Abstract
We carried out comparative DSC and Fourier transform infrared spectroscopic studies of the effects of cholesterol and lanosterol on the thermotropic phase behavior and organization of DPPC bilayers. Lanosterol is the biosynthetic precursor of cholesterol and differs in having three rather than two axial methyl groups projecting from the β-face of the planar steroid ring system and one axial methyl group projecting from the α-face, whereas cholesterol has none. Our DSC studies indicate that the incorporation of lanosterol is more effective than cholesterol is in reducing the enthalpy of the pretransition. Lanosterol is also initially more effective than cholesterol in reducing the enthalpies of both the sharp and broad components of the main phase transition. However, at sterol concentrations of 50 mol %, lanosterol does not abolish the cooperative hydrocarbon chain-melting phase transition as does cholesterol. Moreover, at higher lanosterol concentrations (∼30–50 mol %), both sharp and broad low-temperature endotherms appear in the DSC heating scans, suggestive of the formation of lanosterol crystallites, and of the lateral phase separation of lanosterol-enriched phospholipid domains, respectively, at low temperatures, whereas such behavior is not observed with cholesterol at comparable concentrations. Our Fourier transform infrared spectroscopic studies demonstrate that lanosterol incorporation produces a less tightly packed bilayer than does cholesterol, which is characterized by increased hydration in the glycerol backbone region of the DPPC bilayer. These and other results indicate that lanosterol is less miscible in DPPC bilayers than is cholesterol, but perturbs their organization to a greater extent, probably due primarily to the rougher faces and larger cross-sectional area of the lanosterol molecule and perhaps secondarily to its decreased ability to form hydrogen bonds with adjacent DPPC molecules. Nevertheless, lanosterol does appear to produce a lamellar liquid-ordered phase in DPPC bilayers, although this phase is not as tightly packed as comparable cholesterol/DPPC mixtures.
INTRODUCTION
Cholesterol is a major and essential lipid component of the plasma membranes of the cells of higher animals and is also found in lower concentrations in certain intracellular membranes in vesicular communication with the plasma membrane (1–3). Although cholesterol has a number of different functions in animal cells, one of its primary roles is as a modulator of the physical properties and lateral organization of the plasma membrane lipid bilayer. Thus, many studies of the interaction of cholesterol with phospholipid monolayer and bilayer model membranes have been performed, utilizing a wide range of physical techniques (2,4–7). These studies, most of which have utilized symmetrical-chain, linear-saturated PCs, have established that one of the major effects of cholesterol incorporation on phospholipid monolayer and bilayer model membranes is a broadening and eventual elimination of the cooperative gel-to-liquid-crystalline phase transition and its replacement by a phase with an intermediate degree of organization. Thus, in the liquid-crystalline phase, which would exist at physiological temperatures in the absence of sterols in biological membranes, the presence of cholesterol significantly increases the orientational order of the phospholipid hydrocarbon chains and decreases the cross-sectional area occupied by the phospholipid molecules, while only moderately restricting the rates of phospholipid lateral diffusion or hydrocarbon chain motion. In addition, the presence of cholesterol increases both the thickness and mechanical strength and decreases the permeability of the phospholipid bilayer in the physiologically relevant liquid-crystalline phase. The relatively high rates of intramolecular and intermolecular motion characteristic of phospholipid model membranes in the presence of high levels of cholesterol, coupled with an increased hydrocarbon chain order and a decreased area compressibility, have prompted several workers to postulate the existence of a discrete liquid-ordered or Lo phase in model and biological membranes having cholesterol levels above ∼7–8 mol % (5,8–9). However, as many of the physical properties of model membranes composed of cholesterol and a single phospholipid change smoothly and monotonically with progressive increases in cholesterol concentration up to 50 mol % (2,4–7), the existence of thermodynamically discrete, macroscopic Lo and Ld phases in such binary systems has been questioned (7,10,11), and it has been suggested that the behavior of phospholipid/cholesterol systems can be explained by the formation of various superlattices (12) or molecular complexes (13). However, in model membranes composed of cholesterol, unsaturated PCs, and natural SpMs, the specificity of the interaction of cholesterol for SpM can result in the formation of macroscopic domains enriched in cholesterol and SpMs and depleted in unsaturated PCs, and such domains are thought to form the molecular basis for the possible existence of detergent-insoluble and cholesterol- and SpM-enriched lipid rafts in biological membranes (14–19). Even in such ternary systems, however, the existence of thermodynamically discrete Lo and Ld systems has not been detected by DSC or x-ray diffraction (20) and a number of workers have found the evidence for the existence of relatively large, long-lived lipid rafts in biological membranes unconvincing (21–23). Whatever the biophysical details, it is clear that the presence of cholesterol in biological membranes does modulate a number of different membrane functions, either directly or via its effects on the properties and lateral organization of the phospholipid bilayer (2,24–26).
A number of researchers have investigated the effects of systematic variations in the structure and stereochemistry of the cholesterol molecule on the thermotropic phase behavior, organization, and passive permeability of phospholipid bilayers (2,4,6,7). In general, most structural and stereochemical alterations result in some loss of the ability of the cholesterol molecule to produce its characteristic effects on phospholipid bilayers. Also, sterols must possess an equatorially oriented C3-hydroxy group, a rigid planar fused ring system, and a flexible hydrocarbon side chain at C17 for maximum effect, whereas the degree of unsaturation of the ring system and the size of the alkyl side chain are of less importance. Interestingly, exactly the same structural features are required for exogenous sterols to support the maximum growth of sterol-auxotropic mycoplasma, yeast, and mammalian cells (24–26), confirming that one of the major roles of cholesterol in eukaryotic membranes is to regulate the physical properties of the lipid bilayer.
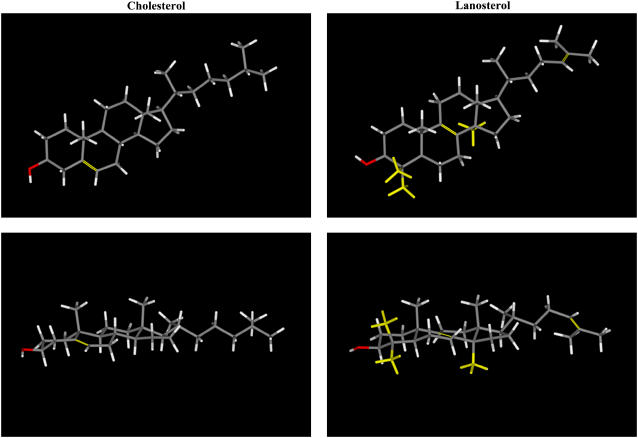
Lanosterol is the first tetracyclic intermediate in the biosynthesis of cholesterol and other sterols in all eukaryotic cells (1). As illustrated in Fig. 1, lanosterol differs from cholesterol in having two extra methyl groups at C4 and an extra axial methyl group at C14, as well as having two double bonds at C8–C9 and C24–C25 rather than a single double bond at C5–C6. Thus, lanosterol, unlike cholesterol, has three rather than two axial methyl groups projecting from the β-face of the planar steroid ring system and one axial methyl group projecting from the α-face, whereas cholesterol has none. These three extra methyl groups on lanosterol give the molecule a rougher surface and a slight bend compared to cholesterol. Also, it is possible that the extra methyl groups at C4 may interfere with efficient hydrogen bonding of the hydroxyl group at C3 (27). Indeed, it has been proposed that the energy-requiring removal of the three extra methyl groups present on the lanosterol molecule is part of the molecular evolution of sterol molecules to produce cholesterol and other sterols with a smoother surface and a more exposed hydroxyl group, which can thus interact more strongly with the phospholipids and sphingolipids in eukaryotic plasma membranes to produce their characteristic ordering effects (28–29). Indeed, studies of the comparative effects of lanosterol and cholesterol on the passive permeability (30–31) and molecular ordering and dynamics (31–42) of phospholipid model membranes have indicated that the lanosterol molecule itself is less ordered and more mobile in phospholipid bilayers than is cholesterol, and that lanosterol is also less potent at reducing the passive permeability and increasing the order of the hydrocarbon chains of the lipid model membranes. Similar results were reported for membranes of the cell wall-less bacteria Mycoplasma capricolum (24,43). In addition, although lanosterol and cholesterol increase the thicknesses of Lα DMPC bilayers to a similar extent, lanosterol is less effective than cholesterol in reducing the thermal area expansion coefficient (44). Moreover, lanosterol is less effective than cholesterol in increasing phospholipid bilayer bending rigidity (45,46) and in increasing the lateral tension and surface viscosity of the bilayer (47). Subtle differences in the location, mobility, and effects of lanosterol and cholesterol on phospholipid bilayers that generally parallel those found experimentally have also been reported using molecular dynamics and Monte Carlo simulations (38,48). Finally, lanosterol has been reported to have a weaker effect than cholesterol in promoting lateral domain formation in lipid-raft-mimetic model membrane systems (49,50).
FIGURE 1.
Molecular models of cholesterol and lanosterol. The top panels show views normal to the plane of the sterol ring and the bottom panels show views parallel to the plane of the sterol ring. The structural differences between the two sterols are highlighted in yellow.
High-sensitivity DSC is a nonperturbing thermodynamic technique that has proven of great value in studies of the effect of cholesterol and cholesterol analogs on the thermotropic phase behavior of phospholipid and sphingolipid bilayers (11,51–57). However, there appears to have been only one high-sensitivity DSC study of lanosterol/phospholipid model membranes to date (38). These workers reported that lanosterol abolished the main phase transition of PPetPC vesicles at all concentrations >12 mol %, whereas cholesterol did so only above concentrations of ∼20 mol %. Moreover, it was reported that lanosterol reduced the gel/liquid-crystalline phase transition temperature of the PPetPC bilayer to a greater extent than cholesterol, although this effect, if real, was small (∼1°C at 10 mol % sterol). Based on their DSC and 2H-NMR studies, Miao et al. (38) proposed that the lanosterol/PPetPC phase diagram is qualitatively different from the cholesterol/DPPC phase diagram, and, subsequently, that lanosterol, unlike cholesterol, is unable to form a Lo phase in phospholipid bilayers (58). However, the calorimetric results reported by Miao et al. are quite different from those obtained in other high-sensitivity DSC studies of disaturated or mixed-chain saturated-unsaturated PC/cholesterol mixtures, where the main phase transition is not abolished until cholesterol concentrations near 50 mol % are reached. Moreover, as we have shown previously (52), the use of a constant sample size and DSC instrumental sensitivity setting can result in a failure to detect and accurately monitor the less energetic and less cooperative chain-melting phase transition occurring at high sterol concentrations. We have thus reinvestigated the effect of lanosterol and cholesterol on the thermotropic phase behavior of the well studied DPPC bilayers using a higher sensitivity calorimeter and an experimental protocol which ensures that the broad, lower enthalpy phase transitions occurring at higher sterol levels are accurately monitored. Moreover, we have also investigated the effects of lanosterol and cholesterol incorporation on the organization of DPPC bilayers by FTIR spectroscopy. Overall, our results indicate that the effects of lanosterol on the thermotropic phase behavior and organization of DPPC vesicles are somewhat different from and more complex than those of cholesterol.
MATERIALS AND METHODS
The DPPC and cholesterol were both obtained from Avanti Polar Lipids (Alabaster, AL), whereas the lanosterol was supplied by Research Plus (Manasquan, NJ). The purities of DPPC and cholesterol were >99% and the purity of the lanosterol was >98%. All organic solvents were of at least analytical-grade quality and were redistilled before use. Samples were prepared in clean glass tubes by mixing appropriate volumes of standard solutions of DPPC and the appropriate sterol in chloroform:methanol (∼95:5, v/v) to obtain the required lipid:sterol ratio. The solvent was then removed with a stream of nitrogen at temperatures near 55°C, such that the lipid:sterol mixtures were cast as thin films on the sides of the tubes. The latter were dried in vacuo overnight to remove the last traces of solvent and were subsequently dispersed in an appropriate volume of deionized water by vigorous vortex mixing at temperatures near 55–60°C. We find that this procedure avoids any fractional crystallization of sterol during sample preparation.
The samples used for the DSC experiments were prepared by dispersing appropriate amounts of the dried lipid/sterol mixture in 1 ml of deionized water. The dispersion was then degassed and 324-μl aliquots were withdrawn for DSC analyses. To ensure better resolution of the broad low-enthalpy thermotropic transitions exhibited by sterol-rich mixtures, the amount of lipid used for DSC measurements was progressively increased with the sterol content of the mixture (52). Typically, samples containing 1–3 mg phospholipid were used at sterol concentrations <5 mol %, 5–8 mg phospholipid at sterol concentrations between 5 and 15 mol %, and 10–15 mg of phospholipid at all higher sterol concentrations. DSC heating and cooling thermograms were recorded with a high-sensitivity Nano II DSC (Calorimetry Sciences, Lindon, UT) operating at a scan rate of 10°C/h. The data acquired were analyzed and plotted with the Origin software package (OriginLab, Northampton, MA). In cases where the DSC thermograms appeared to be a summation of overlapping components, the midpoint temperatures, areas, and widths of the components were estimated with the aid of the Origin nonlinear least-squares curve- and peak-fitting procedures and a custom-coded function based on the assumption that the observed thermogram was a linear combination of components, each of which could be approximated by a reversible two-state transition at thermodynamic equilibrium.
Samples used for FTIR spectroscopic experiments were prepared by dispersing dried lipid/sterol mixtures containing 2–3 mg of phospholipids in 50 μl of distilled water at temperatures near 55–60°C. The paste so formed was then sealed as a thin (25-μm) film between the CaF2 windows of a heatable demountable liquid cell equipped with a 25-μm Teflon spacer. Once mounted in the sample holder of the instrument, sample temperature could be controlled between −20°C and 90°C by means of an external computer-controlled water bath. FTIR spectra were acquired with a Digilab FTS-40 Fourier-transform spectrometer (Biorad, Digilab Division, Cambridge, MA) using data-acquisition and data-processing protocols described by Lewis et al. (59).
RESULTS
The overall pattern of thermotropic phase behavior observed in cholesterol/DPPC and lanosterol/DPPC multilamellar liposomes
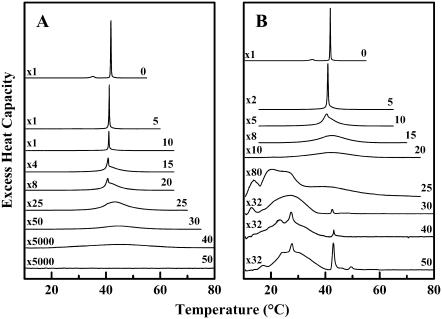
Fig. 2 shows DSC heating scans of DPPC dispersions containing differing concentrations of both sterols. The overall pattern of behavior seen on heating is similar to that reported previously for cholesterol/DPPC mixtures using less sensitive DSC instruments (11,52,53). In the absence of sterols, DPPC heating scans show two sharp endothermic peaks, initially centered at 34°C and 41.2°C, which correspond to the pretransition (Lβ′/Pβ′) and main (Pβ′/Lα) phase transitions, respectively. Increasing the sterol concentration gradually broadens the pretransition and reduces its temperature and enthalpy in both cases. Similarly, in the case of the main phase transition, increasing the sterol concentration initially produces a multicomponent DSC endotherm, consisting of a sharp component that is progressively reduced in temperature, enthalpy, and cooperativity, and a broad component that increases in both temperature and enthalpy but decreases in cooperativity. Thus, with increasing sterol concentrations, the sharp component disappears as the broad component grows. However, there are significant differences in the pattern of thermal events observed in the cholesterol/DPPC (Fig. 2 A) and lanosterol/DPPC (Fig. 2 B) samples, which indicate that the behavior of the latter is more complex. In particular, the DSC thermograms exhibited by the lanosterol-rich DPPC mixtures exhibit a series of sharp endotherms superimposed on the broad components, features that are not observed in any of the cholesterol-containing DPPC mixtures examined. We will therefore focus first on the effect of both sterols on the pretransition and on the major components of main phase transition of DPPC, and address the issue of complex behavior of the lanosterol-rich preparations later.
FIGURE 2.
DSC thermograms illustrating the effect of cholesterol (A) and lanosterol (B) on the gel/liquid-crystalline phase transition of DPPC. The thermograms shown were acquired at the sterol concentrations (mol %) indicated and have all been normalized against the mass of DPPC used. The y axis scaling factors are indicated on the left-hand side of each thermogram.
The effects of cholesterol and lanosterol on the pretransition of DPPC
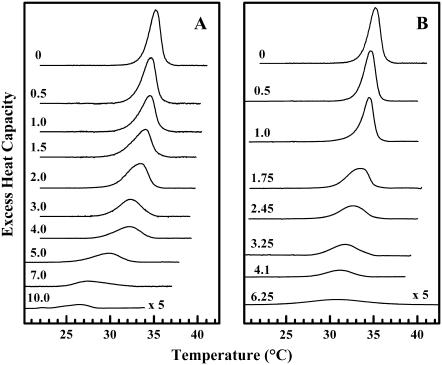
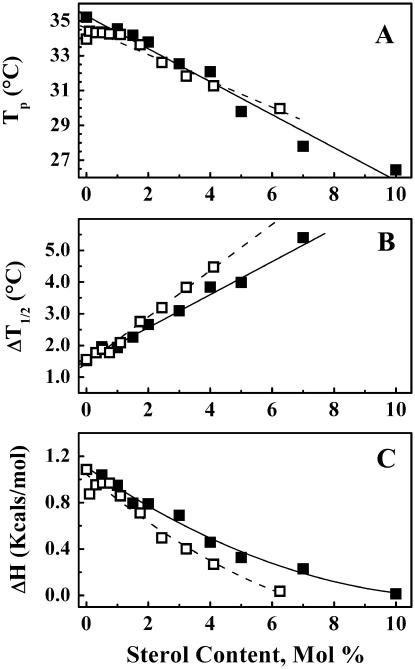
To investigate the disappearance of the pretransition in greater detail, we prepared sterol/DPPC samples with a narrower range of either cholesterol or lanosterol concentrations. The gradual elimination of the pretransition in the DSC heating scans as a function of sterol concentration is shown in Fig. 3 and the derived thermodynamic measurements for both sterol/DPPC systems are presented in Fig. 4. The cholesterol/DPPC and lanosterol/DPPC samples show comparable decreases in the Tp (Fig. 4, A and B) with increasing sterol concentration. Although the values of Tp decrease at similar rates, the values of ΔH (Fig. 4, C and D) and ΔT1/2 (Fig. 4, E and F) decrease and increase, respectively, more rapidly in the lanosterol/DPPC mixtures than in the corresponding cholesterol/DPPC mixtures. Thus, the pretransition is abolished at a lanosterol concentration of ∼7 mol %, whereas it persists up to a cholesterol concentration of ∼10 mol %. Since these changes occur over a narrower range of sterol concentration in the lanosterol/DPPC samples (0–7 mol %) compared to the cholesterol/DPPC samples (0–10 mol %), we can conclude that lanosterol is more effective at abolishing the pretransition than is cholesterol on a molar basis. A possible molecular explanation for this observation will be presented in the Discussion.
FIGURE 3.
DSC thermograms illustrating the effect of cholesterol (A) and lanosterol (B) on the pretransition of DPPC. The thermograms shown were acquired at the sterol concentrations (mol %) indicated and have all been normalized against the mass of DPPC used. The thermograms shown for DPPC/sterol mixtures containing 10 mol % cholesterol and 6.25 mol % lanosterol are plotted on a y axis fivefold expanded relative to all others.
FIGURE 4.
Effect of increases in sterol concentration on the Tp, ΔH, and ΔT½ of the pretransition of DPPC. The symbols (▪) and (□) represent the data from the cholesterol- and lanosterol-containing samples, respectively.
The effects of sterol concentration on the main phase transition of DPPC
The DSC data shown in Fig. 2 indicate that at low to moderate sterol concentrations, both cholesterol- and lanosterol-containing DPPC bilayers exhibit asymmetric thermograms that consist of at least two overlapping thermal events. One of these components is considerably sharper than the others, its peak temperature and cooperativity decrease slightly, but its enthalpy decreases markedly with increasing sterol content. The other component (or components) is considerably broader, its midpoint temperature(s) exhibits a more complex dependence on sterol content, and it is the only component(s) persisting at the higher range of sterol concentrations. This pattern of sterol-concentration-dependent behavior has been observed previously (11,52–53), and the resolved sharp and broad components have been ascribed to the differential melting of sterol-poor and sterol-rich lipid domains, respectively. However, there are significant differences between the sterol-concentration-dependent behaviors exhibited by the cholesterol- and lanosterol-containing preparations, especially with regard to the quantitative aspects of the sterol-concentration dependence of the number and overall properties of the underlying sharp and broad peaks (Fig. 5). This aspect of our experimental observations was further examined through the application of computer-assisting curve- and peak-fitting procedures to deconvolve the observed DSC thermograms into their component peaks, so that the sterol-concentration dependence of each component could be examined and compared (see below).
FIGURE 5.
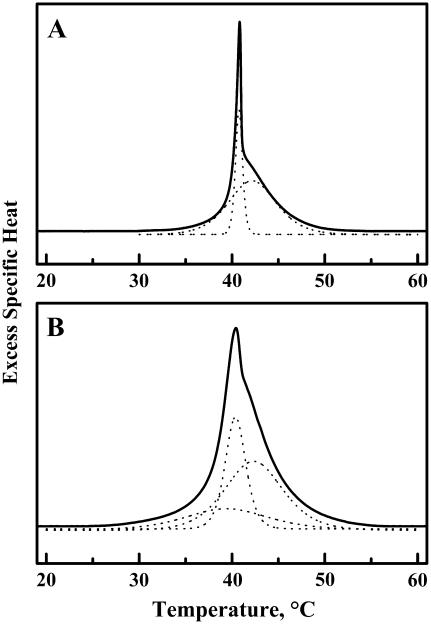
Illustration of the results typically obtained in our peak-fitting deconvolution analyses of the DSC thermograns exhibited by cholesterol-containing (A) and lanosterol-containing (B) DPPC bilayers. The thermograms shown both contained 15 mol % sterol. To facilitate visibility, the fitted curves are slightly displaced along the y axis.
The data presented in Fig. 5 are an example of the results typically obtained in our curve-fitting analyses of the DSC thermograms exhibited by the cholesterol- and lanosterol-containing mixtures. These results indicate that at lower sterol concentrations, the observed DSC thermograms of the cholesterol-containing mixtures can be accurately simulated by a summation of two components (one sharp and one broad), whereas those of the lanosterol-containing preparations can only be accurately simulated if one assumes that the DSC thermograms are summations of one sharp and at least two broad components (see Fig. 5). Moreover, we find that in the higher range of sterol concentrations, the thermograms exhibited by the cholesterol-containing bilayers can be accurately simulated by a single broad, low-enthalpy component, whereas simulation of the thermograms of the lanosterol-containing mixtures requires the assumption of at least two broad, low-enthalpy components (Fig. 5). These observations strongly suggest that bilayers derived from DPPC mixtures of moderate to high lanosterol content contain at least two significant populations of independently melting, lanosterol-rich domains, a possibility also supported by the results of our FTIR spectroscopic studies (see below). We present below a detailed analysis of the effects of variations in cholesterol content on the sharp and broad components of our DSC thermograms.
Effect of cholesterol and lanosterol on the sharp component of the DPPC main phase transition
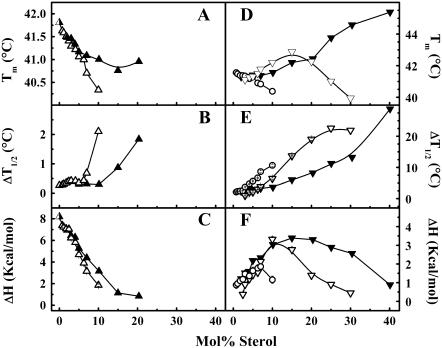
The Tm values obtained from the sharp and broad components of the main phase transition of both the cholesterol/DPPC and lanosterol/DPPC mixtures are shown in Fig. 6. The sharp components of both sterol/DPPC systems (Fig. 6 A) show a gradual decrease in Tm, which closely follows the Tm values obtained for the overall DSC curves. Above 5 mol %, the decrease in the Tm values of the cholesterol/DPPC mixtures levels off and reaches a minimum at 20 mol %, whereas that of the lanosterol/DPPC samples reaches a minimum at 10 mol %.
FIGURE 6.
Thermodynamic parameters for the deconvolved sharp (A–C) and broad (D–F) components obtained from the DSC heating thermograms of the cholesterol/DPPC (▴, ▾) and lanosterol /DPPC (▵, ▿, ○) samples as a function of sterol concentration. The open circles represent the lower-temperature broad component of the lanosterol/DPPC samples. The error bars were typically equal to or smaller than the size of the symbols.
The corresponding values of ΔT1/2 for the sharp components of both sterol/DPPC mixtures (Fig. 6 B) also closely follow those of the overall curve at low sterol concentrations and are virtually identical up to a concentration of 7 mol % in both sterol/DPPC systems. At higher sterol concentrations, the ΔT1/2 values of the sharp component increase sharply for the lanosterol system above 7 mol %, whereas those of the cholesterol/DPPC mixtures increase gradually above 10 mol %, reflecting the different abilities of these sterols to broaden the DPPC main phase transition. In contrast, the pattern of ΔH values for the sharp component of both sterol/DPPC systems is more complex (Fig. 6 C). After the initial drop in enthalpy, there is a small window where the ΔH values for both sterol/DPPC mixtures are indistinguishable from one another and decrease very slowly (Fig. 6 C). At sterol concentrations >3 mol %, there is a more rapid decrease in the ΔH values of the sharp component for both sterol/DPPC mixtures. In the case of the lanosterol/DPPC system, the sharp component is abolished at ∼15 mol %, whereas in the cholesterol/DPPC system it is abolished at ∼25 mol %, again reflecting differences in the ability of each sterol to abolish the main phase transition of DPPC. The possible molecular basis for these differential effects will be addressed in the Discussion.
Effect of cholesterol and lanosterol on the broad component(s) of the DPPC main phase transition
An analysis of the broad components obtained from our deconvolution of the overall DSC thermograms for both sterol/DPPC systems is shown in Fig. 6, D–F. The broad component of the cholesterol/DPPC thermograms shows a linear increase in temperature over the entire range of sterol concentrations (Fig. 6 D). In the lanosterol/DPPC system, our analysis produced two broad peaks using the above-mentioned deconvolution of the overall thermogram. Of those two broad components, the first (BC1) is initially found on the low temperature side of the sharp component. From 3–10 mol %, the BC1 and the sharp component are almost superimposed and their temperature maxima decrease at similar rates, suggesting that these two components are closely related; however, above 10 mol % lanosterol the BC1 is abolished. At 3 mol %, the second broad component (BC2) becomes visible initially on the low-temperature side of the sharp component just below the BC1. At higher lanosterol concentrations, the temperature of the BC2 increases, crossing over to the high temperature side of both the BC1 and the sharp component at ∼4 mol %. This crossover point occurs at a similar sterol concentration in the cholesterol/DPPC mixtures, in agreement with our earlier studies (11,52). The BC2 also increases in temperature more rapidly and at 15 mol % lanosterol is found at a slightly higher temperature than the broad component seen in the corresponding cholesterol/DPPC mixtures. Above 15 mol % lanosterol, the temperature of the BC2 decreases, becoming increasingly difficult to measure against the broad components that appear at lower temperatures and are of unknown origin. The DSC thermograms suggest that the BC2 is completely abolished at a concentration between 30 and 40 mol % lanosterol. Thus, at lower sterol concentrations, the phase transition temperature of the BC2 behaves similarly to the broad component seen in the deconvolved cholesterol/DPPC system. However, at higher sterol concentrations, the decrease in the temperature maximum of the BC2, and for that matter of the BC1 over its entire concentration range, is reminiscent of the behavior seen in the androstenol/DPPC systems, which exhibit androstenol/DPPC immiscibility at higher sterol concentrations (53). These disparities in the temperature maxima may indicate differences in the miscibility and packing of lanosterol and androstenol in DPPC bilayers when compared to the cholesterol/DPPC system, where the sterol and DPPC seem to be freely miscible.
For both sterol/DPPC systems, the ΔT1/2 values of the broad components are significantly larger than those of the sharp component and show a gradual increase with increasing sterol concentration (Fig. 6 E). In the lanosterol/PC system, the ΔT1/2 values of the BC1 are larger than those of the BC2. The BC1 seems to be abolished above 10 mol % lanosterol, yet does not appear to become very broad like the BC2 component or the broad component of the cholesterol/DPPC system. This may be an artifact that arises from the inability of the deconvolution routine to identify small components against a broad, highly energetic second component. The ΔT1/2 values of the lanosterol/DPPC BC2 component increase linearly with increasing sterol concentration and, over the range 10–30 mol %, are significantly greater than those of the cholesterol/DPPC broad component, again indicating that lanosterol has a greater broadening effect on the overall DPPC phase transition than does cholesterol.
The ΔH values of the cholesterol/DPPC broad component gradually increase up to a concentration of ∼15 mol %, above which these steadily decrease and the phase transition is abolished between 40 and 50 mol % (Fig. 6 F). Both of the broad components of the lanosterol/DPPC system seem to follow a similar pattern, reaching a maximum at the same sterol concentration, but with a slightly lower enthalpy value. For the BC1 curve, the ΔH values slowly increase up to ∼6 mol %, above which they decrease. The ΔH values of the BC2 curve increase rapidly with increasing lanosterol concentration and do so at a slightly faster rate than either the BC1 component or the broad component of the cholesterol/DPPC system. At a lanosterol concentration of 15 mol %, the BC2 component has a higher Tm and ΔT1/2 and a similar ΔH compared to the corresponding values for the cholesterol/DPPC broad component. However, above 15 mol %, the ΔH of the BC2 decreases at a faster rate than for the cholesterol/DPPC broad component. Above a lanosterol concentration of 30 mol %, we could not reliably deconvolve the BC2 contribution from that of the broad components of unknown origin seen at lower temperatures (Fig. 2). In the case of the cholesterol/DPPC system, the enthalpy of the broad component crosses that of the sharp component at ∼10 mol %, whereas in the lanosterol/DPPC system those of BC2 first cross BC1 at ∼6 mol % and then cross that of the sharp component, also at ∼10 mol %.
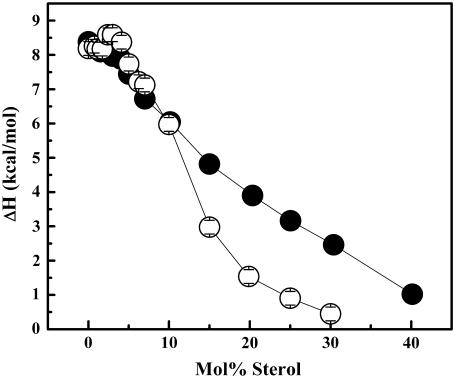
A comparison of all three thermodynamic parameters for both the cholesterol/DPPC and lanosterol/DPPC systems (Fig. 6) shows that the sharp component is reduced more rapidly and thus is eliminated at a lower concentration in the lanosterol/DPPC relative to the cholesterol/DPPC DSC curves, suggesting that lanosterol is better able to perturb the packing of DPPC molecules than is cholesterol. This is supported by the plot of the overall enthalpy for both sterol/DPPC systems shown in Fig. 7.
FIGURE 7.
Overall enthalpy values obtained for cholesterol/DPPC (•) and lanosterol/DPPC (○) mixtures from DSC heating curves. The largest error bar was equal to the symbol diameter.
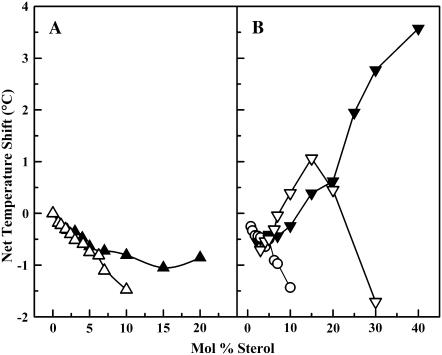
A plot of net temperature shift for both cholesterol- and lanosterol-containing samples is shown in Fig. 8. The temperature shift of the sharp component for the lanosterol/DPPC dispersions is more negative than that of the corresponding cholesterol/DPPC values, as reported by Miao et al. (38). The values for the upper-temperature broad component are similar up to a concentration of 15 mol % sterol, but then differ markedly. For lanosterol, the values decrease above 15 mol %, whereas in the cholesterol samples, they continue to increase. The possible molecular basis for the observed differences in the thermotropic phase behavior of the cholesterol/DPPC and lanosterol/DPPC binary mixtures will be presented in the Discussion.
FIGURE 8.
Net temperature shift of the sharp and broad components of the main chain melting phase transition of cholesterol/DPPC (▴, ▾) and lanosterol/DPPC (▵, ▿, ○) samples obtained from DSC heating thermograms relative to that of pure DPPC. Two broad components exist in the lanosterol samples, and are depicted here as either an open inverted triangle (upper-temperature component) or an open circle (lower-temperature component).
FOURIER TRANSFORM INFRARED SPECTROSCOPIC STUDIES
It is clear from the calorimetric studies presented above that the lanosterol-rich DPPC preparations exhibit a number of broad, lower-temperature endothermic events that were not present in the corresponding cholesterol-rich preparations. Given that the presence of significant amounts of some types of sterols in PC membranes is known to promote the formation of one or more lamellar crystalline phases (11,52), FTIR spectroscopic examinations of the thermotropic phase behavior of representative cholesterol- and lanosterol-containing DPPC preparations were also performed to examine the structural basis of the thermotropic events resolved by DSC.
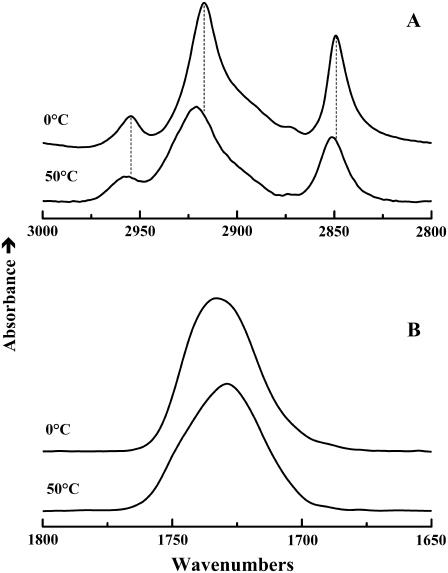
Illustrated in Fig. 9 are FTIR absorption bands illustrating the general features of the temperature-induced changes in the spectra exhibited by the DPPC and the DPPC-sterol mixtures examined. In the 2800–3000 cm−1 region (Fig. 9 A), the dominant features are the conformationally sensitive absorption bands arising predominantly from the symmetric and asymmetric C-H stretching vibrations of the methylene groups on the hydrocarbon chains of DPPC. At temperatures below the onset of the lipid hydrocarbon chain-melting phase transition, these vibrational modes give rise to sharp absorption peaks centered near 2849 and 2916 cm−1, respectively. At the lipid hydrocarbon chain-melting phase transition, there is a discontinuous increase in the width (∼15%) and frequency (∼2–5 cm−1) of these absorption bands, reflecting the increase in the mobility and gauche rotomer content of the lipid hydrocarbon chains, respectively (60–62). In the 1650–1800 cm−1 region (Fig. 9 B), the broad absorption band centered near 1740 cm−1 arises from the C=O stretching vibrations of the ester carbonyl groups at the bilayer polar/apolar interfacial region, and as with most fully hydrated glycerolipid bilayers, this band is resolvable into components centered near 1740 and 1728 cm−1. These latter components arise from populations of “free” and hydrogen-bonded ester carbonyl groups, respectively (60–62, and references cited therein). At the lipid hydrocarbon chain-melting phase transition, there is usually an increase in the relative contribution of the hydrogen-bonded population, reflecting the increased penetration of water into the polar/apolar interfacial region. In these studies, the changes in the FTIR spectra are all correlated with the calorimetrically resolved thermotropic phase changes described above and are typical of the simple Lβ/Lα hydrocarbon chain-melting phase transitions of glycerolipid bilayers. This observation essentially eliminates the possible involvement of DPPC lamellar crystalline phases as contributory factors to the structural changes occurring at the broad, low-temperature thermotropic events exhibited by cholesterol- and lanosterol-rich DPPC mixtures.
FIGURE 9.
Representative temperature-dependent changes in the contours of the FTIR spectra of sterol-containing DPPC bilayers. The data shown were acquired at the temperatures indicated with a cholesterol-containing DPPC mixture (15 mol %) and typify the changes in the band contours observed in the C-H stretching (A) and the C=O stretching (B) regions of the infrared spectrum.
The FTIR data also suggest that lanosterol and cholesterol have qualitatively similar effects on both the hydrocarbon chains and the carbonyl groups of the host DPPC bilayer. At temperatures above the DPPC chain-melting phase transition, the CH2 stretching frequencies of both sterol-DPPC mixtures are reduced relative to DPPC itself, suggesting that both sterols order the hydrocarbon chains of the phospholipid molecules in the liquid-crystalline state, as might be expected from the results of the previous comparative studies summarized in the Introduction. Similarly, at temperatures below the DPPC chain-melting phase transition, the CH2 stretching frequencies of both sterol-DPPC systems are elevated relative to that of DPPC alone, indicating that both sterols disorder the hydrocarbon chains in the gel state. Moreover, the frequencies of both components of the C=O stretching vibrations are lower in the lanosterol- than in the cholesterol-containing DPPC bilayer, suggesting a more hydrated and thus a more loosely packed bilayer. These two observations are compatible with one another and with most previously published studies, which indicate that the lanosterol and cholesterol have broadly similar effects on phospholipid bilayers. However, we generally find that the quantitative aspects of the effect of lanosterol are somewhat less marked than those of the effect of cholesterol.
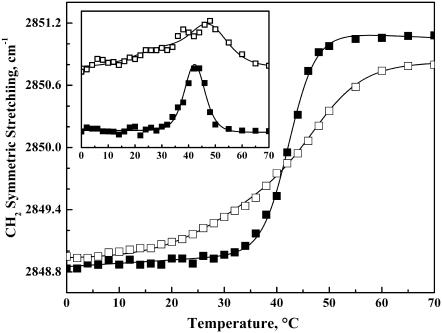
Illustrated in Fig. 10 is a comparison of the temperature-dependent changes in the peak frequencies of the CH2 symmetric stretching bands exhibited by lanosterol- and cholesterol-containing DPPC bilayers at sterol concentrations near 20 and 25 mol %, respectively. These particular sterol levels were studied because they are just above the minimal quantities required to completely abolish the sharp component of the DSC thermograms (Fig. 2). The CH2 symmetric stretching band maxima exhibited by the cholesterol- and lanosterol-containing preparations shift upward as the temperature increases, indicating that hydrocarbon chain-melting processes are involved in the structural changes occurring in these lipid-sterol mixtures. However, the data also highlight a number of significant differences between the two lipid-sterol systems. Specifically, as observed in our DSC measurements, the thermally induced increases in band frequency exhibited by the DPPC-lanosterol mixture occur over a broader temperature range (∼15–55°C) than observed with the DPPC-cholesterol mixture (∼26–52°C). Moreover, the first derivatives of the temperature dependences of the changes in CH2 symmetric stretching band frequency (Fig. 10, inset) suggest that the temperature-dependent changes exhibited by the lanosterol-containing sample consist of two overlapping processes centered near 31°C and 47°C, whereas that exhibited by the DPPC-cholesterol mixture consists of a single process centered near 42°C. These observations thus provide spectroscopic evidence in support of the calorimetrically detected differences in the thermotropic phase behavior exhibited by the cholesterol- and lanosterol-rich DPPC bilayers and, in particular, for the possibility that the lanosterol-rich DPPC bilayers exhibit at least two broad thermotropic processes.
FIGURE 10.
Temperature-dependent changes of the CH2 symmetric stretching band maxima exhibited by DPPC bilayers containing 20 mol % lanosterol (□) and 25 mol % cholesterol (▪). The inset shows the scaled first derivatives of the temperature-dependent plots shown in the main panel.
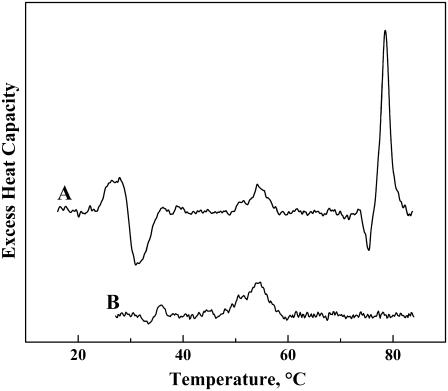
We now return to the issue of the series of sharp, weakly energetic endotherms in the DSC heating thermograms of the lanosterol-rich DPPC samples. In particular, as illustrated in Fig. 2 B, a number of such endotherms are observed in the lanosterol/DPPC containing 30 mol % or more sterol. These endotherms are centered near 22, 27, 43, and 50°C and generally increase in size as the lanosterol content of the sample increases. Moreover, the number and relative sizes of these peaks varies with the thermal history of the sample and, in particular, with the solvent from which the solid is cast, suggesting that lanosterol can crystallize into many polymorphic forms according to the prevailing conditions. However, of particular significance here is the fact that the midpoints of several of these peaks correspond fairly closely to those of endotherms observed upon heating aqueous dispersions of lanosterol alone (Fig. 11). This observation suggests that at least some of these sharp peaks may arise from lanosterol crystallites that may have formed in the lanosterol-rich samples at the lower range of temperatures explored in these experiments. We also note that aqueous dispersions of cholesterol are known to exhibit sharp, weakly energetic endothermic transitions at temperatures near 37 and 77°C, due to transitions between its anhydrous and hydrated crystalline forms. However, similar peaks are absent from all of the cholesterol/DPPC mixtures examined, indicating that cholesterol is fully miscible with DPPC up to concentrations of at least 50 mol %, in marked contrast to lanosterol.
FIGURE 11.
Representative DSC thermograms observed upon heating aqueous dispersions of crystalline lanosterol. The thermograms shown were obtained by heating samples containing 2–3 mg of lanosterol in a Calorimetry Sciences Multi-Cell instrument at scan rates near 56°C/h. The thermograms shown exemplify the type of behavior observed upon initial heating (A) and subsequent heating (B).
DISCUSSION
Although the thermotropic phase behavior of cholesterol/DPPC mixtures has been studied previously by ourselves (11,52) and others (63–65) using various high-sensitivity DSC instruments, we elected to repeat these measurements here on the higher sensitivity calorimeter now available to us to insure that a valid direct comparison between the effects of cholesterol and lanosterol was possible. Indeed, although the measurements of the effects of cholesterol on the main phase transition did not differ qualitatively or quantitatively from our previous studies, this study does establish that the pretransition is actually not completely abolished until the cholesterol content exceeds 10 mol %, whereas previous studies reported that the pretransition could not be detected once cholesterol levels exceed 5–6 mol % (but, see Koynova et al. (66)). We ascribe this difference in findings to the higher sensitivity of the DSC instrument utilized here, as well as to an improved instrumental operating protocol, as described earlier. We note in this regard that a previous x-ray diffraction study of cholesterol/DPPC mixtures also found that the pretransition was abolished at 10 mol % cholesterol, when both the Lβ′ and Pβ′ phases with their tilted hydrocarbon chains, which exist in the absence of cholesterol, are completely replaced by a slightly disordered Lβ-like gel phase with nontilted hydrocarbon chains (67).
The effects of cholesterol and lanosterol on the thermotropic phase behavior of DPPC bilayers differ quantitatively at lower sterol concentrations, and both qualitatively and quantitatively at higher sterol concentrations, despite their rather similar chemical structures. In particular, lanosterol reduces both the enthalpy and the cooperativity of the pretransition of DPPC with increasing sterol concentration more markedly than does cholesterol. As a result, the DPPC pretransition is abolished entirely at a lanosterol concentration of only 6–7 mol % sterol, whereas the pretransition persists until 10 mol % sterol in cholesterol/DPPC mixtures. This finding is not unexpected, since the incorporation of low levels of cholesterol abolishes the hydrocarbon chain tilt required to provide a match between the smaller cross-sectional areas of the hydrocarbon chains relative to the larger cross-sectional area of the DPPC polar headgroup in the gel state, as the cholesterol molecule itself has a very small polar headgroup. Thus, the larger cross-sectional area of the steroid ring system of the lanosterol molecule will make it a more effective spacer of DPPC molecules in the gel-state bilayer, thus relieving the mismatch in the cross-sectional areas of the hydrocarbon chains and DPPC polar headgroups at a lower sterol concentration than does cholesterol. Moreover, the rougher faces of the lanosterol molecule would be expected to produce a greater amount of orientational disorder in the adjacent phospholipid hydrocarbon chains, thus further augmenting the expansion of the DPPC gel-state bilayer and the accommodation of the relatively large polar headgroups of these phospholipid molecules.
Lanosterol also has a similar effect on the sharp component of the DSC endotherm, reducing the temperature, enthalpy, and cooperativity of this component more rapidly than does cholesterol. Thus, the sharp component of the DSC endotherm is abolished at a lanosterol concentration in the DPPC bilayer only ∼15 mol % sterol, whereas the sharp component of the DSC endotherms persists to ∼22–25 mol % in cholesterol/DPPC binary mixtures. Since several of the sharp components of the DSC endotherm probably arise from the fairly cooperative hydrocarbon chain melting of domains highly enriched in DPPC and depleted of sterol (11,52), this result could be explained by a greater perturbation by lanosterol relative to cholesterol of the gel-state organization of these DPPC-enriched domains, by a decrease in the size of these domains, or by a combination of both factors. Again, these effects could be explained by the greater size and rougher surfaces of the lanosterol molecule as described above for the differential effects of lanosterol and cholesterol on the pretransition. In this regard, it is interesting to note that the concentration of lanosterol required to abolish both the pretransition and the sharp component of the main phase transition is ∼⅔ of the concentration of cholesterol required to produce these effects.
The effects of the incorporation of lanosterol and cholesterol on the broad component of the DSC heating runs, which probably arise from the less cooperative chain melting of domains of DPPC enriched in sterol, are also somewhat different, particularly at higher sterol concentrations. In the cholesterol/DPPC mixtures, the temperature of the broad component monotonically increases with increasing sterol concentration, whereas the enthalpy and cooperativity decrease, such that no cooperative phase transition can be detected at 50 mol % sterol. Although a similar qualitative effect is noted with lanosterol at lower sterol concentrations, there appear to be two overlapping broad components in the DSC endotherms between sterol concentrations of 3 and 10 mol %, with one component being shifted upward and the second component being shifted downward with increasing lanosterol levels. Moreover, the single broad component that persists at higher lanosterol concentrations either appears to shift downward in temperature at sterol concentrations above 20–25 mol %, or disappears and is replaced by a different, broad multicomponent endotherm centered at considerably lower temperatures. Although we have not determined the exact structures of the phases formed at moderate to high lanosterol concentrations, we can say from our FTIR spectroscopic results that the low temperature endotherms observed at lanosterol concentrations of 30 mol % and higher are due to a melting of the DPPC hydrocarbon chains. Moreover, DSC scans run at a higher instrumental sensitivity reveal a series of sharp but poorly energetic endotherms superimposed on one or more broad but more strongly energetic endotherms in the temperature range between 10°C and 40°C. Since several of the sharp peaks observed correspond to peaks observed when lanosterol itself is dispersed in water, we suspect that these sharp peaks arise from lanosterol crystallites which have formed in the DPPC bilayer after exposure to low temperature. This observation, together with the fact that a weak, broad chain-melting endotherm can be observed at 50 mol % lanosterol but not at 50 mol % cholesterol, suggests that in contrast to cholesterol, lanosterol is not fully miscible with gel-state DPPC bilayers at sterol concentrations of 30 mol % and higher, and thus that a DPPC chain-melting phase transition can persist at higher sterol concentrations. In this regard we note that Urbina et al. (35) reported NMR evidence for the existence of two separate phases in lanosterol-containing DMPC or DOPC bilayers at sterol concentrations in excess of 30 mol %, whereas only a single phase appeared to exist in the corresponding cholesterol-containing vesicles.
It is of interest to compare our DSC results on binary mixtures of cholesterol and lanosterol with DPPC with previous results of binary mixtures of these two sterols with PPePC, which contains a petroselinoyl rather than a palmitoyl chain at position 2 of the glycerol backbone, the former being an 18C unsaturated fatty acid with a single cis double bond at positions 6–7 of the hydrocarbon chain (38). This comparison is limited to the main phase transition, as PPePC does not exhibit a pretransition in the temperature range examined (5–35°C). Also, although these workers acknowledged that their DSC endotherms at sterol concentrations of 3–12 mol % lanosterol or 3–20 mol % cholesterol were multicomponent, no attempt was made to deconvolve these endotherms into a sharp and broad component, as in this study.
Miao et al. (38) also reported that lanosterol was more effective than cholesterol in reducing the temperature, enthalpy, and cooperativity of the overall main phase transition temperature of PPePC, such that a cooperative chain-melting phase transition was completely abolished at lanosterol and cholesterol concentrations above 12 and 20 mol %, respectively. We see qualitatively similar differences in the comparative effects of lanosterol and cholesterol on the sharp components of our sterol/DPPC mixtures, particularly at sterol concentrations above 5 mol %. However, in our experiments the sharp component of the DSC endotherm persists until lanosterol and cholesterol concentrations of ∼15 and ∼22 mol %, respectively, are reached, a difference which we ascribe to the higher sensitivity of our DSC instrument and our optimized experimental protocol. Moreover, Miao and co-workers were unable to detect the broad, lower enthalpy phase transitions that likely exist in the binary mixtures of both sterols with PPePC at higher sterol concentrations, because their experimental protocol would not have permitted these endotherms to be differentiated from the instrumental baseline, as has unfortunately also been the case in other studies of cholesterol/phospholipid systems (for a discussion of this problem, see McMullen et al. (52)). This being the case, the lanosterol-PPePC phase diagram constructed by Miao et al. (38) is unlikely to be correct. Therefore, the overall results of these two studies can be considered to be qualitatively similar at lower sterol levels but to diverge significantly at higher sterol levels. Since the effect of cholesterol and its analogs on the thermotropic phase behavior of DPPC (52,68) and SOPC (69) bilayers is qualitatively and quantitatively very similar, it is unlikely that the apparent differences in the observed effects of lanosterol and cholesterol on the thermotropic phase behavior of DPPC and PPePC are due simply to the presence of a cis-monounsaturated fatty acid at position 2 of the glycerol backbone in the PC molecule.
Mouritsen and Zuckerman (58) have recently proposed that, in contrast to cholesterol, lanosterol is not able to form a Lo phase in phospholipid bilayer membranes. This proposal seems very unlikely in view of the DSC and FTIR evidence presented here, which indicates that lanosterol and cholesterol produce qualitatively similar changes in the DPPC bilayers, at least at lower sterol concentrations. Moreover, much of the prior literature summarized in the Introduction also does not support this proposal. In fact, lanosterol has been reported to be as effective as cholesterol in increasing the thickness of liquid-crystalline DMPC bilayers (44) and in increasing molecular order and decreasing rates of motion in fluid DOPC bilayers (42). Thus, although lanosterol is generally considered to be somewhat less effective than cholesterol in increasing the order and decreasing the viscosity of phospholipid bilayers, lanosterol nevertheless has a major cholesterol-like effect on these model membranes. Moreover, lanosterol and cholesterol have similar qualitative effects on the permeability and mechanical properties of the host phospholipid bilayer, although the effects of lanosterol are somewhat weaker. In addition, lanosterol is capable of fulfilling the bulk sterol growth requirements of M. capricolum and yeast, providing that very small quantities of cholesterol are also present to meet the more structurally specific sterol metabolic and regulatory requirements of these organisms (32). Therefore, although lanosterol does seem to produce a less ordered Lo state when incorporated into phospholipid bilayer membranes than does cholesterol, there seems little reason to conclude that a Lo state does not form.
It could be argued that the results of studies of the relative abilities of lanosterol and cholesterol to increase the formation of the Lo state in ternary lipid mixtures containing saturated and unsaturated phospholipid and sterols supports the proposal of Mouritsen and Zuckerman (58), since lanosterol seems considerably less effective in this regard than does cholesterol. However, in these studies, a DPPC-rich, DOPC-depleted ordered state forms even in the absence of sterol, so what was being measured was the relative ability of various sterols to facilitate the formation of additional and/or larger ordered domains. Since cholesterol has a higher affinity for saturated as opposed to unsaturated PCs, one might expect that the affinity of lanosterol for saturated phospholipids would be more markedly reduced compared to cholesterol. This is because the additional methyl groups projecting from both the β- and α-faces of the lanosterol molecule would decrease the strength of the van der Waal's interactions between the saturated hydrocarbon chain of the DPPC molecule to a much greater extent than for the unsaturated chains of the DOPC molecule. In addition, the potentially reduced hydrogen bonding ability of lanosterol, due to the partial occlusion of the C3 hydroxyl by the adjacent methyl groups, would also decrease its relative affinity for SpM over PCs, since the sphingosine backbone contains additional sites for hydrogen-bonding interactions with sterols not available with glycerophospholipids. Thus, the markedly decreased ability of lanosterol to facilitate Lo phase formation in lipid-raft-mimetic ternary systems may be due primarily to its relatively much lower affinity for saturated relative to unsaturated phospholipids, and not to its ability to induce the formation of a Lo phase in saturated and unsaturated mixed-chain phospholipids per se.
In summary, our present results and those in the previous literature generally support the Bloch hypothesis that the removal of the three additional methyl groups of the lanosterol molecule does increase its ability to exert its characteristic cholesterol-like effects on the host phospholipid bilayer. However, neither our results nor those of others support the idea that lanosterol does not produce any cholesterol-like effects on phospholipid bilayers generally, nor, in particular, the suggestion that it is incapable of producing a Lo state in such systems. In addition, the results presented here also suggest that a major positive effect of the metabolic conversion of lanosterol to cholesterol and closely related sterols is to increase the miscibility of these compounds with phospholipid bilayer membranes generally, thereby avoiding the possible detrimental effect of sterol lateral phase separation at the high sterol concentrations found in many eukaryotic cell membranes.
Acknowledgments
This work was supported by operating and major equipment grants from the Canadian Institutes of Health Research and by major equipment grants from the Alberta Heritage Foundation for Medical Research.
Abbreviations used: DSC, differential scanning calorimetry; DPPC, dipalmitoylphosphatidylcholine; PC, phosphatidylcholine; DMPC, dimyristoylphosphatidylcholine; DOPC, dioleoylphosphatidylcholine; PPetPC, 1-palmitoyl-2-petroselinoylphosphatidylcholine; SpM, sphingomyelin; FTIR, Fourier transform infrared; NMR, nuclear magnetic resonance; Tp, the pretransition temperature maximum; Tm, the main transition temperature maximum; ΔH, the transition enthalpy; ΔT½, the width of the phase transition at half height, inversely related to the cooperativity of the phase transition; Lβ′ and Lβ, lamellar gel phases with tilted and untilted hydrocarbon chains, respectively; Pβ′, rippled gel phase with tilted hydrocarbon chains; Lα or Ld, lamellar liquid-crystalline or liquid-disordered phase; Lo, lamellar liquid-ordered phase; BC1 and BC2, first and second broad endotherm components of the lanosterol-DPPC DSC thermograms.
References
- 1.Nes, W. R., and M. L. McKean. 1977. Biochemistry of Steroids and Other Isopentenoids. University Park Press, Baltimore, MD.
- 2.Yeagle, P. L. 1988. Cholesterol and the cell membrane. In The Biology of Cholesterol. P. L. Yeagle, editor. CRC Press, Boca Raton, FL. 121–145.
- 3.Liscum, L., and N. J. Munn. 1999. Intracellular cholesterol transport. Biochim. Biophys. Acta. 1438:19–37. [DOI] [PubMed] [Google Scholar]
- 4.Demel, R. A., and B. de Kruijff. 1976. The function of sterols in membranes. Biochim. Biophys. Acta. 457:109–132. [DOI] [PubMed] [Google Scholar]
- 5.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/DPPC mixtures: 2H-nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 6.Finegold, L. (editor). 1993. Cholesterol in Model Membranes. CRC Press, Boca Raton, FL.
- 7.McMullen, T. P. W., and R. N. McElhaney. 1996. Physical studies of cholesterol-phospholipid interactions. Curr. Opin. Colloid Interface Sci. 1:83–90. [Google Scholar]
- 8.Ipsen, J. H., G. Karlstrom, O. G. Mouritsen, H. W. Wennerstrom, and M. Zuckermann. 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 905:162–172. [DOI] [PubMed] [Google Scholar]
- 9.Thewalt, J. L., and M. Bloom. 1992. Phosphatidylcholine:cholesterol phase diagrams. Biophys. J. 63:1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinl, H., T. Brumm, and T. M. Bayerl. 1992. Changes in the physical properties of the liquid-ordered phase with temperature in binary mixtures of DPPC with cholesterol: a 2H-NMR, FTIR, DSC, and neutron scattering study. Biophys. J. 61:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMullen, T. P. W., and R. N. McElhaney. 1995. New aspects of the interactions of cholesterol and dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim. Biophys. Acta. 1234:90–98. [DOI] [PubMed] [Google Scholar]
- 12.Somerharju, P., J. A. Virtanen, and K. H. Cheng. 1999. Lateral organization of membrane lipids. The superlattice view. Biochim. Biophys. Acta. 1440:32–48. [DOI] [PubMed] [Google Scholar]
- 13.McConnell, H. J., and A. Radhakrishman. 2003. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta. 1610:159–173. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. [DOI] [PubMed] [Google Scholar]
- 15.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- 16.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science. 290:1721–1726. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Graton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470–477. [DOI] [PubMed] [Google Scholar]
- 19.Silvius, J. R. 2003. Role of cholesterol in lipid raft formation: lessons from model systems. Biochim. Biophys. Acta. 1610:174–183. [DOI] [PubMed] [Google Scholar]
- 20.Gandhavadi, M., D. Allende, A. Vidal, S. A. Simn, and T. J. McIntosh. 2002. Structure, composition and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 82:1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edidin, M. 2003. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257–283. [DOI] [PubMed] [Google Scholar]
- 22.Munro, S. 2003. Lipid rafts: elusive or illusive? Cell. 115:377–388. [DOI] [PubMed] [Google Scholar]
- 23.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 2004. Cholesterol-phospholipid interactions, the liquid-ordered phase and lipid rafts in model and biological membranes. Curr. Opin. Colloid Interface Sci. 8:459–468. [Google Scholar]
- 24.Dahl, C. E., and J. S. Dahl. 1988. Cholesterol and cell function. In The Biology of Cholesterol. P. L. Yeagle, editor. CRC Press, Boca Raton, FL. 148–153.
- 25.McElhaney, R. N. 1992. Membrane structure. In Mycoplasmas: Molecular Biology and Pathogenesis. J. B. Baseman, L. R. Finch, J. Maniloff, and R. N. McElhaney, editors. American Society for Microbiology, Washington, D.C. 113–155.
- 26.McElhaney, R. N. 1992. Membrane function. In Mycoplasmas: Molecular Biology and Pathogenesis. J. B. Baseman, L. R. Finch, J. Maniloff, and R. N. McElhaney, editors. American Society for Microbiology, Washington, D.C. 259–287.
- 27.Duax, W. L., Z. Wawrzak, J. F. Griffin, and C. Cheer. 1988. Sterol conformation and molecular properties. In Biology of Cholesterol, P. L. Yeagle, editor. C.R.C. Press, Boca Raton, FL. 1–18.
- 28.Bloch, K. 1965. The biological synthesis of cholesterol. Science. 150:19–28. [DOI] [PubMed] [Google Scholar]
- 29.Bloch, K. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14:47–92. [DOI] [PubMed] [Google Scholar]
- 30.Demel, R. A., K. R. Bruckdorfer, and L. L. M. van Deenen. 1972. The effect of sterol structure on the permeability of liposomes to glucose, glycerol and Rb. Biochim. Biophys. Acta. 225:321–330. [DOI] [PubMed] [Google Scholar]
- 31.Yeagle, P. L., R. B. Martin, L. K. Lala, H.-K. Lin, and K. Bloch. 1977. Differential effects of cholesterol and lanosterol on artificial membranes. Proc. Natl. Acad. Sci. USA. 74:4924–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahl, C., J. Dahl, and K. Bloch. 1980. Effect of alkyl-substituted precursors of cholesterol on artificial and natural membranes and on the viability of Mycoplasma capricolum. Biochemistry. 19:1462–1467. [DOI] [PubMed] [Google Scholar]
- 33.Dahl, C. E. 1981. The effect of sterol structure on acyl chain ordering in phosphatidylcholine vesicles: a deuterium nuclear magnetic resonance and electron spin resonance study. Biochemistry. 20:7158–7161. [DOI] [PubMed] [Google Scholar]
- 34.Yeagle, P. L. 1985. Lanosterol and cholesterol have different effects on phospholipid acyl chain ordering. Biochim. Biophys. Acta. 815:33–36. [DOI] [PubMed] [Google Scholar]
- 35.Urbina, J. A., S. Pekerar, H. B. Lee, J. Patterson, B. Montez, and E. Oldfield. 1995. Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: a comparative study using 2H-, 13C- and 31P-NMR spectroscopy. Biochim. Biophys. Acta. 1238:163–176. [DOI] [PubMed] [Google Scholar]
- 36.Endress, E., S. Bayerl, K. Prechtel, C. Maier, R. Merkel, and T. M. Bayerl. 2002. The effect of cholesterol, lanosterol and ergosterol on lecithin bilayer mechanical properties: a solid-state NMR and micropipet study. Langmuir. 18:3293–3299. [Google Scholar]
- 37.Endress, E., H. Heller, H. Casalta, M. F. Brown, and T. M. Bayerl. 2002. Anisotropic motion and molecular dynamics of cholesterol, lanosterol and ergosterol in lecithin bilayers studied by quasi-elastic neutron scattering. Biochemistry. 41:13078–13086. [DOI] [PubMed] [Google Scholar]
- 38.Miao, L., M. Nielsen, J. Thewalt, J. H. Ipsen, M. Bloom, M. J. Zuckermann, and O. G. Mouritsen. 2002. From lanosterol to cholesterol: structural evolution and differential effects on lipid bilayers. Biophys. J. 82:1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez, G. V., E. M. Dykstra, S. Lope-Predrafita, and M. F. Brown. 2004. Lanosterol and cholesterol-induced variations in bilayer elasticity probed by 2H-NMR relaxation. Langmuir. 20:1043–1046. [DOI] [PubMed] [Google Scholar]
- 40.Huster, D., A. Scheidt, K. Arnold, A. Herrmann, and P. Muller. 2005. Desmosterol may replace cholesterol in lipid membranes. Biophys. J. 88:1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheidt, H. A., D. Huster, and K. Gawrisch. 2005. Diffusion of cholesterol and its precursors in lipid membranes studies by 1H pulsed field gradient magic angle spinning NMR. Biophys. J. 89:2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korstanje, L. J., G. van Ginkel, and Y. K. Levine. 1990. Effects of steroid molecules on the dynamical structure of dioleoylphosphatidylcholine and digalactosyldiacyglycerol bilayers. Biochim. Biophys. Acta. 1022:155–162. [DOI] [PubMed] [Google Scholar]
- 43.Huang, T. H., A. J. DeSiero, and Q. X. Yang. 1991. Effect of cholesterol and lanosterol on the structure and dynamics of the cell membrane of Mycoplasma capricolumn. Deuterium nuclear magnetic resonance study. Biophys. J. 59:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pencer, J., M.-P. Nieh, T. Harroun, S. Krueger, C. Adams, and J. Katsaras. 2005. Bilayer thickness and the thermal response of dimyristoylphosphatidylcholine unilamellar vesicles containing cholesterol, ergosterol and lanosterol: a small angle neutron scattering study. Biochim. Biophys. Acta. 1720:84–91. [DOI] [PubMed] [Google Scholar]
- 45.Henrikson, J., A. C. Rowat, and J. H. Ipsen. 2004. Vesicle fluctuation analysis of the effects of sterols on membrane bending rigidity. Eur. Biophys. J. 33:732–741. [DOI] [PubMed] [Google Scholar]
- 46.Petrache, H. I., D. Harries, and V. A. Parsegian. 2005. Alteration of lipid membrane rigidity by cholesterol and its metabolic precursors. Macromol. Symp. 219:39–50. [Google Scholar]
- 47.Hildenbrand, M. F., and T. H. Bayerl. 2005. Differences in the modulation of collective membrane motions by ergosterol, lanosterol and cholesterol: a dynamic light scattering study. Biophys. J. 88:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smondyrev, A. M., and M. L. Berkowitz. 2001. Molecular dynamics simulation of the structure of dimyristoylphosphatidylcholine bilayers with cholesterol, ergosterol, and lanosterol. Biophys. J. 80:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, X., and E. London. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 39:843–849. [DOI] [PubMed] [Google Scholar]
- 50.Beattie, M. E., S. L. Veatch, B. J. Stottrup, and S. L. Keller. 2005. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 89:1760–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McElhaney, R. N. 1982. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem. Phys. Lipids. 30:229–259. [DOI] [PubMed] [Google Scholar]
- 52.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 1993. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 32:516–522. [DOI] [PubMed] [Google Scholar]
- 53.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 1994. Comparative differential scanning calorimetric and FTIR and 31P-NMR spectroscopic studies of the effects of cholesterol and androstenol on the thermotropic phase behavior and organization of phosphatidylcholine bilayers. Biophys. J. 66:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 1999. Calorimetric and spectroscopic studies of the effects of cholesterol on the thermotropic phase behavior and organization of a homologous series of linear saturated phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1416:119–134. [DOI] [PubMed] [Google Scholar]
- 55.McMullen, T. P. W., R. N. A. H. Lewis, and R. N. McElhaney. 2000. Differential scanning calorimetric and Fourier transform infrared spectroscopic studies of the effects of cholesterol on the thermotropic phase behavior and organization of a homologous series of linear saturated phosphatidylserine bilayer membranes. Biophys. J. 79:2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohvo-Rekila, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. [DOI] [PubMed] [Google Scholar]
- 57.Mannock, D. A., T. J. McIntosh, X. Jang, D. F. Covey, and R. N. McElhaney. 2003. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayers. Biophys. J. 84:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mouritsen, O. G., and M. J. Zuckerman. 2004. What's so special about cholesterol? Lipids. 39:1101–1113. [DOI] [PubMed] [Google Scholar]
- 59.Lewis, R. N. A. H., I. Winter, M. Kriechbaum, K. Lohner, and R. N. McElhaney. 2001. Studies of the structure and organization of cationic lipid membranes: calorimetric, spectroscopic and x-ray diffraction studies of linear saturated P-O-ethyl phosphatidylcholines. Biophys. J. 80:1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis, R. N. A. H., and R. N. McElhaney. 1996. FTIR spectroscopy in the study of hydrated lipids and lipid bilayer membranes. In Infrared Spectroscopy of Biomolecules H. H. Mantsch and D. Chapman, editor. John Wiley and Sons, New York. 159–202.
- 61.Lewis, R. N. A. H., and R. N. McElhaney. 1998. The structure and organization of phospholipid bilayers as revealed by infrared spectroscopy. Chem. Phys. Lipids. 96:9–21. [Google Scholar]
- 62.Lewis, R. N. A. H., and R. N. McElhaney. 2002. Vibrational spectroscopy of lipids. In Handbook of Vibrational Spectroscopy, Vol. 5. J. M. Chalmers and P.R. Griffiths, editors. John Wiley and Sons, Chichester, United Kingdom. 3447–3464.
- 63.Estep, T. N., D. B. Mountcastle, R. L. Biltonen, and T. E. Thompson. 1978. Studies of the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 17:1984–1989. [DOI] [PubMed] [Google Scholar]
- 64.Mabrey, S., P. L. Mateo, and J. M. Sturtevant. 1978. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 17:2464–2468. [DOI] [PubMed] [Google Scholar]
- 65.Genz, A., J. F. Holzwarth, and T. Y. Tsong. 1986. The influence of cholesterol on the main phase transition of unilamellar dipalmitoylphosphatidylcholine vesicles. A differential scanning calorimetry and iodine laser T-jump study. Biophys. J. 50:1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koynova, R. D., A. I. Boyanov, and B. G. Tenchov. 1985. On the phase diagram of an L-dipalmitoylphosphatidylcholine-cholesterol mixture. FEBS Lett. 187:65–68. [Google Scholar]
- 67.McIntosh, T. J. 1978. Effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 513:43–58. [DOI] [PubMed] [Google Scholar]
- 68.McMullen, T. P. W., C. Vilcheze, R. N. McElhaney, and R. Bittman. 1995. Differential scanning calorimetric study of the effect of sterol side chain length and structure on dipalmitoylphosphatidylcholine thermotropic phase behavior. Biophys. J. 69:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vilcheze, C., T. P. W. McMullen, R. N. McElhaney, and R. Bittman. 1996. The effect of side chain analogues of cholesterol on the thermotropic phase behavior of 1-stearoyl-2-oleoyl-phosphatidylcholine bilayers: a differential scanning calorimetric study. Biochim. Biophys. Acta. 1279:235–242. [DOI] [PubMed] [Google Scholar]