudson JI, Pope HG. A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacol Bull. 1994;30:179–86. [PubMed] [Google Scholar]
- 21.Reich JH, Vasile RG. Effect of personality disorders on the treatment outcome of axis I conditions: an update. J Nerv Ment Dis. 1993;181:475–84. doi: 10.1097/00005053-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Coryell W, Leon A, Winokur G, Endicott J, Keller M, Akiskal H, et al. Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry. 1996;153:483–9. doi: 10.1176/ajp.153.4.483. [DOI] [PubMed] [Google Scholar]
- 23.Keller MB, Yonkers KA, Warshaw MG, Gollan J, Massion AO, White K, et al. Remission and relapse in subjects with panic disorder and panic with agoraphobia: a prospective short-interval naturalistic follow-up. J Nerv Ment Dis. 1994;182:290–6. doi: 10.1097/00005053-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Warshaw MG, Keller MB, Stout RL. Reliability and validity of the longitudinal interval follow-up evaluation for assessing outcome of anxiety disorders. J Psychiatr Res. 1994;28:531–45. doi: 10.1016/0022-3956(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 25.Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive-Compulsive Scale. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- 26.National Institute of Mental Health. Special feature: rating scales and assessment instruments for use in pediatric psychopharmacology research. Psychopharmacol Bull. 1985:21. [PubMed] [Google Scholar]
- 27.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 28.Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- 29.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID): I. History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 30.Williams JBW, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM III-R Personality Disorders (SCID-II) Part I: description. J Personal Disord. 1995;9:83–91. [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW, Davies M, Borus J, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multi-site test-retest reliability study. J Personal Disord. 1995;9:92–104. [Google Scholar]
- 33.Phillips KA, Atala KD, Pope HG. Diagnostic instruments for body dysmorphic disorder. New research program and abstracts, American Psychiatric Association 148th Annual Meeting; Miami. 1995. p. 157. [Google Scholar]
- 34.Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry. 1998;155:102–8. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- 35.Cox D, Oakes D. New York: Chapman and Hall; 1984. Analysis of survival data. [Google Scholar]
- 36.Phillips KA, Nierenberg AA, Brendel G, Fava M. Prevalence and clinical features of body dysmorphic disorder in atypical major depression. J Nerv Ment Dis. 1996;184:125–9. doi: 10.1097/00005053-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Phillips KA, Najjar F. An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry. 2003;64:715–20. doi: 10.4088/jcp.v64n0615. [DOI] [PubMed] [Google Scholar]
- 38.Leon AC, Keller MB, Warshaw MG, Mueller TI, Solomon DA, Coryell W, et al. Prospective study of fluoxetine treatment and suicidal behavior in affectively ill subjects. Am J Psychiatry. 1999;156:195–201. doi: 10.1176/ajp.156.2.195. [DOI] [PubMed] [Google Scholar]
- 39.Warshaw MG, Keller MB. The relationship between fluoxetine use and suicidal behavior in 654 subjects with anxiety disorders. J Clin Psychiatry. 1996;57:158–66. [PubMed] [Google Scholar]
- 40.Geerlings SW, Beekman AT, Deeg DJ, Twisk JW, Van Tilburg W. Duration and severity of depression predict mortality in older adults in the community. Psychol Med. 2002;32:609–18. doi: 10.1017/s0033291702005585. [DOI] [PubMed] [Google Scholar]
- 41.Steketee G, Eisen J, Dyck I, Warshaw M, Rasmussen S. Predictors of course in obsessive-compulsive disorder. Psychiatry Res. 1999;27:229–38. doi: 10.1016/s0165-1781(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 42.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–53. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 43.Phillips KA, McElroy SL. Personality disorders and traits in patients with body dysmorphic disorder. Compr Psychiatry. 2000;41:229–36. doi: 10.1053/comp.2000.7429. [DOI] [PubMed] [Google Scholar]
- 44.Hollander E, Cohen LJ, Simeon D. Body dysmorphic disorder. Psychiatr Ann. 1993;23:359–64. [Google Scholar]
- 45.Phillips KA, McElroy SL, Hudson JI, Pope HG., Jr Body dysmorphic disorder: an obsessive compulsive spectrum disorder, a form of affective spectrum disorder, or both? J Clin Psychiatry. 1995;56(S):41–52. [PubMed] [Google Scholar]
- 46.McElroy SL, Phillips KA, Keck PE., Jr Obsessive-compulsive spectrum disorders. J Clin Psychiatry. 1994;55(S):33–51. [PubMed] [Google Scholar]
- 47.Kasahara Y. Seoul, Korea: East Asia Academy of Cultural Psychiatry; 1987. Social phobia in Japan. Social phobia in Japan and Korea, Proceedings of the First Cultural Psychiatry Symposium Between Japan and Korea. [Google Scholar]


