Abstract
Acute rheumatic fever is a serious autoimmune sequel of Streptococcus pyogenes infection. This study shows that serotype M3 and M18 S. pyogenes isolated during outbreaks of rheumatic fever have the unique capability to bind and aggregate human basement membrane collagen type IV. M3 protein is identified as collagen-binding factor of M3 streptococci, whereas M18 isolates bind collagen through a hyaluronic acid capsule, revealing a novel function for M3 protein and capsule. Following in vivo mouse passage, conversion of a nonencapsulated and collagen-binding negative M1 S. pyogenes into an encapsulated, collagen-binding strain further supports the crucial role of capsule in mediating collagen binding. Collagen binding represents a novel colonization mechanism, as it is demonstrated that S. pyogenes bind to collagen matrix in vitro and in vivo. Moreover, immunization of mice with purified recombinant M3 protein led to the generation of anti–collagen type IV antibodies. Finally, sera from acute rheumatic fever patients had significantly increased titers of anti–collagen type IV antibodies as compared with healthy controls. These findings may suggest a link between the potential of rheumatogenic S. pyogenes isolates to bind collagen, and the presence of collagen-reactive autoantibodies in the serum of rheumatic fever patients, which may form a basis for post-streptococcal rheumatic disease. These anti-collagen antibodies may form a basis for poststreptococcal rheumatic disease.
Introduction
Group A streptococci (GAS) are able to cause acute rheumatic fever (ARF) and subsequent rheumatic heart disease (RHD) after infection in humans. Since ARF and RHD constitute the major cause of mortality due to heart disease below the age of 50 worldwide (1), the pathogenesis of this disease has been the focus of interdisciplinary research. The pathogenic mechanism, however, by which certain GAS but not others evoke autoimmune inflammatory processes remains unresolved.
It is generally believed that autoimmunity following streptococcal infection is responsible for the organ damage in ARF and RHD patients (2). The current concept that attempts to explain how streptococci trigger a host-directed autoimmune response is based on heart cross-reactivity induced by molecular mimicry — the presence of common epitopes in bacteria and the human host (3, 4). Besides streptococcal membrane peptides (5) and the group A carbohydrate (6), M protein is the favorite molecule in this concept, since myosin or other heart–cross-reactive epitopes have been identified (7–9). ARF is a multifocal inflammatory disease that may affect the heart (carditis), joints (arthritis), CNS (chorea), skin, and subcutaneous areas. These clinical manifestations could be explained by the presence of several distinct, tissue-specific autoantigens, or by a ubiquitous host antigen that might serve as autoimmune target in several tissues. In this scenario, extracellular host factors present in basement membrane represent attractive candidates in rheumatic disease. Beside this, ECM proteins are targets for Streptococcus pyogenes during the initial infection process, enabling the organism to adhere, colonize, and evade the host defense mechanisms (4, 10, 11). This study now analyzes the interaction of S. pyogenes with collagen type IV (CIV), one of the major constituents of basement membrane (12), since it (a) is an attractive target for pathogen colonization, (b) is a labile factor involved in a series of autoimmune syndromes observed in humans and animals (13–16), and (c) appears to be affected during rheumatic disease (17). Our aim was thus to elaborate how GAS interact with human CIV, to identify the bacterial factors involved, and to define their biological function. Based on these findings, the further aim was to analyze the potential to generate anti-collagen antibodies in mice, and to test sera from ARF patients for the presence of collagen-reactive autoantibodies.
Methods
Streptococcal strains and culture conditions.
S. pyogenes strains representing 43 M types (M types 1–6, 11–14, 17–19, 22, 23, 25, 28, 30, 33, 41, 42, 44, 49, 52–54, 56–59, 63, 65, 68–70, 74–78, 80, 81, and 89) were collected worldwide from various infections. Rheumatic fever–associated M type 3 and M type 18 GAS isolates were collected during outbreaks of ARF in the US (18). M3 S. pyogenes C203 (ATCC12384; American Type Culture Collection, Rockville, Maryland, USA) and its M-negative variant C203S (ATCC14289; American Type Culture Collection) as well as the M1 blood isolate KTL3 (19) were also used. The Streptococcus gordonii strain was described previously (20). Streptococci were grown as described previously (11).
Collagen-binding assays.
Streptococci were suspended in PBS to give 5 × 108 bacteria per ml. Then, 1 × 108 bacteria were incubated with 30 ng (20 nCi) of 125I-labeled CIV isolated from placenta (Calbiochem; Merck Biosciences GmbH, Schwalbach, Germany) for 45 minutes at 22°C. Bacteria were processed and binding was assessed as described previously (11). For removal of capsule, 1 × 108 bacteria were incubated with 20 μl (60 U) hyaluronidase (AppliChem GmbH, Darmstadt, Germany) for 45 minutes at 37°C, washed three times in 1 ml phosphate-buffered saline pH 7.5 containing 0.05% tween 20 (PBST), and resuspended in a final volume of 250 μl PBST. To denature proteins on the streptococcal surface, we suspended bacteria in PBS, boiled them for 15 minutes at 100°C in a water bath, and tested them for collagen binding. We performed ligand overlay assays by spotting purified M proteins (5 μg, 1 μg, or 0.2 μg) and glutathione-S-transferase (GST) on nitrocellulose membrane or blotting M proteins following SDS-PAGE. Filters were processed as described previously (20). In pulldown experiments, 50 μg pure recombinant GST-M fusion protein was mixed with 0.5 μg soluble radiolabeled CIV in a total volume of 70 μl PBS. The mixture was incubated for 45 minutes at 22°C prior to addition of 100 μl of a 50% glutathione sepharose solution in PBS. The mixture was incubated 30 minutes at 22°C, beads were washed three times with 1 ml PBS and precipitated, and the amount of sepharose-bound CIV/M protein complex was determined by analysis of the pellet in a gamma counter.
Polymorphonuclear cell–binding assay.
Streptococci were suspended in PBS to give 1 × 108 bacteria per ml. From this suspension, 0.25 ml was either incubated with 10 μg soluble CIV alone, or preincubated with 10 μg soluble collagen prior to addition of 10 μg soluble fibronectin. Streptococci were washed and labeled, and polymorphonuclear (PMN) cell binding was assessed by flow cytometry as described previously (11). The percentage of fluorescent PMN cells was used as a measure of attached and/or phagocytosed streptococci.
Electron microscopy.
For field emission scanning electron microscopy (FESEM) of streptococci, 10 μg CIV was added to 2.5 × 107 bacteria suspended in 250 μl PBST, and incubated at 22°C for 30 minutes. After washing, samples were fixed and processed as described previously (21). Collagen fibers were prepared from mouse tail, transferred into 1 ml DMEM, and incubated with 1 × 108 bacteria for 4 hours at 37°C. Fibers were excessively washed and processed for FESEM analysis. For pre-embedding immunogold labeling of bound collagen, bacteria were incubated with a 1:100 dilution of an anti-collagen antibody (Progen Biotechnik GmbH, Heidelberg, Germany) and protein A/G–coated colloidal gold particles (10 nm in diameter; British BioCell International Ltd., Cardiff, United Kingdom), fixed with 2% glutaraldehyde and 5% formaldehyde, dehydrated with ethanol, and embedded in LRWhite resin following the scheme recommended by the manufacturer (London Resin Co., Reading, United Kingdom). Collagen gold complexes were generated by addition of 10 μg soluble CIV to 1 ml of colloidal gold particles (15 nm in diameter, pH 5.5), incubated for 30 minutes at 22°C, and centrifuged. The gold pellet was dissolved in PBS containing 0.5 mg polyethylene glycol (molecular weight 20,000). Bacteria were incubated with collagen gold complexes for 1 hour at 30°C, washed, and adsorbed onto carbon-coated Formvar (Sigma-Aldrich, Taufkirchen, Germany) films. After air-drying, uncoated samples were examined by low-voltage (1.5 kV) field emission scanning electron microscopy using a DSM982 Gemini FESEM; Zeiss). M18 capsule was visualized by a lysine-acetate–based formaldehyde-glutaraldehyde ruthenium red–osmium embedding procedure according to the method of Fassel et al. (22).
Cloning, purification, and characterization of M proteins.
For generation of GST–M protein or histidine-tagged fusions, emm3, emm6, and emm18 gene fragments were amplified from the chromosomal DNA of the respective serotype GAS via PCR using the following primers: 5′-GCAGACAGTAGGATCCGATGCTAGGAGTG-3′ (M3), 5′-TCAAACAGAAGGAT-CCGCAGCACCCCTTA-3′ (M18), 5′-GAAGTTAGTGGATCCGTGTTTCCTAGG-3′ (M6), and 5′-CACCTGTTGAGTCGACCTGTCTCTTAGTT-3′ (M3.1, M18, and M6 reverse). A C-terminally truncated subclone of M3 was generated using reverse primer 5′-GACGGCTTGCTGCGACGATTTGTTTTTCTT-3′ (M3.2 reverse) Standard cloning techniques were used to generate constructs in the pGEX6P-1 vector system (Amersham Biosciences Europe GmbH, Freiburg, Germany) or pQE30 vector (QIAGEN GmbH, Hilden, Germany). Proteins were expressed, purified under native conditions, and processed for cleavage and removal of the GST part following the manufacturer’s protocol.
Mouse infection experiments.
For mouse passage and in vivo localization of S. pyogenes, streptococci were grown in Todd Hewitt broth to an OD600 of 0.3. Bacteria were washed and resuspended in PBS, and a single dose of 5 × 108 streptococci suspended in 0.1 ml PBS was injected subcutaneously into 6-week-old pathogen-free female BALB/c mice (Harlan Winkelmann GmbH, Borchen, Germany). After 72 hours, streptococci were reisolated from blood. To lyse erythrocytes, 200 μl blood was mixed with 5 ml deionized water and added to 45 ml broth. Bacteria were then grown for 18 hours at 37°C. Streptococci were analyzed for capsule expression in transmission electron microscopy, and for collagen binding as described above. For in vivo localization of S. pyogenes, skin was taken from areas of local abscess formation. Samples were fixed with 2% glutaraldehyde and 3% formaldehyde in cacodylate buffer (0.1 M cacodylate, 0.09 M sucrose, 0.01 M CaCl2, 0.01 M MgCl2 [pH 6.9]) for 2 hours and washed with cacodylate buffer. For FESEM analysis, samples were dehydrated with a graded series of acetone and critical point dried with liquid CO2, using a CPD040 (BAL-TEC, Balzers, Liechtenstein). Samples were fractured, and fracture faces were sputter-coated with a thin gold film and examined in a DSM982 Gemini FESEM (Zeiss) at 5 kV using an Everhart-Thornley secondary electron detector (Zeiss) and an inlens secondary electron detector (Zeiss) at a 50:50 ratio. For transmission electron microscopy analysis, samples were fixed with osmium, dehydrated with acetone, and embedded in Spurr’s epoxy resin. Ultrathin sections were counterstained with uranyl acetate and lead citrate prior to observation in a transmission electron microscope (TEM910; Zeiss) at an acceleration voltage of 80 kV.
Immunization of mice and detection of collagen-reactive antibodies.
Pathogen-free 8-week-old female BALB/c mice were immunized intraperitoneally with 100 μg recombinant purified M3 protein (n = 5) or PBS suspended in 100 μl Freund’s incomplete adjuvant per dose at days 1, 7, and 14. At day 21, serum samples of each group were collected, pooled, and tested in ELISA. To absorb M3-reactive antibodies, 10 μl serum pool was diluted 1:50 in PBS, and incubated for 2 hours with 2 mg GST–M3 protein immobilized on agarose. Antibodies bound to immobilized M3 protein were removed by centrifugation prior to ELISA analysis. To determine anti-collagen antibody titers, plates (Greiner, Frickenhausen, Germany) were coated overnight at 4°C with anti–human CIV rabbit serum (Progen Biotechnik GmbH) diluted 1:100 in coating buffer, blocked with 2% BSA in PBS, and incubated with CIV (2 μg/ml in PBS) for 1 hour at room temperature. Mouse sera (diluted 1:800 in PBS) were added to wells and incubated for 1 hour at room temperature. After washing, a 1:1,000 dilution of HRP-conjugated goat anti-mouse Ig (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) was added and incubated for 1 hour. Antibody binding was detected using 2,2-azino-di-[3-ethylbenzthiazoline sulfonate] diammonium salt (ABTS) tablets (Boehringer Ingelheim GmbH, Mannheim, Germany) as substrate. The absorbance was determined at 405 nm in triplicates.
Patient sera and detection of human antibodies.
Sera were obtained from acute-stage ARF patients as validated by the Jones criteria. All patients presented with carditis and arthritis. Control sera were taken from healthy individuals or uncomplicated-pharyngitis patients from the same geographical area 2–4 weeks after onset of illness. For determination of myosin or streptolysin O–reactive antibodies, plates were coated overnight at 4°C with either 1 μg/ml human heart myosin (Quartett Immunodiagnostika und Biotechnologie GmbH, Berlin, Germany) or streptolysin O (Sigma-Aldrich) in PBS (pH 7.6). Collagen coating was performed as described above. Patient sera (diluted 1:50 to 1:50,000 in serial dilutions in PBS) were added to wells and incubated overnight at 4°C. After washing, a 1:5,000 dilution of HRP-conjugated rabbit anti-human IgG (Sigma-Aldrich) was added and incubated for 1 hour at 37°C. Antibody binding was detected as described above. The cutoff titer for CIV was defined as 165 calculated from sera from healthy controls. Anti-GAS cell wall polysaccharide antibodies were detected as described by Thakur and Prakash (23). Serum titers were obtained in independent assays and normalized against ARF3, a rheumatic fever serum included in all tests for standardization.
Results
Serotype M3 and M18 streptococci bind collagen.
A collection of streptococcal isolates (n = 125) was tested for the ability to bind radiolabeled human CIV. These isolates represented 43 different M types from throat, wound, skin infection, invasive disease, or ARF. The majority of GAS isolates did not bind collagen (less than 5% binding), whereas 10% (n = 13) of the isolates showed strong (80–100%) binding activity toward CIV. Analyses of the M serotypes of the collagen-binding strains revealed that they belonged to either type M3 or type M18. To further validate these findings, we tested a collection of M type 3 (n = 5) or highly mucoid M type 18 (n = 5) GAS serotypes isolated during ARF outbreaks in the US for CIV binding. All of these ARF-associated M3 and M18 streptococcal isolates bound between 80% and 90% CIV.
Collagen aggregation affects PMN cell binding.
To characterize the physiology of GAS in the presence of collagen, we analyzed M3 and M18 streptococci after incubation with CIV via FESEM. All M3 (n = 6) and M18 (n = 6) GAS tested revealed a rough and clumping phenotype due to matrix-like association of collagen on the streptococcal surface, whereas nonbinding strains exhibited a non-clumping and smooth phenotype (Figure 1, a and b) that was also displayed by all isolates in the absence of CIV (data not shown). Immunogold labeling of collagen demonstrated that the rough phenotype indeed reflects collagen aggregation, as gold particles were associated on the bacterial surface only in the presence of CIV (Figure 1c). Since aggregation of streptococci was shown to inhibit phagocytosis (11), binding of M3 streptococci to human PMN cells was tested following collagen and fibronectin incubation. Bacteria preincubated with either fibronectin or collagen alone were bound by PMN cells as untreated bacteria, whereas streptococci precoated with both matrix proteins formed large aggregates and PMN cell binding was inhibited to 74% (PMN cell binding of S. pyogenes was 46% ± 0.1% SD without coating, 12% ± 0.3% SD with addition of collagen and fibronectin, and 45% ± 0.5% SD with addition of either fibronectin or collagen). These results show that collagen aggregation alone is sufficient to mediate aggregation, and that collagen and fibronectin binding synergistically protect the pathogen from PMN cell binding and, thus, from subsequent ingestion.
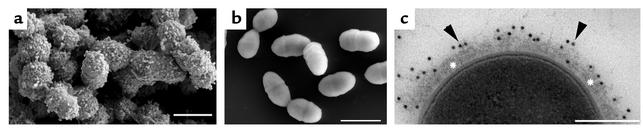
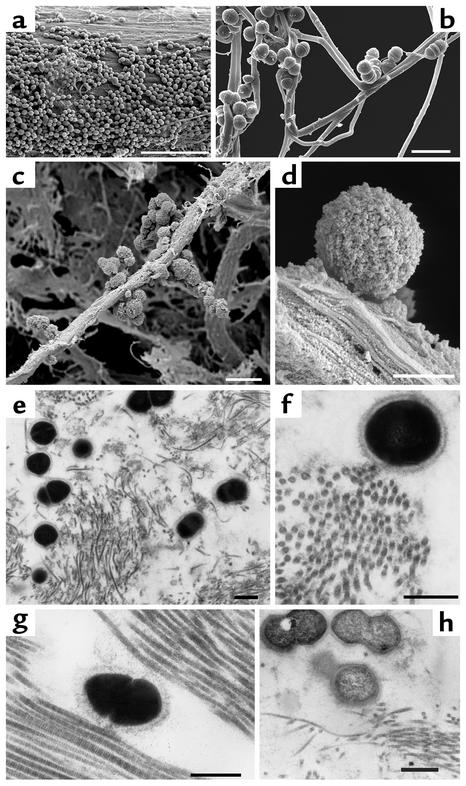
Figure 1.
Collagen matrix association of GAS. FESEM shows a thick CIV network on the surface of a collagen-binding M3 strain 86764 (18) isolated from a rheumatic fever patient (a) that is absent in strains that do not bind CIV (A40; b). Transmission electron microscopy of collagen-binding serotype M3 GAS 86764 shows collagen deposition on the surface using immunogold labeling (c, arrowheads). Asterisks indicate aggregated CIV on the streptococcal surface. Bars, 1 μm (a and b) and 0.25 μm (c).
Streptococcal M3 protein binds collagen.
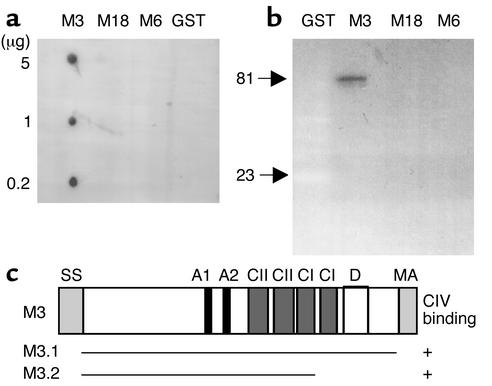
To determine whether M proteins of serotype M3 and M18 GAS are the molecular factors that mediate collagen aggregation on the streptococcal surface, we cloned the emm genes in Escherichia coli and tested pure recombinant M proteins in ligand overlay assays and pulldown experiments. M3 protein reacted with 125I-labeled CIV, whereas M18 protein and M6 protein, another recombinant M protein, as well as GST showed no reaction in ligand overlay assays (Figure 2, a and b). A C-terminally truncated M3 fragment (Figure 2c) was expressed as histidine-tagged fusion protein and also bound CIV (data not shown). To analyze the CIV-binding potential of native M proteins, a liquid-phase binding assay was developed. This pulldown assay revealed that 60% (± 0.4% SD) of the total amount of CIV could be coprecipitated with M3 protein, but that CIV could not be coprecipitated with M18 (1.5% ± 0.2% SD), M6 (0.6% ± 0.3% SD), or CIV alone (0.4% ± 0.2% SD). These results indicate that M3 protein, but not M18 protein, aggregates CIV. The role of M3 protein in collagen binding is further supported by our finding of an M protein–negative variant of the M3 S. pyogenes strain C203 that was unable to bind CIV, while the parental M3 protein-producing strain bound CIV (data not shown). Competition assays using radiolabeled collagen revealed that M3 was unable to block collagen binding to streptococci. We hypothesized that M3 protein could be built in the collagen complex on the streptococcal surface, since collagen appears to be aggregated in a multimolecular complex rather than bound by a single domain, and, indeed, labeled M3 bound back to the collagen-coated streptococci (data not shown).
Figure 2.
CIV binding of M3 protein. (a and b) Ligand overlay assay of GST and GST-fused recombinant M proteins with soluble radiolabeled CIV. (a) Spot membrane; (b) Western blot. Molecular masses (kDa) of M3 and GST are indicated. (c) Model of M3 protein and the CIV-binding subfragments.
Hyaluronic acid capsule mediates collagen binding of M18 streptococci.
A common feature of M18 strains is the permanently observed high degree of encapsulation (Figure 3a). We therefore examined whether the hyaluronic acid (HA) polysaccharide capsule is responsible for CIV aggregation. Collagen deposition on the capsular surface of the rheumatogenic M18 GAS strain 87282 (18) was visualized using collagen-coated gold particles by low-voltage FESEM (Figure 3, b and c). Specific degradation of the HA capsule through hyaluronidase treatment of streptococci revealed complete loss of whole-cell collagen-binding activity in all M18 strains, reflected by a decrease of binding activity from 90.1% (± 0.4% SD) to the level of nonbinding control strains, 4.2% (± 0.8% SD), in the radioactive binding assay. Hyaluronidase treatment of serotype M3 strain 86764, however, did not affect collagen-binding activity (data not shown). Electron microscopic analysis of the hyaluronidase-treated M18 streptococci revealed loss of capsule (Figure 3d) and a massive decrease (up to 100%) of capsule-associated collagen gold particles (Figure 3e). Heat denaturing of potential capsule-associated surface proteins revealed intact capsule and collagen-binding activity (data not shown). These results demonstrate that HA capsule is crucial for the collagen binding and aggregation in serotype M18, but not serotype M3, S. pyogenes isolates.
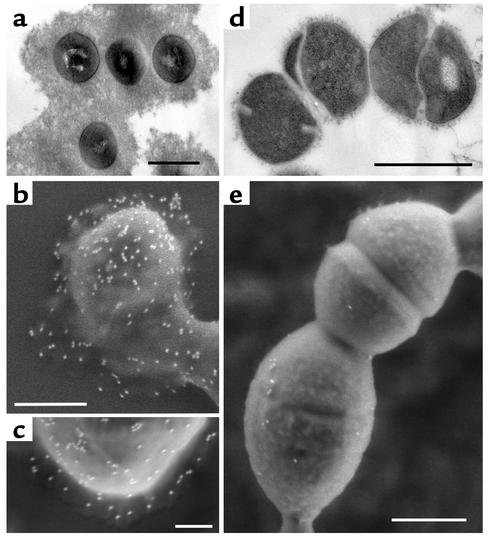
Figure 3.
Visualization of streptococcal capsule and binding of CIV gold complexes to M18 GAS. M18 strain 87282 S. pyogenes associated with ARF exhibit a thick capsule (a), and loss of capsule after hyaluronidase treatment (d). Low-voltage FESEM (1.5 kV) reveals binding of CIV gold complexes on M18 capsular structures (b and c), and highly reduced binding of CIV gold complexes on hyaluronidase-treated bacteria (e). Bars, 1 μm (a and d), 0.5 μm (b and e), and 0.2 μm (c).
In vivo conversion of M1 GAS to an encapsulated and collagen-binding phenotype.
To further support the finding that capsule is a collagen-binding factor of GAS, a nonencapsulated and collagen-binding negative serotype M1 strain was subjected to mouse passage. The first culture grown from blood of the infected animal revealed the typical fuzzy structure on the streptococcal surface that is characteristic for HA capsule expression (Figure 4) and that was absent in the strain grown in medium only. The mouse-passaged M1 strain bound collagen as efficiently as a passaged M18 strain (Figure 4d) but, in contrast to M18 GAS, completely lost its capsule and binding potential after one more cultivation step in medium (data not shown). These results again suggest that encapsulation is essential for collagen binding, and that other S. pyogenes serotypes may acquire the potential to bind collagen under in vivo conditions.
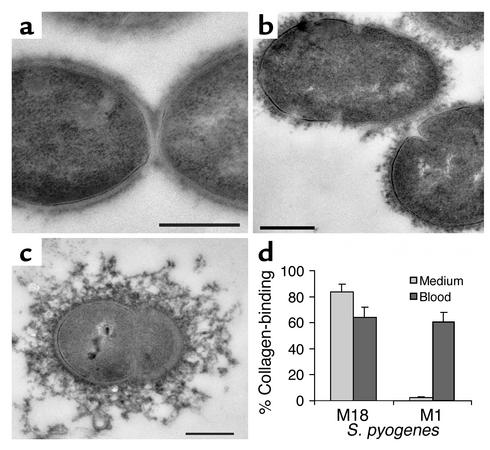
Figure 4.
Capsule expression and collagen-binding activity of a mouse-passaged serotype M1 S. pyogenes. Transmission electron microscopic images show M1 S. pyogenes strain KTL3 grown directly in medium (a) or cultivated from blood of infected mice (b). (c) A highly encapsulated mouse-passaged M18 strain 87282. (d) Collagen-binding activities are shown for M18 and M1 isolates grown in medium (light gray bars) or cultivated from blood of infected mice (dark gray bars). Collagen binding is expressed as a ratio of bound radiolabeled CIV to streptococci. Bars, 0.25 μm (a–c).
S. pyogenes colonizes collagen matrix in vitro and in vivo.
To investigate the biological relevance of collagen binding, we tested the potential of GAS to adhere to a complex collagen matrix in a cell-free system. M3 and M18 strains but no other serotypes grown in culture were able to attach to and associate with collagen fibers in vitro (Figure 5, a and b). A further aim was thus to localize collagen-binding S. pyogenes cells in infected animals using a mouse skin-infection model. Three days after initial infection, electron microscopic analysis of skin samples revealed M3, M18, and M1 streptococci attached to collagen (Figure 5, c–h). As in the in vitro studies, bacteria were found to be aggregated and attached to collagen bundles and intimately associated with collagen fibers in vivo. Moreover, the M18 and the M1 isolates appeared to bind to collagen via their capsule (Figure 5, e–g). These in vitro and in vivo data indicate that collagen binding and aggregation represent a novel mechanism of S. pyogenes colonization to ECM.
Figure 5.
S. pyogenes colonizes collagen in vitro and in vivo. FESEM analysis shows S. pyogenes colonizing collagen type I bundles (a) and fibers (b) in vitro. For in vivo localization, mice were infected subcutaneously with M3, M18, and M1 S. pyogenes. Infected skin was analyzed by FESEM analysis, showing S. pyogenes aggregated on and attached to collagen fibers (c and d). Analysis of skin sections by transmission electron microscopy shows intimate in vivo binding of streptococci to collagen. Serotypes shown are M3 (a, b, and h), M18 (c and g), and M1 (d–f). Bars, 10 μm (a), 2 μm (b), 2.5 μm (c), and 0.5 μm (d–h).
M3 protein induces anti-collagen antibodies in mice.
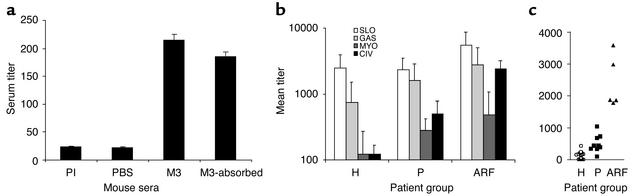
M3 streptococci are frequently associated with outbreaks of rheumatic disease. Since M3 protein is shown in this study to directly interact with CIV, the potential of M3 to evoke an anticollagen response in mice was analyzed. Mice were immunized intraperitoneally with pure recombinant M3 protein. All animals immunized with recombinant M3 (n = 5) had significantly elevated levels of CIV-reactive serum antibodies as compared with control mice (n = 5; Figure 6a). Absorption of M3 protein–reactive antibodies from serum revealed only a slight decrease in collagen reactivity. These data indicate that M3 protein has the potential to elicit a specific immune response toward basement membrane collagen, but they also suggest that only a small population of collagen antibodies cross-reacts with both proteins.
Figure 6.
CIV-specific serum responses. (a) Serum Ig response in M3 protein–immunized mice. Reactivity was determined for preimmune serum pool (PI), serum pool of PBS-immunized (PBS) or M3 protein–immunized (M3) mice, and M3 serum pool after absorbing M3-reactive antibodies (M3-absorbed). (b) CIV-specific serum responses in patients and controls. Sera were from healthy controls (H, n = 27), pharyngitis patients (P, n = 9), or ARF patients (ARF, n = 5). Antigens were streptolysin O (SLO), GAS polysaccharide (GAS), human heart myosin (MYO), and CIV. Bars represent mean IgG titers of three experiments ± SD. (c) Anticollagen titer of individual patient sera. Titers are shown for each individual serum collected from ARF patients (triangles), pharyngitis patients (squares), or healthy individuals (circles).
Collagen-reactive autoantibodies are present in sera of ARF patients.
To assess whether CIV-specific serum antibody titers are elevated in ARF patients, human sera were tested for the presence of anti-CIV antibodies. Sera were obtained from ARF patients (n = 5), pharyngitis patients (n = 9), and healthy individuals (n = 27). In addition to collagen-reactive IgG titers (means ± SD), titers against GAS cell wall polysaccharides, streptolysin O, and human cardiac myosin were determined (Figure 6b). As compared with the pharyngitis group, anti-CIV titers (P < 0.005, two-tailed unpaired Student’s t test) but not antimyosin titers (P = 0.49) were significantly increased in the ARF patient group. Furthermore, the ratio of anti-CIV to anti–streptolysin O titers was twice as high in ARF patients (0.43) as in pharyngitis patients (0.21). In contrast, the ratio antimyosin to anti–streptolysin O titers was not altered in the ARF group (0.09) as compared with the pharyngitis group (0.12). These data indicate a specific increase in anti-collagen antibody levels in ARF patients as compared with levels of antibodies against the other bacterial and host antigens tested, thereby clearly supporting the potential role of anti-CIV antibodies in ARF.
Discussion
M3 and M18 S. pyogenes isolates were found to be associated with rheumatic fever outbreaks and invasive disease (18). A common factor or a common function, however, that would link these two serotypes in their pathogenic capability but separate them from others has not been documented. This study now highlights a common theme of these two important streptococcal serotypes: their ability to bind and aggregate collagen.
M3 protein, a membrane-anchored streptococcal surface protein (24, 25), and HA capsule were identified in this study as the CIV-binding and -aggregating factors of GAS isolated from ARF patients. Cpa, the only collagen-binding protein so far described, binds to immobilized collagen type I but is not expressed in M3 and M18 streptococci (26, 27). In addition to collagen, GAS bind a variety of host factors, such as fibronectin, laminin, fibrinogen, Ig’s, and albumin (4). The matrix protein fibronectin plays an important role in mediating streptococcal adherence on the surface of epithelial cells (10, 20, 28, 29).
Since collagen is the major constituent of the ECM and basement membrane (12), collagen-binding molecules such as M3 protein and HA capsule may be defined as adherence and colonization factors. This is supported by our finding that S. pyogenes cells expressing M3 protein or capsule are able to colonize collagen matrix in vitro and in vivo (Figure 5). Streptococci were also shown here to intimately associate with collagen fibrils via their HA capsule. Streptococcal HA is structurally identical to human HA, a ubiquitously expressed extracellular polymer that functionally acts as an ECM-modeling molecule. Collagen type VI microfibrils were shown to be associated with human HA (30), suggesting that encapsulated GAS mimic matrix-assembly mechanisms of their host. However, commercial (dry, denatured) HA did not competitively block collagen binding at all after reconstitution in solution (K. Dinkla, unpublished observations). This may be due to structural denaturation of this fragile polyanionic stealth molecule during the purification process and the failure to acquire its native structure following rehydration.
The stable HA capsule is a characteristic of M18 streptococci, which may be explained by the genetic background of this serotype. HasA promoter activity of M18 strains was shown to be enhanced (31). A hyaluronidase (HylP2), homologous to that of serotype M18 GAS, remains intracellular, allowing the bacterium to accumulate extracellular HA (32). Interestingly, HylP2 and HylM18 differ from the hyaluronidases of other GAS in that the former lack a collagen-like stretch (33). According to our results on HA/collagen interaction, HylM18 therefore may be unable to bind HA and may not be cotransported, which would explain both its intracellular localization and the highly encapsulated M18 phenotype.
Besides enabling the organism to adhere to collagen matrix, coating of the bacteria with CIV allows them to form aggregates (Figures 1a and 5c) and, thus, may protect them from phagocytosis. This was shown for strains with certain M proteins that interact with each other (34) and for strains showing SfbI protein–mediated matrix-protein recruitment (11). The present study reveals further evidence that complex matrix recruitment impairs PMN cell binding and protects from phagocytosis in vitro. M proteins in general are important antiphagocytic factors that have been shown to impair phagocytosis through binding to fibrinogen (35). Thus, collagen binding displayed by M3 protein may elucidate how streptococci bypass host defense mechanisms. Since M3 and M18 isolates are often associated with invasive disease, the role of collagen binding may be important in the development of deep-tissue invasion such as cellulitis and fasciitis. This is supported by the observation that M3 and M18 streptococci colocalize with collagen in subdermal tissue in vivo, as demonstrated in the mouse model.
With respect to post-streptococcal rheumatic disease, epidemiological data led to two leading concepts, a “serotype concept” of rheumatogenicity in which certain M serotypes are frequently associated with ARF, and a “strain concept” that is supported by the finding that, within a given M serotype, only particular strains appear to cause ARF (36, 37). Our findings may fit in both concepts: M3- and HA-mediated CIV binding of serotype M3/M18 streptococci supports the serotype concept, while the strain concept may apply to other serotypes in which the degree of in vivo HA encapsulation and, thus, CIV binding may vary. However, it is also possible that the mechanism(s) by which other serotypes produce disease is completely different from those reported here.
In this study, conversion of a serotype M1 isolate into an encapsulated, collagen-binding organism by in vivo mouse passage was demonstrated. This is in agreement with reports that M1 and other serotypes isolated from ARF patients are rich in M protein and highly mucoid when freshly isolated (1, 18, 37, 38). Recombinant M1 protein did not bind collagen (K. Dinkla, unpublished observations), supporting the concept that, as in M18 isolates, capsule is important for collagen binding. In contrast to M18 streptococci, capsule association and collagen-binding potential were completely lost after one more recultivation step, demonstrating that M1 rapidly loses capsule in vitro.
For one of the collagen-binding factors, M3 protein, we demonstrate that it can induce collagen-reactive antibodies in mice (Figure 6a). Only a slight reduction of the collagen-reactive antibody titer was observed following absorption of M3-reactive antibodies, revealing that only a small population of collagen-reactive antibodies cross-reacts with M protein. Thus, although structural similarity (mimicry) between M3 protein and collagen may exist, this does not explain the presence of collagen-reactive antibodies that do not cross-react with M3 protein. Binding per se may represent the key event that leads to generation of CIV-reactive antibodies. Upon M3 protein binding, changes in the conformation of CIV may expose cryptic epitopes that become accessible for antibody binding, a phenomenon observed in Goodpasture syndrome, a CIV-based autoimmune disease (39).
Our results indicate that ARF patients, too, show an increase in CIV-reactive IgG antibody titers (Figure 6b). It may be possible that collagen-like proteins (40–43) of GAS elicit anti-collagen antibodies, since the Scl proteins share primary sequence homology with human collagen and have been shown to form triple helices (44). However, the presence of Scl’s by itself cannot explain the M serotype dependency in ARF. What role the anti-collagen antibodies identified here play in triggering post-streptococcal inflammatory processes remains to be determined. Causing arthritis is one attractive hypothesis, since ARF-related arthritis is generally believed to be induced by antibodies. Accordingly, M protein–cross-reactive anti-Hsp65 antibodies were shown to recognize collagen type II (45) and can induce experimental arthritis.
To summarize, the data of this study indicate that GAS with high rheumatogenic potential bind and aggregate human collagen type IV in vitro and in vivo. A novel function is presented for M3 protein and HA capsule. It is further demonstrated that M3 protein induces anti-collagen autoantibodies in mice and that patients suffering from ARF possess elevated levels of collagen-reactive antibodies. Besides its potential role in ARF, collagen binding may enhance the overall potential of S. pyogenes to cause disease and may thereby contribute to the immunopathological processes observed in the susceptible host.
Acknowledgments
We thank D.R. Johnson for M3 and M18 GAS strains, H. Vohra and A. Grover for human sera, and K. Mummenbrauer, E. Müller, and N. Janze for excellent technical assistance. We are grateful to M. Rasmussen and L. Björck for providing S. pyogenes KTL3. We also thank A. Nerlich and O. Goldmann for technical support. S.R. Talay gratefully acknowledges funding through the Dorothea-Erxleben-Programme given by the Ministry of Science and Culture (Niedersachsen, Germany).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: group A streptococci (GAS); acute rheumatic fever (ARF); rheumatic heart disease (RHD); collagen type IV (CIV); phosphate-buffered saline pH 7.5 containing 0.05% tween 20 (PBST); glutathione-S-transferase (GST); polymorphonuclear (PMN); field emission scanning electron microscopy (FESEM); hyaluronic acid (HA).
References
- 1.Bisno AL. Group A streptococcal infections and acute rheumatic fever. N. Engl. J. Med. 1991;325:783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- 2.Veasy LG, Hill HR. Immunologic and clinical correlation in rheumatic fever and rheumatic heart disease. Pediatr. Infect. Dis. J. 1997;16:400–407. doi: 10.1097/00006454-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan MH, Svec KH. Immunologic relation of streptococcal and tissue antigens. III. Presence in human sera of streptococcal antibody cross reactive with heart tissue: association with streptococcal infection, rheumatic fever, and glomerulonephritis. N. Engl. J. Med. 1964;119:651–666. doi: 10.1084/jem.119.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Rijn I, Zabriskie JB, McCarty M. Group A streptococcal antigens cross-reactive with myocardium: purification of heart reactive antibody and isolation and characterization of the streptococcal antigen. J. Exp. Med. 1977;146:579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein I, Halpern B, Robert L. Immunological relationship between streptococcus A polysaccharide and the structural glycoproteins of heart valve. Nature. 1967;213:44–47. [Google Scholar]
- 7.Dale JB, Beachey EH. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J. Exp. Med. 1985;161:113–122. doi: 10.1084/jem.161.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale JB, Beachey EH. Sequence of myosin-cross-reactive epitopes of streptococcal M protein. J. Exp. Med. 1986;164:1785–1790. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham MW, et al. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J. Immunol. 1989;143:2677–2683. [PubMed] [Google Scholar]
- 10.Chhatwal, G.S., and Preissner, K.T. 2000. Extracellular matrix interactions with gram-positive pathogens. In Gram-positive pathogens. V.A. Fischetti, R.P. Novick, J.J. Ferretti, D.A. Portnoy, and J.I. Rood, editors. ASM Press. Washington, DC, USA. 78–86.
- 11.Dinkla K, et al. Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol. Microbiol. 2003;47:861–869. doi: 10.1046/j.1365-2958.2003.03352.x. [DOI] [PubMed] [Google Scholar]
- 12.Kühn K. Basement membrane (type IV) collagen. Matrix Biol. 1994;14:439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Wieslander J, et al. Goodpasture antigen of the glomerular basement membrane: localization to noncollagenous region of type IV collagen. Proc. Natl. Acad. Sci. U. S. A. 1984;81:3838–3842. doi: 10.1073/pnas.81.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart JM, Watson WC, Kang AH. Collagen autoimmunity and arthritis. FASEB J. 1988;2:2950–2956. doi: 10.1096/fasebj.2.14.3053308. [DOI] [PubMed] [Google Scholar]
- 15.Kraetsch HG, et al. Cartilage specific autoimmunity in rheumatoid arthritis: characterization of a triple helical B cell epitope in the integrin-binding domain of collagen type II. Eur. J. Immunol. 2001;31:1666–1673. doi: 10.1002/1521-4141(200106)31:6<1666::aid-immu1666>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Morello R, et al. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat. Genet. 2001;27:205–208. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- 17.Wagner BM. Rheumatic fever as a collagen disease. Proc. Rudolf Virchow Med. Soc. City N. Y. 1969;27:189–200. [PubMed] [Google Scholar]
- 18.Johnson DR, Stevens DL, Kaplan EL. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J. Infect. Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen M, Müller HP, Björck L. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding α2-macroglobulin. J. Biol. Chem. 1999;274:15336–15344. doi: 10.1074/jbc.274.22.15336. [DOI] [PubMed] [Google Scholar]
- 20.Talay SR, et al. Co-operative binding of human fibronectin to SfbI protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol. 2000;2:521–535. doi: 10.1046/j.1462-5822.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 21.Molinari G, et al. The role played by the group A streptococcal regulator Nra on bacterial interactions with epithelial cells. Mol. Microbiol. 2001;40:99–114. doi: 10.1046/j.1365-2958.2001.02373.x. [DOI] [PubMed] [Google Scholar]
- 22.Fassel TA, Mozdziak PE, Sanger JR, Edmiston CE. Superior preservation of the staphylococcal glycocalyx with aldehyde-ruthenium red and select lysine salts using extended fixation times. Microsc. Res. Tech. 1998;41:291–297. doi: 10.1002/(SICI)1097-0029(19980515)41:4<291::AID-JEMT2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Thakur A, Prakash K. Detection of antibody to C-carbohydrate of group A streptococci with enzyme-treated whole bacterial cells as antigen for ELISA. J. Med. Microbiol. 1996;45:214–218. doi: 10.1099/00222615-45-3-214. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt K-H, Mann K, Cooney J, Köhler W. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol. Med. Microbiol. 1993;7:135–144. doi: 10.1111/j.1574-695X.1993.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 25.Hong K. Human IgG binding ability of streptococcal M3 protein: its related complement activation-dependent M3 protein polymerization. FEMS Immunol. Med. Microbiol. 1997;18:163–174. doi: 10.1111/j.1574-695X.1997.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 26.Podbielski A, Woischnik M, Leonard BA, Schmidt KH. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 1999;31:1051–1064. doi: 10.1046/j.1365-2958.1999.01241.x. [DOI] [PubMed] [Google Scholar]
- 27.Kreikemeyer B, Beckert S, Braun-Kiewnick A, Podbielski A. Group A streptococcal RofA-type global regulators exhibit a strain-specific genomic presence and regulation pattern. Microbiology. 2002;148:1501–1511. doi: 10.1099/00221287-148-5-1501. [DOI] [PubMed] [Google Scholar]
- 28.Talay SR, Valentin-Weigand P, Jerlström PG, Timmis KN, Chhatwal GS. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joh D, Wann ER, Kreikemeyer B, Speziale P, Hook M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 30.Kielty CM, Whittacker SP, Grant ME, Shuttleworth CA. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J. Cell Biol. 1992;118:979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberti S, Ashbaugh CD, Wessels MR. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol. Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 32.Hynes WL, Hancock L, Ferretti JP. Analysis of a second bacteriophage hyaluronidase gene from Streptococcus pyogenes: evidence for a third hyaluronidase involved in extracellular enzymatic activity. Infect. Immun. 1995;63:3015–3020. doi: 10.1128/iai.63.8.3015-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marciel AM, Kapur V, Musser JM. Molecular population genetic analysis of a Streptococcus pyogenes bacteriophage-encoded hyaluronidase gene: recombination contributes to allelic variation. Microb. Pathog. 1997;22:209–217. doi: 10.1006/mpat.1996.9999. [DOI] [PubMed] [Google Scholar]
- 34.Frick I-M, Mörgelin M, Björck L. Virulent aggregates of Streptococcus pyogenes are generated by homophilic protein-protein interactions. Mol. Microbiol. 2000;37:1232–1247. doi: 10.1046/j.1365-2958.2000.02084.x. [DOI] [PubMed] [Google Scholar]
- 35.Ringdahl U, et al. A role for the fibrinogen-binding regions of streptococcal M proteins in phagocytosis resistance. Mol. Microbiol. 2000;37:1318–1326. doi: 10.1046/j.1365-2958.2000.02062.x. [DOI] [PubMed] [Google Scholar]
- 36.Stollerman GH. Rheumatogenic group A streptococci and the return of rheumatic fever. Adv. Intern. Med. 1990;35:1–26. [PubMed] [Google Scholar]
- 37.Stollerman GH. Rheumatic fever. Lancet. 1997;349:935–942. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 38.Veasy LG, et al. Resurgence of acute rheumatic fever in the intermountain area of the United States. N. Engl. J. Med. 1987;316:421–427. doi: 10.1056/NEJM198702193160801. [DOI] [PubMed] [Google Scholar]
- 39.Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. J. Biol. Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- 40.Rasmussen M, Eden A, Björck L. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect. Immun. 2000;68:6370–6377. doi: 10.1128/iai.68.11.6370-6377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukomski S, et al. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect. Immun. 2000;68:6542–6553. doi: 10.1128/iai.68.12.6542-6553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukomski S, et al. Identification and characterization of a second extracellular collagen-like protein made by group A Streptococcus: control of production at the level of translation. Infect. Immun. 2001;69:1729–1738. doi: 10.1128/IAI.69.3.1729-1738.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasmussen M, Björck L. Unique regulation of SclB: a novel collagen-like surface protein of Streptococcus pyogenes. Mol. Microbiol. 2001;40:1427–1438. doi: 10.1046/j.1365-2958.2001.02493.x. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Keene DR, Bujnicki JM, Hook M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J. Biol. Chem. 2002;277:27312–27318. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]
- 45.Quinn A, Shinnik TM, Cunningham MW. Anti-Hsp65 antibodies recognize M proteins of group A streptococci. Infect. Immun. 1996;64:818–824. doi: 10.1128/iai.64.3.818-824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]