Abstract
Calcineurin is a calcium-activated protein phosphatase that is the target of the immunosuppressants cyclosporin A and FK506. In T cells, calcineurin controls nuclear import of the NF-AT transcription factor and gene activation. In plants and fungi, calcineurin functions in stress responses (e.g., temperature, cations, and pH) and is necessary for the virulence of the fungal pathogen Cryptococcus neoformans. Here we show that calcineurin is also required for the virulence of another major fungus that is pathogenic to humans, Candida albicans. C. albicans calcineurin mutants had significantly reduced virulence in a murine model of systemic infection. In contrast to its role in C. neoformans, calcineurin was not required for C. albicans survival at 37°C. Moreover, C. albicans calcineurin mutant strains exhibited no defects in known Candida virulence traits associated with host invasion, including filamentous growth, germ tube formation, and adherence to and injury of mammalian cells. C. albicans calcineurin mutant strains failed to colonize and grow in the kidneys of infected animals and were unable to survive when exposed to serum in vitro. Our studies illustrate that calcineurin has evolved to control aspects of the virulence of two divergent fungal pathogens via distinct mechanisms that can be targeted to achieve broad-spectrum antifungal action.
Candida albicans is the most common cause of invasive fungal disease. Interestingly, C. albicans is also a component of the normal human biota that resides on mucosal surfaces, including the gastrointestinal tract and the oral and vaginal mucosae. Superficial colonization of immunocompetent or immunocompromised patients by the fungus can lead to oral thrush, vulvovaginitis, esophagitis, and cutaneous infections. Patients who have undergone abdominal surgery or organ transplantation, have late-onset diabetes, are taking broad-spectrum antibiotics, or are neutropenic are all at increased risk for serious invasive disease (6, 43, 58). Often, the first stage of invasive disseminated infection is candidemia, a bloodstream infection with Candida that can be associated with foreign-body catheters or entry of yeast through a damaged intestinal mucosa (reviewed in reference 7). Candidemia can progress to acute or chronic hematogenously disseminated candidiasis, in which organs such as the liver, kidney, and spleen are involved. During the progression from a common commensal organism to a pathogen, C. albicans faces a number of environments in vivo that require it to sense and respond to the extracellular milieu for survival.
A great deal is known about the events leading to the pathogenic transition of C. albicans, especially about those pathways important for tissue invasion: the morphological switch from yeast-like growth to filamentous growth, expression of adherence molecules in the cell wall, and protease production (7, 11, 25, 42, 54, 55). Germ tube production, an early stage of filamentation, plays a multifunctional role in virulence. The germ tube is the site of localization for proteins such as the adherence protein Hwp1 (53) and the secreted aspartyl proteinases Sap4 to Sap6 (28). Also, filament production by the fungus inside macrophages causes lysis of the macrophages and thus allows the fungus to survive this host defense (14, 38). Afilamentous mutants of C. albicans have markedly attenuated virulence, although it is not known whether filamentous structures are themselves essential or whether it is the loss of specific proteins associated with the hyphal state that causes the attenuation of virulence (5, 15, 23, 26, 38). Disruption of genes encoding the adhesins and proteases that are expressed by filamentous C. albicans is known to reduce the virulence of C. albicans. Mutant strains lacking the adhesin Hwp1 have attenuated virulence in an oroesophageal model of candidiasis in immunodeficient mice (56). Mutants lacking the Sap1 to -6 proteases also have attenuated virulence and, in some instances, demonstrate either a reduced capacity to invade host tissues or enhanced clearance from host organs (19, 29, 50). Many of the proteins involved in germ tube formation, adhesion, and host cell damage are dependent on host cues, such as high pH and high temperature, for their expression (16, 19, 47, 48), and thus the ability to sense these environmental cues is essential for the virulence of C. albicans.
A candidate for such a sensor is calcineurin, a highly conserved protein that is important for mediating stress responses in both plants and fungi. Calcineurin is composed of two distinct subunits, the catalytic A and the regulatory B subunit. Calcineurin B binds to an α-helical extension of calcineurin A distinct from the active site. In response to calcium influx, calmodulin binds to calcineurin A and blocks the action of the autoinhibitory C-terminal domain of calcineurin A, resulting in the formation of the active calcineurin complex (reviewed in references 2, 27, 34, and 49). Calcineurin is important for responses to environmental temperature stress, including low (Arabadopsis thaliana and Schizosaccharomyces pombe) and high (Cryptococcus neoformans) temperatures (35, 44, 62). Calcineurin also functions in response to cation stress in A. thaliana, C. neoformans, and Saccharomyces cerevisiae (9, 13, 35, 44). Additionally, calcineurin is also important for response to and growth in alkaline pH media in both S. cerevisiae and C. neoformans (44, 51). Thus, we hypothesized that calcineurin might function in C. albicans to sense host cues to enable host tissue invasion and disseminated infection.
Calcineurin has recently been identified in C. albicans and shown to play a key role in enabling cells to survive membrane perturbation by drugs that target ergosterol biosynthesis (12, 45). The resulting model is that calcineurin function becomes essential when cell integrity is compromised. Based on these observations, we hypothesized that calcineurin might also be necessary to maintain the viability of C. albicans during the stresses inherent in infection. Here we show that calcineurin is indeed important for virulence in a murine tail-vein injection model of candidiasis. Histological examination of the renal parenchyma of infected mice reveals that calcineurin is required to establish tissue infection. However, analysis of the known virulence traits important for organ invasion did not uncover an explanation for the inability of strains lacking calcineurin to produce renal infection. Instead, we found that calcineurin is important for survival in serum. Thus, we conclude that calcineurin plays a key role early in infection, during candidemia, a critical stage of the C. albicans infectious cycle.
MATERIALS AND METHODS
Strains and media.
Strains used in this study are listed in Table 1. Strains were grown on either yeast extract-peptone-dextrose (YPD) rich medium, defined synthetic dextrose medium, prepared as previously described (52), or fetal bovine serum (FBS) (Sigma). FK506 (Fujisawa), niacin (Sigma), or thiamine (Sigma) was added to media at the concentrations described.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| SC5314 | Wild type | 26a |
| CAF2-1 | URA3/ura3::imm434 | 20a |
| DAY185 | ura3::imm434/ura3::imm434 his1::hisG::HIS1/his1::hisG arg4::hisG::ARG4-URA3/arg4::hisG | 15 |
| DAY364 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU/cnb1::ARG4 | 12 |
| DAY365 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU/cnb1::ARG4 | 12 |
| JRB64 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU/cnb1::ARG4 | This study |
| JRB71 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU/cnb1::ARG4 | This study |
| MCC85 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG::CNB1-HIS1/his1::hisG arg4::hisG/arg4::hisG cnb1::UAU/cnb1::ARG4 | 12 |
Strains MCC85, DAY364, and DAY365 have been described previously (12). To create prototrophic calcineurin mutants for in vivo analysis, plasmid pGEM-HIS1 was linearized by SacI-EagI digestion (59). The linearized fragment was transformed into the calcineurin B mutant strains DAY364 and DAY365 (both ura3/ura3 arg4/arg4 his1/his1 cnb1::URA3/cnb1::ARG4) (12) by the lithium acetate procedure (30), and transformants were selected on synthetic dextrose medium lacking histidine. The prototrophic strains JRB64 (from DAY364) and JRB71 (from DAY365) were isolated after this transformation and have the genotype ura3/ura3 arg4/arg4 HIS1/his1 cnb1::URA3/cnb1::ARG4. His+ isolates were grown on YPD medium for several generations and then tested on synthetic dextrose medium lacking histidine to ensure that the HIS1 gene had been stably integrated into the genome.
Virulence in a murine model.
The virulence of the cnb1/cnb1 mutant C. albicans strains was tested in a murine tail vein injection model. Two tests were performed, one with 5 animals in each injection group and a second with 10 animals in each group. Strains were grown overnight in Sabouraud's agar, and a light suspension of each was made in Sabouraud's broth. One drop of this suspension was added to 50 ml of Sabouraud's broth, which was then shaken at 250 rpm overnight at 35°C. Cells were washed three times, resuspended in phosphate-buffered saline (PBS), and counted with a hemocytometer. The cells were then diluted to yield 3 × 106 CFU per 200 μl and injected intravenously through the tail vein into outbred ICR mice. Inoculum concentrations were verified by culture and ranged from 2.46 × 106 to 3.12 × 106 cells per inoculum in the 5-mouse experiment (Fig. 1A) and from 1.74 × 106 to 4.2 × 106 cells per inoculum in the 10-mouse experiment (Fig. 1B). The end point for survival was determined by clinical signs of pain or distress, at which point animals were sacrificed by CO2 asphyxiation.
FIG. 1.
Calcineurin is essential for the virulence of C. albicans. The wild-type strain CAF2 (diamonds), the cnb1/cnb1 calcineurin B mutants JRB64 (squares) and JRB71 (triangles), and the cnb1/cnb1+ CNB1 calcineurin B reconstituted strain MCC85 (multiplication signs) were used to infect groups of 5 (A) or 10 (B) mice each by lateral tail vein injection, and survival was monitored over time. The survival of mice infected with calcineurin mutant strains was significantly different from that of mice infected with the wild-type strain or the reconstituted mutant strain (P < 0.05), except between JRB64 and MCC85 in panel A. No significant difference was noted between the two calcineurin mutant strains or between the wild-type and reconstituted strains in either experiment (P > 0.05).
Adherence to epithelial cells.
Adherence of yeast cells was measured using a buccal epithelial cell (BEC) protocol modified from the work of Panagoda et al. and of Kimura and Pearsall (33, 46). Wild-type, calcineurin B mutant, and reconstituted strains were grown overnight in liquid YPD medium, washed twice with PBS, and then resuspended in PBS at a concentration of 107 cells per ml. BECs were harvested from healthy human subjects by gently rubbing the inside of the cheek with sterilized cotton-tipped swabs (Fisher), and cells were suspended in PBS. These cells were washed twice in PBS and then resuspended in PBS at a concentration of 105 cells per ml. For the adherence tests, 0.5 ml of the epithelial suspension and 0.5 ml of the yeast inoculum were mixed in Eppendorf tubes and incubated at 37°C for 1 h with shaking. As a control, 0.5 ml of the BEC suspension or 0.5 ml of the C. albicans suspensions were mixed with 0.5 ml of PBS and incubated for 1 h at 37°C with shaking. Following incubation, all samples were filtered (Ultra-ware 25-mm microfiltration system; Kontes Glass Company, Vineland, N.J.) through 10-μm-pore-size polycarbonate filters (Millipore, Bedford, Mass.). To remove unattached yeast cells, the filters were washed twice with 10 ml of PBS. Filters were then removed from the filtration apparatus, gently pressed onto glass slides, and then carefully pulled off of the slides to leave the epithelia and attached yeast cells attached to the slides. Slides were air dried, heat fixed, Gram stained (Fisher Protocol Gram Stain Stat Pack; Fisher Scientific, Pittsburgh, Pa.), and mounted with Permount (Fisher Scientific). The number of C. albicans cells attached to the first 100 BECs was counted.
Endothelial cell injury.
The endothelial cells used in this study were harvested from human umbilical cord veins by the method of Jaffe et al. (32). Cells were grown in M-199 medium supplemented with 10% FBS, 10% defined bovine calf serum, and 2 mM l-glutamine with penicillin and streptomycin. Cells were grown in 24-well plates coated with gelatin and incubated at 37°C under 5% CO2. Endothelial cell damage caused by SC5314, JRB64, and MCC85 was measured by a chromium release assay as previously described (20). A total of 105 C. albicans cells in RPMI 1640 medium were added to each well in a 24-well tissue culture plate and incubated for 3 h. All experiments were performed in triplicate and repeated twice.
Histology.
Healthy BALB/c mice were infected by tail vein injection with ∼3 × 106 cells of strain CAF2 (wild type), JRB64 (cnb1/cnb1), JRB71 (cnb1/cnb1), or MCC85 (cnb1/cnb1 + CNB1) per 200 μl. Two animals from each infection group were sacrificed on day 1 postinfection. Both kidneys were harvested from each sacrificed animal and fixed in zinc formalin fixative (Polysciences, Inc. Warrington, Pa.). Fixed kidneys were processed and embedded in paraffin. Renal sections were subsequently prepared and subjected to staining with methenamine silver (Grocott's modification) to visualize yeast cells or with hematoxylin and eosin to visualize host cells. Processing and staining were performed at the Duke University Medical Center Immunohistology Research Laboratory, Durham, N.C. Renal sections were examined under a Nikon Eclipse E400 microscope at ×200, and photographs were taken with a Nikon Coolpix digital camera.
Survival in serum.
Fungal strains were grown overnight in rich liquid medium at 30°C, washed twice in PBS, and counted with a hemocytometer. Cells were diluted into FBS, porcine serum, or sheep serum (Sigma) at a concentration of 2.5 × 103 cells/ml and allowed to grow for the indicated time at 30°C with agitation. In certain experiments, the vitamins niacin and/or thiamine (Sigma), yeast extract (Difco), or peptone (Difco) was added to the serum at the specified concentrations, after which the serum solution was filtered through a Millex-GV syringe-driven filter unit with a pore size of 0.22 μm (Millipore) and then incubated with yeast. Following incubation, cells were diluted appropriately and 100 μl was plated onto YPD plates such that there were approximately 100 to 500 CFU per plate, except in the case of the calcineurin mutants of C. albicans, which with no dilution had only 1 to 2 CFU after 24 h of incubation in serum.
Statistical analysis.
Analysis of the virulence experiments was performed with Kruskal-Wallis and Dunn's multiple comparison tests. Endothelial cell injury was analyzed with a single-factor analysis of variance. Analysis of the data for yeast survival in serum and adherence to BECs was performed by using an unpaired, two-tailed Student t test.
RESULTS
C. albicans calcineurin mutants have attenuated virulence.
In divergent microorganisms, calcineurin senses and responds to a variety of stresses similar to those present in mammalian hosts. In particular, calcineurin is essential for the virulence of the pathogenic basidiomycete C. neoformans (13, 21, 44). This led us to hypothesize that calcineurin might also be important for the virulence of C. albicans. To test this hypothesis, we generated an isogenic series of prototrophic calcineurin mutants lacking the regulatory B subunit (cnb1/cnb1) and a strain in which calcineurin B was reconstituted (cnb1/cnb1+ CNB1) (Table 1) (12). Virulence studies were performed in which groups of 5 or 10 ICR outbred mice were infected intravenously with ∼3 × 106 cells of the wild-type strain (CAF2), the cnb1/cnb1 mutant strains (JRB64 and JRB71), or the cnb1/cnb1 + CNB1 reconstituted strain (MCC85).
All of the mice infected with the wild-type C. albicans strain succumbed to lethal infection by day 8 postinfection, whereas the survival of mice infected with either of two different independent calcineurin B mutant strains was dramatically prolonged (Fig. 1). Of 30 animals infected with the calcineurin B mutant strains, 27 survived until the experiment was terminated on day 30 postinfection. Three of the 30 mice infected with the calcineurin mutant strains died during the course of the experiment, but the survival time for 2 of the 3 mice was longer than that of any of the animals infected with the wild-type strain. Importantly, reintroduction of the wild-type calcineurin B gene via integration at the HIS1 locus restored virulence, and all of the mice infected with the reconstituted strain died during the course of both virulence experiments, with a survival rate that approached that of animals infected with the wild-type strain (Fig. 1). In summary, calcineurin plays a critical role in virulence in the murine tail vein injection model of candidiasis.
Calcineurin is dispensable for high-temperature growth of C. albicans.
Calcineurin is essential for optimal growth of C. neoformans strains at 37°C, and as a consequence, C. neoformans calcineurin mutant strains are avirulent (13, 21, 44). Wild-type and calcineurin mutant strains of C. albicans were analyzed for growth at 30, 37, and 42°C on rich and minimal media (Fig. 2 and data not shown). The C. albicans cnb1/cnb1 calcineurin B mutant strains did not exhibit a growth defect at any temperature tested (22 to 42°C) (Fig. 2 and data not shown). Thus, in contrast to the requirements of C. neoformans, calcineurin is not required for growth at elevated temperatures in C. albicans, and its impact on virulence must therefore be attributable to a distinct molecular mechanism.
FIG. 2.
Calcineurin is not required for growth of C. albicans at 37°C. Wild-type and calcineurin B mutant strains of C. albicans and C. neoformans were grown at 30 and 37°C on rich medium for 72 h and photographed.
Calcineurin is not required for germ tube formation.
Several virulence attributes, including germ tube formation, adherence to host cells, and host cell invasion, have been shown to play a role in tissue colonization and infection by C. albicans (5, 15, 19, 23, 26, 36, 38). Adhesins and proteases important for host cell adherence and invasion are localized to the germ tube (28, 53, 57), and the hyphal structure itself may promote invasion of host cells. High temperature, alkaline pH, and other undefined in vivo cues signal a change in morphology from yeast to filamentous growth in C. albicans. Two pathways have been implicated in this dimorphic switch, and additional regulatory inputs likely also participate (reviewed in references 10 and 42). Because calcineurin is important for growth under high-salt, alkaline pH, and elevated-temperature conditions in other fungi (9, 13, 35, 44, 51, 62), we tested whether calcineurin controls the hyphal growth of C. albicans and thereby contributes to virulence.
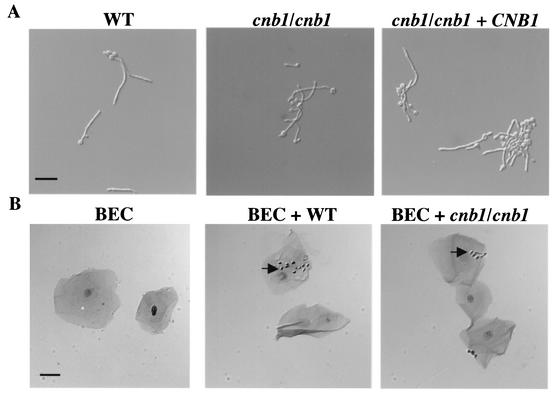
Hyphal differentiation was analyzed in vitro on solid or liquid media. There were no differences between the calcineurin mutants and the wild-type strain in the ability to filament on solid, filamentation-inducing media (Lee's, 10% serum, and spider media) at 30 and 37°C (data not shown). The calcineurin mutants did exhibit a filamentation defect at 25°C, but only on spider medium (data not shown). Calcineurin mutants also exhibited no filamentation defect in liquid media containing 10% FBS either with regard to the number of cells producing germ tubes or with regard to the length of the germ tubes and hyphae formed (Fig. 3A and data not shown). In summary, calcineurin is not required for germ tube formation or filamentous growth under most conditions in vitro.
FIG. 3.
Calcineurin is not required for germ tube formation or adherence to epithelial cells. (A) Wild-type (WT) (SC5314), calcineurin mutant (cnb1/cnb1) (JRB64), and calcineurin reconstituted (cnb1/cnb1+ CNB1) (MCC85) strains were grown in rich liquid medium with 10% FBS at 37°C for 2.5 h, and germ tubes produced by all three strains were visualized by Nomarski optics and photographed at a magnification of ×200. At this stage, 89.6% of wild-type, 82.5% of calcineurin mutant, and 87.0% of reconstituted cells had procuced germ tubes (only the difference between the data for wild-type and calcineurin mutant cells was statistically significant [P = 0.03]). (B) A total of 5 × 106 wild-type (SC5314) or calcineurin mutant (JRB64) cells were incubated with 5 × 104 human BECs. Cells were Gram stained, visualized with a Zeiss Axioscop 2 plus microscope, and photographed at a magnification of ×400. Arrows indicate representative C. albicans cells attached to epithelial cells. Bars, 25 μm.
Calcineurin is not necessary for host cell adherence.
C. albicans adheres to host cells to avoid clearing from the mucosal surface, and adherence is an essential initiating event for establishing infection during invasive candidiasis (54). An in vitro test was performed with human BECs to compare the abilities of calcineurin B-null mutant and wild-type cells to adhere to epithelial cells. C. albicans strains were incubated with human BECs in PBS, and the number of adherent yeast cells was quantified after 1 h of coincubation. The number of yeast cells per BEC for wild-type versus calcineurin mutant strains did not differ significantly (81.5 yeast cells per 100 BECs for the wild type and 142 yeast cells per 100 BECs for the calcineurin mutant; P = 0.21) (Fig. 3B). This assay was also performed in TC199 cell culture medium, and no significant difference was noted between wild-type and mutant adherence, even though adherence was greatly enhanced in TC199 medium compared to that in PBS (data not shown). Calcineurin, therefore, is not essential for C. albicans adherence to host cells.
Calcineurin is largely dispensable for endothelial cell injury.
The next stage of tissue infection by C. albicans involves invasion of host cells to promote host cell injury, evade the host immune defense, and scavenge nutrients from host cells. Here, endothelial cell injury was monitored with a 51Cr release assay (20). Wild-type C. albicans, a calcineurin-null mutant, and the reconstituted mutant strain were incubated with umbilical vein endothelial cells preloaded with 51Cr, and release of 51Cr from endothelial cells was measured after a 3-h incubation with fungal cells. The calcineurin mutant strain exhibited a defect in endothelial cell injury in this assay (20% less than that with the wild type) (Fig. 4). This statistically significant (P = 0.001 for comparison with the wild type) but modest defect might contribute to the observed virulence defect of the mutant in the murine whole-animal model. However, it is unlikely that the defect in endothelial cell injury displayed by the calcineurin mutant strains is sufficient to explain the avirulent phenotype in the whole-animal model. Supporting this assertion, the extent of endothelial cell injury caused by the reconstituted mutant strain was not statistically significantly different from that caused by the wild type (P = 0.9) and was only slightly different from that caused by the calcineurin mutant strain (P = 0.029) (Fig. 4), even though this strain is nearly as virulent as the wild type in the murine model. It should also be noted that, though tested in a more susceptible mouse model at a lower inoculum, the rim101/rim101 mutant strain, which exhibits a more severe defect in endothelial cell wounding, led to a median survival time of only 10 days (15), compared to the almost avirulent calcineurin mutant strains, which had a much less severe endothelial cell injury defect.
FIG. 4.
Calcineurin mutants exhibit a modest defect in endothelial cell wounding. A marker-matched wild-type strain, DAY185, was compared with the calcineurin B cnb1/cnb1 mutant strain JRB64 and the cnb1/cnb1 + CNB1 reconstituted strain MCC85 for the ability to wound endothelial cells, as measured by 51Cr release. The rim101/rim101 mutant served as a control based on its established defect in endothelial cell wounding. The level of wounding by the wild-type strain was taken as 100%. Error bars, standard errors of the means for six replicate tests.
Calcineurin mutants fail to colonize and grow in the kidneys.
C. albicans infections frequently start as candidemia that can progress to acute hematogenously disseminated candidiasis and result in the infection of many organs. The murine tail vein injection model mimics aspects of this disease progression in that the fungus is first introduced into the bloodstream and then disseminates to cause disease in organs including the kidney and liver. In this model of disease progression, acute infection of the kidney appears to be the main cause of attributable mortality (1, 3, 4).
To monitor disease progression in animals infected with either wild-type or calcineurin B mutant strains, and to determine whether the mutant strains might have an in vivo defect in filamentation that was not detected in our in vitro assays, kidneys were harvested and subjected to histopathological analysis. Mice infected with the wild-type strain displayed multiple aggregates of filamentous cells, approximately 75 to 250 μm in diameter, in the kidney (Fig. 5). Significantly fewer fungal cells were present in the kidneys of mice infected with the calcineurin B mutant strain, and these were typically observed as single cells in the renal section rather than as aggregates (Fig. 5). However, the mutant strains did produce filaments in vivo, thus demonstrating that the calcineurin mutant virulence defect is not likely attributable to a filamentation defect in vivo. On the other hand, these observations indicate that calcineurin is required for C. albicans to initiate and maintain infection in the renal parenchyma. This inability to grow within the kidney likely contributes to the marked attenuation in virulence of the mutant strains.
FIG. 5.
Calcineurin is important for renal infiltration during C. albicans infection. Histological analysis of renal tissue 1 day postinfection demonstrated a marked decrease in C. albicans levels in the renal parenchyma of animals infected with the cnb1/cnb1 calcineurin mutant strain. Kidneys of animals infected with the wild-type strain (WT) (left) had multiple nodules of infection approximately 75 to 250 μm in diameter, whereas kidneys of animals infected with the cnb1/cnb1 calcineurin mutant (JRB64) (right) contained fewer fungi, which were typically present as single cells or in small nodules of infection (arrow).
Calcineurin is essential for survival in serum.
Calcineurin is important for virulence and growth within the kidney, yet calcineurin mutants appear to have only minor defects in the known steps preceding tissue invasion. We therefore focused on an even earlier stage of the infection cycle, candidemia. In the murine tail vein injection model of candidiasis, one of the first environments that the yeast cells encounter is the blood. Blood is composed of host cells and plasma, which constitutes about 55% of whole blood. We therefore tested whether calcineurin is important for survival in this medium. This was accomplished by testing the survival of wild-type, calcineurin mutant, and reconstituted strains for persistence in serum.
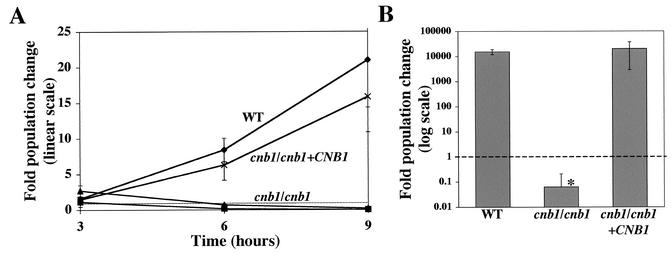
Calcineurin mutants exhibited a defect in survival in 100% FBS relative to survival of the wild-type and reconstituted strains. After 3 h in serum, there was no difference in growth between the wild type and calcineurin mutants. However, by 6 h of incubation, the wild-type and reconstituted strain populations had increased six- to eightfold whereas the population of the calcineurin mutant strains had decreased, and this trend continued at 9 h (Fig. 6A). Finally, after 24 h of incubation, the mutant cells were essentially inviable while the wild-type and reconstituted strain populations had increased more than 14,000-fold (Fig. 6B). Thus, we conclude that calcineurin is crucial for the survival of C. albicans in serum. This finding was not specific to FBS, because similar observations were made with porcine, sheep and murine sera (data not shown). In addition, a wild-type strain grown in FBS with 1 μg of the calcineurin inhibitor FK506/ml was as sensitive to serum as the calcineurin B mutant strain. This finding reinforces the conclusion that calcineurin is essential for survival in serum.
FIG. 6.
Calcineurin is required for C. albicans survival in serum. A wild-type strain (WT) (diamonds), calcineurin mutant (cnb1/cnb1) strains JRB64 (squares) and JRB71 (triangles), and the calcineurin reconstituted (cnb1/cnb1 + CNB1) strain MCC85 (multiplication signs) were tested for survival in 100% FBS. The fold change in population size was determined by comparing the CFU at each time point with the initial CFU. Values below the dashed line represent a fold change below 1, indicating cell death. (A) WT, calcineurin mutant (cnb1/cnb1), and reconstituted (cnb1/cnb1 + CNB1) strains at ∼2,500 cells/ml were incubated in 100% FBS at 30°C. Aliquots were collected at 3, 6, and 9 h postincubation and grown on YPD medium for 18 h at 37°C. The CFU at each time point was compared to the CFU at time zero and was recorded as fold population change. Error bars, standard errors of the means for three to six repetitions. (B) Aliquots of cultures grown for 24 h in serum were grown on YPD medium for 12 h. CFU was measured and compared to CFU at time zero. The difference between the calcineurin mutant and both the WT and reconstituted strains was statistically significant (P < 0.05), whereas there was no statistically significant difference between the WT and reconstituted strains (P > 0.05). Error bars, standard errors of the means for six repetitions.
Why is calcineurin required for growth in serum?
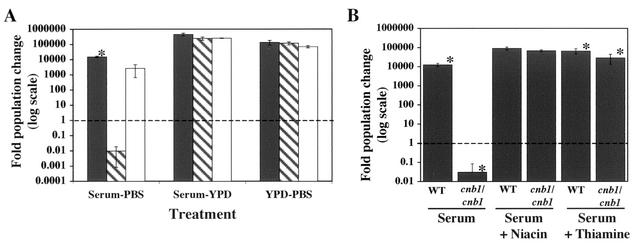
Calcineurin is essential for survival in serum, but it is not known whether serum contains an active component that is killing the fungal cells or lacks a component that is required for the growth and survival of calcineurin mutant cells. We tested whether serum lacks components necessary for the survival of calcineurin mutants by nutrient addition experiments. The growth of wild-type, calcineurin mutant, and reconstituted strains of C. albicans was measured in FBS diluted 1:1 with PBS or YPD rich medium; YPD diluted 1:1 with PBS was used as a control. The growth of strains in the 1:1 dilution of serum with PBS was similar to that in undiluted FBS after 24 h of incubation, but replacement of PBS with YPD liquid medium led to an almost complete recovery of calcineurin mutant growth such that the difference in growth between the wild-type and the calcineurin mutant strains was less than 2-fold, compared to an approximately 1,000,000-fold difference in serum alone at 24 h (Fig. 7A).
FIG. 7.
Medium supplementation restores growth of calcineurin mutants in serum. (A) Wild-type (SC5314) (shaded bars), calcineurin mutant (JRB64) (hatched bars), and reconstituted (MCC85) (open bars) strains were grown for 24 h at 30°C in serum diluted 1:1 with PBS or YPD, or in YPD diluted 1:1 with PBS. Statistically significant differences were noted between the wild-type strain and both the calcineurin mutant strain (P < 0.005) and the reconstituted strain (P = 0.006) but not between the calcineurin mutant and reconstituted strains (P > 0.05) in the serum-PBS experiment. Also, no significantly significant difference in growth was found between the samples in the serum-YPD and YPD-PBS experiments (P > 0.05). (B) Wild-type (WT) (SC5314) and calcineurin mutant (cnb1/cnb1) (JRB64) strains were grown for 24 h at 30°C in serum either alone or supplemented with either 5.95 mg of niacin/ml or 5.3 mg of thiamine/ml. Fold changes in population size were measured by comparing the CFU at the conclusion of the experiments with the initial CFU. Differences in growth between the wild-type and calcineurin mutant strains in both the serum-alone (P < 0.001) and thiamine addition (P = 0.004) experiments were statistically significant. Values below the dashed line represent a fold change below 1, indicating cell death.
YPD medium is composed of peptone, yeast extract, glucose, and water. When tested individually, none of these components, used at the concentrations present in YPD, had any obvious effects on the growth of the calcineurin mutants in serum, but a 10-fold increase in the yeast extract concentration partially complemented the growth defect of the calcineurin mutants (∼2-fold difference in fold population growth between the wild type and the calcineurin mutant). Peptone restored growth only weakly (data not shown). Peptone and yeast extract are similar in composition, but several differences exist, most notably in the concentrations of certain vitamins. Niacin, para-aminobenzoic acid, pantothenic acid, pyridoxine, riboflavin, and thiamine are ∼25- to 5,000-fold more abundant in yeast extract than in peptone. Supplementation of serum with niacin and thiamine restored the growth of calcineurin mutants in serum, but only at supraphysiologic levels, concentrations ∼1,000-fold higher than those at which they are present in rich YPD medium and approximately 1.5 × 105-fold (niacin) (31) to 1 × 106-fold (thiamine) (17) higher than those in human blood (Fig. 7B). Niacin and thiamine had no appreciable affect on the growth of calcineurin mutants in serum at ∼1, 10, and 100-fold their concentrations in YPD (data not shown). Therefore, absence of vitamins might contribute to the growth defect of calcineurin mutants in serum, but other factors must contribute as well.
Candidates for an active lethal factor in serum include complement components, antibodies, or antimicrobial peptides. Size fractionation of serum excluded the possibility that complement and antibodies were the active factor, because FBS that was size fractionated to exclude components of >3 kDa retained the activity that kills calcineurin mutants of C. albicans (data not shown). In addition, serum that was boiled for 15 min and pelleted to remove denatured proteins still retained the ability to kill C. albicans calcineurin mutants (data not shown). Hence, an active component in serum, if any, must be heat stable and <3 kDa.
DISCUSSION
We have discovered that calcineurin, a calcium-regulated signaling enzyme conserved throughout evolution, is essential for the virulence of C. albicans. Sanglard and collegues have independently reached a similar conclusion (personal communication). We find that calcineurin is not required for hyphal development in vitro or in vivo or for adherence to host cells, two well-studied processes important for virulence. Calcineurin mutants have a modest defect in endothelial cell injury, but it is not likely of sufficient magnitude to confer the marked reduction in virulence. Histopathological analysis revealed that calcineurin is required for dissemination and growth in the renal parenchyma. Calcineurin was found to be necessary for survival in serum, and we propose that calcineurin mutants fail to survive long enough in the bloodstream to disseminate efficiently and cause significant disease in target tissues for mortality such as the kidneys. The necessity of calcineurin for survival in serum is interesting in light of a recent study exploring the expression profile of C. albicans exposed to human blood in vitro or mouse blood in vivo (22). A number of classes of genes were discovered to be upregulated in serum or blood, including genes involved in glycolysis, the glyoxylate cycle, and fatty acid metabolism. It will be of interest to determine whether calcineurin plays a role in regulating any of these processes that could be important for growth and survival in blood.
We propose two models to explain the sensitivity of calcineurin mutants of C. albicans to serum. In the first, serum lacks one or more components necessary for the proliferation of calcineurin mutant cells. In the second, an active factor in serum kills calcineurin mutants. Our studies reveal that 1:1 supplementation of serum with YPD rich medium, addition of 100 mg of yeast extract/ml to serum (10-fold the level in YPD), or addition of supraphysiologic levels of the vitamins thiamine and niacin rescues the growth of calcineurin mutants, providing support for the first model. Furthermore, our studies reveal that boiling serum and passing it though a 3-kDa filter does not destroy activity against calcineurin mutants, eliminating an involvement of most serum proteins, including complement and antibodies.
In contrast to the nutrient addition studies, the rapid loss of CFU by the calcineurin mutants in serum suggests an active killing component. In support of this second hypothesis, calcineurin mutant cells incubated overnight at 30°C in PBS, which lacks nutrients, persist in this medium (data not shown). Thus, the phenotype of the calcineurin mutants cannot simply be due to a lack of nutrients in the serum. The addition of supraphysiologic nutrients to serum in the nutrient addition experiments might simply promote growth and mask the effect of active factors, which could include small antimicrobial peptides (AMPs) with molecular weights below 3 kDa. AMPs, such as the platelet-produced antimicrobial peptides, are known to have antifungal capabilities (61). AMPs have little to no tertiary structure, are heat stable, have broad activity against a number of organisms, including bacteria, fungi, and some viruses, and generally act by disrupting the pathogen membrane (reviewed in references 24 and 60). The membrane disruption activity of the AMPs is reminiscent of the membrane disruption caused by fluconazole, which is lethal to strains lacking calcineurin but only fungistatic to strains with active calcineurin (12). Thus, AMPs in serum might be contributing to the growth defect of calcineurin mutants in serum via a mechanism similar to the azole-hypersensitive phenotype observed with C. albicans cnb1/cnb1 calcineurin mutants. It is also possible that both the active factor and nutrient models are correct and that their effects compound to lead to the rapid killing of calcineurin mutants. Further studies will be necessary to elucidate in detail the role of calcineurin in promoting the growth and survival of C. albicans in serum.
Although calcineurin is highly conserved, calcineurin mutants of the distantly related pathogenic basidiomycete C. neoformans and the closely related ascomycete S. cerevisiae exhibit distinct phenotypes. Calcineurin is required for C. albicans to survive in serum, but not for the survival of C. neoformans or S. cerevisiae (J. R. Blankenship and J. Heitman, unpublished data). In contrast, calcineurin is required for survival at 37°C in C. neoformans but not in C. albicans or S. cerevisiae (13, 21, 44). Thus, the calcineurin signaling cascade has evolved to control aspects of virulence of two divergent pathogens via distinct molecular mechanisms. It is also clear from these findings that one cannot extrapolate from model yeasts or even from other human fungal pathogens to establish the cellular functions of these conserved signaling molecules; rather, studies must be conducted with each organism.
Calcineurin is the target of the immunosuppressive and antifungal drugs cyclosporine A and FK506 (tacrolimus) (37). Less-immunosuppressive analogs of these drugs that retain their antifungal activity have been identified, and several groups have explored the potential of these drugs for anti-Candida therapy (12, 39-41, 45). These drugs have no effect on the growth of C. albicans and S. cerevisiae in rich media but exhibit a potent fungicidal synergism when combined with azoles (12, 18, 39, 41). Edlind and colleagues have shown that the activity of the calcineurin target Crz1 is important for synergy between calcineurin inhibitors and azoles in S. cerevisiae (18). However, other studies suggest that a Crz1-independent role of calcineurin in responding to endoplasmic reticulum stress is responsible for the synergism between azoles and calcineurin inhibitors in S. cerevisiae (8). In our studies, the growth defect of calcineurin mutants in serum was recapitulated by exposing wild-type cells to the calcineurin inhibitor FK506 in serum. Thus, the utility of calcineurin inhibitors in treating Candida infections could be expanded. These studies highlight the potential use of less-immunosuppressive analogs of calcineurin inhibitors for treatment of candidal infections and illustrate that screening drugs for antifungal activity in serum rather than rich medium has the potential to reveal novel classes of antifungal agents for therapeutic intervention.
Acknowledgments
We thank Dana Davis for providing strain DAY185, Dominique Sanglard for sharing data prior to publication, and Christina Hull, Debbie Fox, and Alan Goldstein for comments on the manuscript. We thank the nurses at Harbor-UCLA Medical Center for collecting umbilical cords and Angela Sanchez for assistance with the damage assays.
These studies were supported in part by T32 training grant AI07392 from the NIH (F.L.W.), RO1 grant AI50438 from NIAID (to J.H.), PO1 grant AI44975 to the Duke University Mycology Research Unit, NIH grants RO1-AI28388 and RO1 PA-98-100 (to J.R.P.), and Public Health Service grant MO1 RR00425 (to S.G.F.). S. G. Filler is aBurroughs Wellcome Fund New Investigator in Molecular Pathogenic Mycology. J. Heitman is an associate investigator of the Howard Hughes Medical Institute and a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology.
REFERENCES
- 1.Anaissie, E., H. Pinczowski, and D. Louria. 1993. Candida infections in experimental animals, p. 43-57. In G. Bodey (ed.), Candidiasis. Pathogenesis, diagnosis, and treatment. Raven Press, New York, N.Y.
- 2.Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell Regul. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 3.Ashman, R., A. Fulurija, and J. Papadimitriou. 1996. Strain-dependent differences in host response to Candida albicans infection in mice are related to organ susceptibility and infectious load. Infect. Immun. 64:1866-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashman, R., and J. Papadimitriou. 1989. Genetic regulation of pathogenesis and host responses in fungal infection, p. 347-371. In E. Kurstak and G. Marquis (ed.), Immunology of fungal infection. Marcel Dekker, New York, N.Y. [PubMed]
- 5.Bahn, Y. S., and P. Sundstrom. 2001. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J. Bacteriol. 183:3211-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck-Sague, C. M., and W. R. Jarvis. 1993. Secular trends in the epidemiology of nosocomical fungal injections in the United States, 1980-1990. National Nosocomial Infections Surveillance System. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 7.Berman, J., and P. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla, M., K. K. Nastase, and K. W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21:2343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breuder, T., C. S. Hemenway, N. R. Movva, M. E. Cardenas, and J. Heitman. 1994. Calcineurin is essential in cyclosporin A- and FK506-sensitive yeast strains. Proc. Natl. Acad. Sci. USA 91:5372-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, A. 2002. Morphogenetic signaling pathways in Candida albicans, p. 95-106. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 11.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 12.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz, M. C., R. A. L. Sia, M. Olson, G. M. Cox, and J. Heitman. 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 68:982-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187-218. [DOI] [PubMed] [Google Scholar]
- 15.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, R. E., G. C. Icke, J. Thom, and W. J. Riley. 1984. Intestinal absorption of thiamin in man compared with folate and pyridoxal and its subsequent urinary excretion. J. Nutr. Sci. Vitaminol. (Tokyo) 30:475-482. [DOI] [PubMed] [Google Scholar]
- 18.Edlind, T., L. Smith, K. Henry, S. Katiyar, and J. Nickels. 2002. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol. Microbiol. 46:257-268. [DOI] [PubMed] [Google Scholar]
- 19.Felk, A., M. Kretschmar, A. Albrecht, M. Schaller, S. Beinhauer, T. Nichterlein, D. Sanglard, H. C. Korting, W. Schafer, and B. Hube. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filler, S. G., J. N. Swerdloff, C. Hobbs, and P. M. Luckett. 1995. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63:976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox, D. S., M. C. Cruz, R. A. Sia, H. Ke, G. M. Cox, M. E. Cardenas, and J. Heitman. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39:835-849. [DOI] [PubMed] [Google Scholar]
- 22.Fradin, C., M. Kretschmar, T. Nichterlein, C. Gaillardin, C. d'Enfert, and B. Hube. 2003. Stage-specific expression of Candida albicans in human blood. Mol. Microbiol. 47:1523-1543. [DOI] [PubMed] [Google Scholar]
- 23.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 24.Gallo, R. L., M. Murakami, T. Ohtake, and M. Zaiou. 2002. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 110:823-831. [DOI] [PubMed] [Google Scholar]
- 25.Ghannoum, M. A. 2000. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13:122-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghannoum, M. A., B. Spellberg, S. M. Saporito-Irwin, and W. A. Fonzi. 1995. Reduced virulence of Candida albicans PHR1 mutants. Infect. Immun. 63:4528-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolution of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 27.Hemenway, C. S., and J. Heitman. 1999. Calcineurin: structure, function, and inhibition. Cell Biochem. Biophys. 30:115-151. [DOI] [PubMed] [Google Scholar]
- 28.Hube, B., M. Monod, D. A. Schofield, A. J. Brown, and N. A. Gow. 1994. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol. Microbiol. 14:87-99. [DOI] [PubMed] [Google Scholar]
- 29.Hube, B., D. Sanglard, F. C. Odds, D. Hess, M. Monod, W. Schafer, A. J. Brown, and N. A. Gow. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob, R. A., M. E. Swendseid, R. W. McKee, C. S. Fu, and R. A. Clemens. 1989. Biochemical markers for assessment of niacin status in young men: urinary and blood levels of niacin metabolites. J. Nutr. 119:591-598. [DOI] [PubMed] [Google Scholar]
- 32.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura, L. H., and N. N. Pearsall. 1978. Adherence of Candida albicans to human buccal epithelial cells. Infect. Immun. 21:64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klee, C., H. Ren, and X. Wang. 1998. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273:13367-13370. [DOI] [PubMed] [Google Scholar]
- 35.Kudla, J., Q. Xu, K. Harter, W. Gruissem, and S. Luan. 1999. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 96:4718-4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leidich, S. D., A. S. Ibrahim, Y. Fu, A. Koul, C. Jessup, J. Vitullo, W. Fonzi, F. Mirbod, S. Nakashima, Y. Nozawa, and M. A. Ghannoum. 1998. Cloning and disruption of CaPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J. Biol. Chem. 273:26078-26086. [DOI] [PubMed] [Google Scholar]
- 37.Liu, J., J. D. Farmer, W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 38.Lo, H.-J., J. R. Köhler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 39.Maesaki, S., P. Marichal, M. A. Hossain, D. Sanglard, H. V. Bossche, and S. Kohno. 1998. Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 42:747-753. [DOI] [PubMed] [Google Scholar]
- 40.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine; a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 43.Odds, F. C. 1988. Candida and candidosis: a review and bibliography, 2nd ed. Baillière Tindall, London, United Kingdom.
- 44.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors in Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panagoda, G. J., A. N. Ellepola, and L. P. Samaranayake. 2001. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses 44:29-35. [DOI] [PubMed] [Google Scholar]
- 47.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 80:1483-1521. [DOI] [PubMed] [Google Scholar]
- 50.Sanglard, D., B. Hube, M. Monod, F. C. Odds, and N. A. Gow. 1997. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Arino. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319-1333. [DOI] [PubMed] [Google Scholar]
- 52.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 53.Staab, J. F., C. A. Ferrer, and P. Sundstrom. 1996. Developmental expression of a tandemly repeated, proline-and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J. Biol. Chem. 271:6298-6305. [DOI] [PubMed] [Google Scholar]
- 54.Sundstrom, P. 1999. Adhesins in Candida albicans. Curr. Opin. Microbiol. 2:353-357. [DOI] [PubMed] [Google Scholar]
- 55.Sundstrom, P. 2002. Adhesion in Candida spp. Cell Microbiol. 4:461-469. [DOI] [PubMed] [Google Scholar]
- 56.Sundstrom, P., E. Balish, and C. M. Allen. 2002. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J. Infect. Dis. 185:521-530. [DOI] [PubMed] [Google Scholar]
- 57.Sundstrom, P. M., and G. E. Kenny. 1984. Characterization of antigens specific to the surface of germ tubes of Candida albicans by immunofluorescence. Infect. Immun. 43:850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 59.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeaman, M. R., and A. S. Bayer. 1999. Antimicrobial peptides from platelets. Drug Resist. Updat. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 61.Yeaman, M. R., A. S. Ibrahim, J. E. Edwards, Jr., A. S. Bayer, and M. A. Ghannoum. 1993. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob. Agents Chemother. 37:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida, T., T. Toda, and M. Yanagida. 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107:1725-1735. [DOI] [PubMed] [Google Scholar]