Abstract
The 5′-untranslated leaders of mitochondrial mRNAs appear to localize translation within the organelle. VAR1 is the only yeast mitochondrial gene encoding a major soluble protein. A chimeric mRNA bearing the VAR1 untranslated regions and the coding sequence for pre-Cox2p appears to be translated at the inner membrane surface. We propose that translation of the ribosomal protein Var1p is also likely to occur in close proximity to the inner membrane.
The yeast mitochondrial genome codes for eight major polypeptides, seven of which are components of respiratory complexes located in the mitochondrial inner membrane (28). The eighth translation product, Var1p, is a soluble protein and a structural component of the small subunit of the mitochondrial ribosome, necessary for ribosome assembly (9, 12, 26, 27). Translation of many, if not all, mRNAs encoded by the yeast mitochondrial genome depends on the action of nuclear-encoded, inner membrane-bound, mRNA-specific translational activators (4, 7, 8, 13, 15, 17). These activators are thought to help target translation of mitochondrially encoded mRNAs to sites of respiratory complex assembly on the inner membrane through their interactions with mRNA 5′-untranslated leaders (UTLs) (4, 17, 21).
Little is known about the location of the VAR1 mRNA translation or its activation. For example, is the VAR1 mRNA translated at the surface of the inner membrane despite the fact that its product is assembled into the ribosome? Our laboratories have shown previously that synthesis of the membrane proteins Cox2p and Cox3p, subunits of the multimeric enzyme cytochrome c oxidase, can be directed by chimeric mRNAs bearing the untranslated regions (UTRs) of the VAR1 mRNA (21). However, the vast majority of Cox2p and Cox3p synthesized under these conditions was rapidly degraded, leading to decreased steady-state levels of the proteins and decreased respiratory growth rates. Thus, the VAR1 UTRs of the chimeric mRNAs apparently caused mislocalized synthesis of Cox2p and Cox3p, reducing the efficiency with which they are assembled into cytochrome c oxidase (21).
Cox2p is synthesized as a larger precursor, pre-Cox2p (23), whose N-terminal 15 amino acids are removed by the Imp proteolytic complex after translocation through the inner membrane to the intermembrane space (1, 18, 19, 22). Pulse-labeled Cox2p translated from the chimeric var1::COX2 mRNA exhibited the same electrophoretic mobility as the wild-type protein (21). If pre-Cox2p encoded by the chimeric mRNA were synthesized in a soluble matrix milieu by free ribosomes, it might be subsequently clipped at the N or C terminus by nonspecific matrix proteases to yield a species with the same mobility as mature Cox2p. However, if pre-Cox2p encoded by the chimeric mRNA were translated at the inner membrane, then its N terminus could be rapidly translocated through the membrane and processed by Imp in the intermembrane space. To distinguish these possibilities, we asked whether the deletion of IMP1, which encodes a catalytic subunit of the Imp complex, would prevent processing of pre-Cox2p translated from the var1::COX2 mRNA.
We constructed strains (Table 1), using standard methods (3, 5, 10), that contained a nuclear gene supplying Var1p on a plasmid (pAM2) (20), the COX2 coding sequence inserted at the VAR1 locus, and a replacement of the endogenous COX2 coding sequence by the mitochondrial reporter gene ARG8m (24), encoding an arginine biosynthetic enzyme. Control strains had ARG8m in place of VAR1 and the wild-type COX2 gene. Mitochondrial translation was followed in vivo by pulse-labeling cells with [35S]methionine for 10 min in the presence of cycloheximide as described previously (3), except that cells were grown initially in synthetic complete medium lacking uracil with 2% raffinose and then shifted to liquid 1% yeast extract-2% Bacto Peptone-2% raffinose for 5 h, and protease inhibitors (EDTA-free Complete; Roche) were added during cell disruptions.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Nuclear genotype | Mitochondrial genotype | Source |
|---|---|---|---|
| AFC6a | MATaura3-52 leu2-3,112 his3Δ arg8::hisG lys2 pAM2 | rho+ cox2::ARG8m var1::COX2 | This study |
| AFC15a | MATaura3-52 leu2-3,112 his3Δ arg8::hisG lys2 pet111::LEU2 pAM2 | rho+ cox2::ARG8m var1::COX2 | This study |
| SCS193a | MATaura3-52 leu2-3,112 his3Δ arg8::hisG lys2 imp1::kanMX4 | rho+ | Scott Saracco |
| AFC12b | MATa/α ura3-52/− ade2-101/+ leu2-3,112/+ his3Δ/+ arg8::hisG/arg8::hisG lys2/+ kar1-1/+ pAM2 | rho+ var1::ARG8m | This study |
| AFC13b | MATa/α ura3-52/− -ade2-101/+ leu2-3,112/+ his3Δ/+ arg8::hisG/arg8::hisG lys2/+ kar1-1/+ pAM2 | rho+ cox2::ARG8m var1::COX2 | This study |
| AFC19b | MATa/α ura3-52/− ade2-101/+ leu2-3,112/+ his3Δ/+ arg8::hisG/arg8::hisG lys2/+ kar1-1/+ imp1::kanMX4/imp1::kanMX4 pAM2 | rho+ cox2::ARG8m var1::COX2 | This study |
Congenic to D273-10B.
From mating of strains congenic to D273-10B and strains congenic to DBY947.
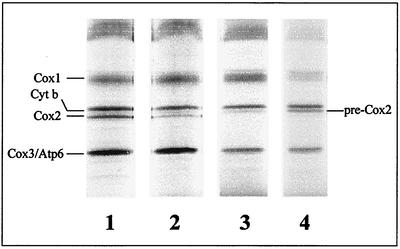
Autoradiography of pulse-labeled proteins from diploid strains, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, confirmed that the var1::COX2 mRNA was translated and that the labeled product had the same mobility as mature wild-type Cox2p (Fig. 1, lanes 1 and 2). Labeling of the var1::COX2 mRNA translation product was reduced relative to that of the COX2 mRNA product. A similar though less-pronounced reduction in labeling after 10 min was previously observed (21). The strains used in that study were apparently triploid, based on the extremely low viability of meiotic spores they produced (unpublished results), which could account for relative differences in gene expression (6).
FIG. 1.
Imp1p is required for processing of pre-Cox2p translated from the var1::COX2 mRNA. Mitochondrial proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after radioactive pulse-labeling of the indicated strains (Table 1) for 10 min and detected by autoradiography. Lane 1, AFC12 [COX2 var1::ARG8m]; lane 2, AFC13 [cox2::ARG8m var1::COX2]; lane 3, AFC19 imp1::kanMX4 [cox2::ARG8m var1::COX2]; lane 4, SCS193 imp1::kanMX4 [COX2].
Labeling of cells carrying the same nuclear and mitochondrial genomes, but homozygous for an imp1::kanMX4 deletion, revealed accumulation of unprocessed pre-Cox2p (Fig. 1, lane 3). The electrophoretic mobility of this pre-Cox2p was identical to that produced by a haploid imp1::kanMX4 mutant containing wild-type mitochondrial DNA (Fig. 1, lane 4). Similar results were obtained when the labeling pulse was shortened to 5 min, although overall incorporation of label was lower (unpublished data).
Thus, processing of newly synthesized pre-Cox2p generated by translation of the chimeric var1::COX2 mRNA is dependent upon Imp1p, whose activity is located in the intermembrane space (18, 22). These results strongly suggest that, despite the presence of VAR1 5′- and 3′-UTRs on the chimeric mRNA which mislocalize pre-Cox2p synthesis (21), the N terminus of newly synthesized pre-Cox2p is readily translocated through the inner membrane. Apparently, the export apparatus in the inner membrane that translocates the N terminus (11) has ready access to the var1::COX2 mRNA translation product. Based on the observation that other Saccharomyces cerevisiae mitochondrial 5′-UTLs are involved in localizing translation, we propose that the wild-type VAR1 mRNA may also be translated at the surface of the inner membrane. A similar proposal has been made previously based on kinetic studies of mitochondrial protein synthesis (14).
However, our data are also consistent with the possibility that pre-Cox2p could be synthesized in a soluble milieu but rapidly and efficiently translocated through the inner membrane.
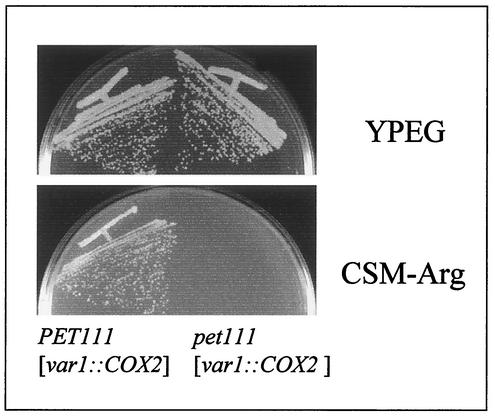
Translation of the wild-type COX2 mRNA is activated through its 5′-UTL by the nuclearly coded membrane protein Pet111p (7, 16). However, there are also sites regulating translation of COX2 within the coding sequence (2), as well as an amino acid sequence similarity between Cox2p and Pet111p that could underlie interactions involving these proteins (25). We therefore asked whether we could detect any effect of a pet111 deletion mutation on expression of the var1::COX2 chimeric gene. While pet111 deletion prevented phenotypic expression of the cox2::ARG8m gene in the control strain as expected, it did not further reduce the slow respiratory growth rate of the var1::COX2 strain (Fig. 2) or synthesis of Cox2p from the chimeric mRNA in pulse-labeling experiments (unpublished results).
FIG. 2.
Growth of var1::COX2 strains on respiratory carbon sources is independent of Pet111p. The var1::COX2 strain AFC6, left, and an isogenic pet111 null mutant AFC15, right, were streaked on complete synthetic medium lacking arginine and on complete medium containing ethanol and glycerol as carbon sources (YPEG) and incubated at 30°C for 3 and 5 days, respectively.
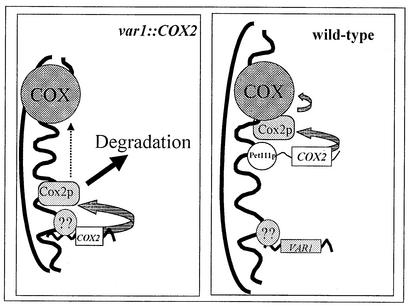
In conclusion, our evidence taken together with previous studies argues that the UTRs of the VAR1 mRNA direct translation of coding sequence information to sites at or in close proximity to the membrane. We speculate that membrane-associated mitochondrial synthesis of Var1p could help nucleate the ribosome assembly process, which must also involve proteins imported through the inner membrane from the cytoplasm. However, these ribosome assembly sites would be distinct from locations where Cox2p can be efficiently assembled into the cytochrome c oxidase holoenzyme. The diagram in Fig. 3 summarizes this proposal, which suggests that Var1p is also likely to be translated at the surface of the inner membrane via the action of an as-yet-unidentified membrane-bound translational activator, while translation of the COX2 mRNA is localized elsewhere on the membrane by its interaction with Pet111p. Thus, we propose the existence of distinct sites of assembly for mitochondrial ribosomes and cytochrome c oxidase.
FIG. 3.
Model for the translation of the var1::COX2 chimeric mRNA and wild-type COX2 and VAR1 mRNAs in yeast mitochondria. We propose that the VAR1 mRNA 5′-UTL directs synthesis of Cox2p on the matrix side of the inner membrane through the action of an unknown VAR1 translational activator. Reduced incorporation of Cox2p into cytochrome c oxidase leads to rapid degradation of most of the newly synthesized protein.
Acknowledgments
We thank Scott Saracco for strain SCS193.
A.F. is the recipient of a fellowship from the Pasteur Institute-Cenci Bolognetti Foundation. This work was supported by a grant, GM58590, from the NIH to T.L.M. and T.D.F.
REFERENCES
- 1.Behrens, M., G. Michaelis, and E. Pratje. 1991. Mitochondrial inner membrane protease 1 of Saccharomyces cerevisiae shows sequence similarity to the Escherichia coli leader peptidase. Mol. Gen. Genet. 228:167-176. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefoy, N., N. Bsat, and T. D. Fox. 2001. Mitochondrial translation of Saccharomyces cerevisiae COX2 mRNA is controlled by the nucleotide sequence specifying the pre-Cox2p leader peptide. Mol. Cell. Biol. 21:2359-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnefoy, N., and T. D. Fox. 2001. Genetic transformation of Saccharomyces cerevisiae mitochondria. Methods Cell Biol. 65:381-396. [DOI] [PubMed] [Google Scholar]
- 4.Fox, T. D. 1996. Genetics of mitochondrial translation, p. 733-758. In J. W. B. Hershey, M. B. Matthews, and N. Sonnenberg (ed.), Translational control. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 5.Fox, T. D., L. S. Folley, J. J. Mulero, T. W. McMullin, P. E. Thorsness, L. O. Hedin, and M. C. Costanzo. 1991. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 194:149-165. [DOI] [PubMed] [Google Scholar]
- 6.Galitski, T., A. J. Saldanha, C. A. Styles, E. S. Lander, and G. R. Fink. 1999. Ploidy regulation of gene expression. Science 285:251-254. [DOI] [PubMed] [Google Scholar]
- 7.Green-Willms, N. S., C. A. Butler, H. M. Dunstan, and T. D. Fox. 2001. Pet111p, an inner membrane-bound translational activator that limits expression of the Saccharomyces cerevisiae mitochondrial gene COX2. J. Biol. Chem. 276:6392-6397. [DOI] [PubMed] [Google Scholar]
- 8.Grivell, L. A., M. Artal-Sanz, G. Hakkaart, L. de Jong, L. G. Nijtmans, K. van Oosterum, M. Siep, and H. van der Spek. 1999. Mitochondrial assembly in yeast. FEBS Lett. 452:57-60. [DOI] [PubMed] [Google Scholar]
- 9.Groot, G. S., T. L. Mason, and N. Van Harten-Loosbroek. 1979. Var1 is associated with the small ribosomal subunit of mitochondrial ribosomes in yeast. Mol. Gen. Genet. 174:339-342. [DOI] [PubMed] [Google Scholar]
- 10.Guthrie, C., and G. R. Fink (ed.). 1991. Guide to yeast genetics and molecular biology, vol. 194. Academic Press, San Diego, Calif.
- 11.Hell, K., W. Neupert, and R. A. Stuart. 2001. Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 20:1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudspeth, M. E., W. M. Ainley, D. S. Shumard, R. A. Butow, and L. I. Grossman. 1982. Location and structure of the var1 gene on yeast mitochondrial DNA: nucleotide sequence of the 40.0 allele. Cell 30:617-626. [DOI] [PubMed] [Google Scholar]
- 13.Islas-Osuna, M. A., T. P. Ellis, L. L. Marnell, T. M. Mittelmeier, and C. L. Dieckmann. 2002. Cbp1 is required for translation of the mitochondrial cytochrome b mRNA of Saccharomyces cerevisiae. J. Biol. Chem. 277:37987-37990. [DOI] [PubMed] [Google Scholar]
- 14.Marzuki, S., and A. R. Hibbs. 1986. Are all mitochondrial translation products synthesized on membrane-bound ribosomes? Biochim. Biophys. Acta 866:120-124. [DOI] [PubMed] [Google Scholar]
- 15.McMullin, T. W., and T. D. Fox. 1993. COX3 mRNA-specific translational activator proteins are associated with the inner mitochondrial membrane in Saccharomyces cerevisiae. J. Biol. Chem. 268:11737-11741. [PubMed] [Google Scholar]
- 16.Mulero, J. J., and T. D. Fox. 1993. PET111 acts in the 5′-leader of the Saccharomyces cerevisiae mitochondrial COX2 mRNA to promote its translation. Genetics 133:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naithani, S., S. A. Saracco, C. A. Butler, and T. D. Fox. 2003. Interactions among COX1, COX2, and COX3 mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane of Saccharomyces cerevisiae. Mol. Biol. Cell 14:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunnari, J., T. D. Fox, and P. Walter. 1993. A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science 262:1997-2004. [DOI] [PubMed] [Google Scholar]
- 19.Pratje, E., G. Mannhaupt, G. Michaelis, and K. Beyreuther. 1983. A nuclear mutation prevents processing of a mitochondrially encoded membrane protein in Saccharomyces cerevisiae. EMBO J. 2:1049-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchirico, M., A. Tzellas, T. D. Fox, H. Conrad-Webb, P. S. Perlman, and T. L. Mason. 1995. Relocation of the unusual VAR1 gene from the mitochondrion to the nucleus. Biochem. Cell. Biol. 73:987-995. [DOI] [PubMed] [Google Scholar]
- 21.Sanchirico, M. E., T. D. Fox, and T. L. Mason. 1998. Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J. 17:5796-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider, A., M. Behrens, P. Scherer, E. Pratje, G. Michaelis, and G. Schatz. 1991. Inner membrane protease I, an enzyme mediating intramitochondrial protein sorting in yeast. EMBO J. 10:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevarino, K. A., and R. O. Poyton. 1980. Mitochondrial membrane biogenesis: identification of a precursor to yeast cytochrome c oxidase subunit II, an integral polypeptide. Proc. Natl. Acad. Sci. USA 77:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steele, D. F., C. A. Butler, and T. D. Fox. 1996. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc. Natl. Acad. Sci. USA 93:5253-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strick, C. A., and T. D. Fox. 1987. Saccharomyces cerevisiae positive regulatory gene PET111 encodes a mitochondrial protein that is translated from an mRNA with a long 5′ leader. Mol. Cell. Biol. 7:2728-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terpstra, P., and R. A. Butow. 1979. The role of var1 in the assembly of yeast mitochondrial ribosomes. J. Biol. Chem. 254:12662-12669. [PubMed] [Google Scholar]
- 27.Terpstra, P., E. Zanders, and R. A. Butow. 1979. The association of var1 with the 38S mitochondrial ribosomal subunit in yeast. J. Biol. Chem. 254:12653-12661. [PubMed] [Google Scholar]
- 28.Tzagoloff, A., and A. M. Myers. 1986. Genetics of mitochondrial biogenesis. Annu. Rev. Biochem. 55:249-285. [DOI] [PubMed] [Google Scholar]