Abstract
A PCR-based approach was developed to recover nitric oxide (NO) reductase (norB) genes as a functional marker gene for denitrifying bacteria. norB database sequences grouped in two very distinct branches. One encodes the quinol-oxidizing single-subunit class (qNorB), while the other class is a cytochrome bc-type complex (cNorB). The latter oxidizes cytochrome c, and the gene is localized adjacent to norC. While both norB types occur in denitrifying strains, the qnorB type was also found in a variety of nondenitrifying strains, suggesting a function in detoxifying NO. Branch-specific degenerate primer sets detected the two norB types in our denitrifier cultures. Specificity was confirmed by sequence analysis of the norB amplicons and failure to amplify norB from nondenitrifying strains. These primer sets also specifically amplified norB from freshwater and marine sediments. Pairwise comparison of amplified norB sequences indicated minimum levels of amino acid identity of 43.9% for qnorB and 38% for cnorB. Phylogenetic analysis confirmed the existence of two classes of norB genes, which clustered according to the respective primer set. Within the qnorB cluster, the majority of genes from isolates and a few environmental clones formed a separate subcluster. Most environmental qnorB clones originating from both habitats clustered into two distinct subclusters of novel sequences from presumably as yet uncultivated organisms. cnorB clones were located on separate branches within subclusters of genes from known organisms, suggesting an origin from similar organisms.
Nitric oxide (NO) is produced by prokaryotes as an intermediate during respiratory denitrification, when oxidized nitrogen compounds are used as alternative electron acceptors under oxygen-limited conditions (50). The ability to denitrify is widespread among a variety of phylogenetically unrelated organisms and was presumably acquired through horizontal gene transfer. Thus, molecular studies based on functional marker genes coding for key enzymes of the denitrification process have the potential to reflect structures of denitrifying communities in environmental samples. Gene-specific primer sets were developed previously to detect narG (16), narH (26), nirK and nirS (3, 17), and nosZ (34), but a gene-specific primer set to target nitric oxide reductase genes was lacking. Nitric oxide reductase is encoded by the norB gene and catalyzes the reduction of NO to N2O, which represents an unusual reaction in biology, the formation of an N-N bond. The existence of a separate enzyme, apart from nitrite reductase, that catalyzes NO reduction was demonstrated by mutational disruption of nitrite reductase genes (nirK and nirS) and thereby resolved the complete denitrification pathway in vivo (46, 53).
Genes coding for both the small and large subunits of nitric oxide reductase, norC and norB, respectively, were retrieved from denitrifying bacteria, including Paracoccus halodenitrificans (30), Pseudomonas spp. (1, 52), Alcaligenes faecalis (20), and Rhodobacter sphaeroides (2). The mature protein accepts electrons from cytochrome c and therefore was designated cNor. For the denitrifier Ralstonia eutropha harboring a different but homologous class of nitric oxide reductases, however, the adjacent norC-encoded subunit was lacking, and the predicted protein showed instead an N-terminal extension with homology to norC (9). This single-component type of norB gene was designated qNor because electrons are transferred from quinol. Both gene types are localized on the genome. Interestingly, Ralstonia eutropha H16 has a second very similar copy of this gene on a megaplasmid (10). Genes of the qnorB type were also discovered during genome annotation in a variety of nondenitrifying, mostly pathogenic organisms such as Neisseria meningitidis and Mycobacterium avium. Complementation studies of an NO reductase-deficient mutant of Ralstonia eutropha with the norB gene of a Synechocystis sp. also demonstrated the presence of a potentially functional norB gene within the nondenitrifying cyanobacterium (5). Detoxification of NO produced by the accompanying microflora or by macrophages during the host defense was proposed as the function of nitric oxide reductases in nondenitrifying organisms (29).
In the present study, we report on the development of distinct norB-specific primer sets to detect the two types of nitric oxide reductase genes and explore the genetic diversity of these gene types in pure cultures and environmental samples.
MATERIALS AND METHODS
Bacterial strains.
To evaluate the specificity of the newly designed norB primers, denitrifying strains and nondenitrifying controls (see Table 2) were grown aerobically at 27°C in nutrient broth (Merck, Darmstadt, Germany) with the following exceptions: the Rhizobium sp. was grown on yeast extract medium (YEM [43]), Hyphomicrobium zavarzinii IFAM ZV-622T was grown on 337-B1 medium (14) with 0.5% methanol, Roseobacter denitrificans was grown on oligotrophic medium (PYGV [38]) supplemented with 25‰ artificial seawater (21), and Blastobacter denitrificans was grown on peptone yeast extract glucose medium (PYGV without vitamins). Nondenitrifying strains were grown on Luria broth (31).
TABLE 2.
Amplification of norB genes from denitrifying strains and sediment samples with selected primer sets qnorB and cnorB
| Strain | Source or referencea | Denitrifi- cationb | PCR products obtained with primer setc:
|
Database sequence | Amplification with primer set: | ||||
|---|---|---|---|---|---|---|---|---|---|
| qnorB 2F-5R (262 bp) | qnorB 2F-7R (637 bp) | cnorB 1F-6R (578 bp) | cnorB 2F-6R (389 bp) | cnorB 2F-7R (454 bp) | |||||
| Alcaligenes sp. | DSM 30128 | + | + | + | − | − | − | − | qnorB |
| Alcaligenes faecalis | DSM 30030 | + | + | + | − | − | − | − | qnorB |
| Alcaligenes xylosoxidans | NCIMB 11015 | + | + | + | − | − | − | − | qnorB |
| Ralstonia eutropha H16 | DSM 428 | + | + | + | − | − | − | + | qnorB |
| Synechocystis sp. strain PCC6803 | R. Cramm, Humboldt Universität, Berlin, Germany | − | + | + | − | − | − | + | qnorB |
| Alcaligenes faecalis A15 | H. Bothe, Universität, Köln, Cologne, Germany | + | − | − | − | + | + | − | cnorB |
| Azoarcus tolulyticus Td-3 | Fries et al. (13) | + | − | − | ++ | + | + | − | cnorB |
| Azoarcus tolulyticus Tol-4 | Zhou et al. (48) | + | − | − | ++ | + | + | − | cnorB |
| Azoarcus tolulyticus Td-17 | Fries et al. (13) | + | − | − | ++ | + | + | − | cnorB |
| Azospirillum brasilense Sp7 | DSM 1690 | + | − | − | ++ | + | + | − | cnorB |
| Blastobacter denitrificans | DSM 1113 | + | − | − | ++ | (+)+ | + | − | cnorB |
| “Corynebacterium nephridii” | ATCC 11425 | + | − | − | + | + | + | − | cnorB |
| Denitrifier | IFAM 3698 | + | − | − | ++ | + | + | − | cnorB |
| Hyphomicrobium zavarzinii | ATCC 27496 | + | − | − | + | + | + | − | cnorB |
| Ochrobactrum anthropi GSF M26 | M. Schloter, GSF, Neuher- berg, Germany | + | − | − | + | + | + | − | cnorB |
| Paracoccus denitrificans Pd1222 | ATCC 19367 | + | − | − | (+) | ++ | + | + | cnorB |
| Paracoccus halodenitrificans | IFO 14912 | + | − | − | ++ | + | + | − | cnorB |
| Pseudomonas aeruginosa | NCTC 6750 | + | − | − | − | + | + | − | cnorB |
| Pseudomonas fluorescens AK | Michotey et al. (24) | + | − | − | + | + | + | − | cnorB |
| Pseudomonas sp. strain G-179 | Ye et al. (47) | + | − | − | + | + | + | + | cnorB |
| Pseudomonas stutzeri | ATCC 14405 | + | − | − | ++ | + | + | − | cnorB |
| Pseudomonas stutzeri JM300 | Coyne et al. (8) | + | − | − | ++ | + | + | − | cnorB |
| Rhodobacter sphaeroides | Satoh et al. (33) | + | − | − | − | (+)+ | + | + | cnorB |
| Roseobacter denitrificans | ATCC 33942 | + | − | − | ++ | + | + | + | cnorB |
| Thauera aromatica K172 | DSM 6984 | + | − | − | + | ++ | ++ | − | cnorB |
| Pantoea aerogenes | DSM 3493 | − | − | − | − | − | − | − | qnorB/cnorB |
| Enterobacter cloacae | NCIMB 11463 | − | − | − | − | − | − | − | qnorB/cnorB |
| Escherichia coli | DSM 498 | − | − | − | − | − | − | − | qnorB/cnorB |
| Freshwater sediment | Red Cedar River, Mich. | ND | + | + | − | + | + | − | qnorB/cnorB |
| Marine sediment | Washington margin, Wash. | ND | + | + | − | + | ++ | − | qnorB/cnorB |
ATCC, American Type Culture Collection; DSM, Deutsche Sammlung von Mikroorganismen; GSF, National Research Center for Environment and Health; IFO, Institute for Fermentation, Osaka, Japan; NCIMB, National Collection of Industrial and Marine Bacteria; NCTC, National Collection of Type Cultures.
Data from the literature.
+, PCR product of expected size; −, no amplification; ++, PCR product of expected size and extra band of unexpected size; (+), weak PCR product; (+)+, weak PCR product of expected size and extra band of unexpected size. The expected size of the PCR product is shown in parentheses for each primer set. ND, not determined.
Primer design.
norB nucleotide sequences available from the EMBL sequence database and from annotated genes of genome sequencing projects were translated to amino acids, and deduced amino acids were aligned automatically with the ClustalW function (http://www.ebi.ac.uk/clustalw/). Additionally, amino acid alignments were edited manually, and nucleotide sequences were aligned accordingly. Degenerate primers were designed to target conserved regions of the two groups of norB genes separately and were designated qnorB and cnorB.
DNA extractions and PCR conditions.
Genomic DNA from pure cultures was extracted as described by Gliesche et al. (15). DNA extracts from Azoarcus sp. strains and from Thauera aromatica K172 were kindly provided by Sabine A. Rech (San José State University, San José, Calif.) and John K. Davis (Michigan State University, East Lansing Mich.), respectively. Total DNA from a freshwater sediment sample from the Red Cedar River (East Lansing, Mich.) was extracted according to the method of Purdy et al. (28) immediately after sampling. A marine sediment sample from the Washington margin of the Pacific Ocean (water depth, 1,936 m; sediment core section from a depth of 0.5 to 1.0 cm) was kept frozen at −20°C until total DNA was extracted with the freeze-thaw procedure of van Elsas and Smalla (42). In addition, a proteinase K treatment (50 μl of a 20-mg/ml solution) was performed after incubation with sodium dodecyl sulfate. DNA was quantified and analyzed spectrophotometrically by taking measurements at 230, 260, and 280 nm. The purity of the DNA was high, as judged by the ratios of absorption at 260 nm and 280 nm of 1.71 to 1.84 for the Red Cedar River samples (n = 5) and 1.68 and 1.82 for the Washington margin samples (n = 2).
Amplification of norB fragments from 50 ng of pure culture DNA extract or 100 ng of environmental DNA extract was performed in 50-μl reactions containing 50 pmol of each primer, 200 μM each deoxynucleoside triphosphate (Gibco-BRL, Gaithersburg, Md.), 400 ng of bovine serum albumin (Roche Molecular Biochemicals, Indianapolis, Ind.) μl−1, 1.5 mM MgCl2, and 1 U of Taq polymerase (Sigma Chemical Co., St. Louis, Mo.) in 1× reaction buffer, provided with the enzyme. After 5 min of denaturation, 40 PCR cycles were done, including 10 initial cycles of 30 s of denaturation at 95°C, 40 s of primer annealing with a touch down from 57 to 52.5°C, primer extension of 1 min at 72°C, and an additional 30 cycles with a constant annealing temperature of 55°C. PCR products (10 μl) were analyzed on 2% (wt/vol) agarose gels (Gibco-BRL) and visualized by UV excitation after staining with ethidium bromide (0.5 mg liter−1).
Cloning and screening of environmental clones.
PCR products of the expected size from environmental DNA extracts obtained with primer sets qnorB2F-7R and cnorB2F-6R were eluted from agarose gels with the QIAquick gel extraction kit (Qiagen, Chatsworth, Calif.). Eluted PCR products were ligated into the pCR2.1 vector, and subsequently cells provided with the TA cloning kit (Invitrogen, San Diego, Calif.) were transformed according to the manufacturer's instructions. Inserts of the proper size were detected by PCR performed with a small amount of cells from 100 randomly chosen white colonies and the appropriate norB primer set under the conditions described previously. Clones (30 to 35) containing an insert of the proper size were screened by restriction fragment length polymorphism (RFLP). PCR products (5 μl) were hydrolyzed in two separate reactions with 3 U each of restriction endonucleases MspI and RsaI for the qnorB primer set and HhaI and MspI for the cnorB primer set. Restriction fragments were separated on 3.5% Metaphor agarose gels as described previously (49). Subsequently, RFLPs were compared with the GelCompar software (Applied Maths, Kortrijk, Belgium) by applying the unweighted pair group method with arithmetic averages and the Jaccard algorithm. The resulting clusters were additionally compared by eye.
Sequencing.
norB PCR products from pure cultures and inserts from clones amplified with vector-specific primers were purified with the QIAquick PCR purification kit (Qiagen, Chatsworth, Calif.). Both strands were sequenced directly from 70 ng of PCR product with primers used for PCR amplification for pure cultures and vector-specific primers (M13 reverse and T7 Promoter) for clones and the ABI Big Dye terminator kit version 2.0 (Applied Biosystems, Weiterstadt, Germany) according to the manufacturer's instructions. Subsequently, excess primers and dye terminators were removed with Autoseq G-50 columns (Amersham-Pharmacia Biotech, Freiburg, Germany), and cycle sequencing reactions were analyzed with an ABI 377 DNA sequencer (Applied Biosystems).
Phylogenetic analysis.
Forward and reverse strand norB sequences were assembled with the Seqman II software (DNAstar Inc. Madison, Wis.) and aligned to sequences from the EMBL database with the ARB Fast aligner feature (http://www.arb-home.de). For phylogenetic analysis, a filter was applied, including 100 deduced amino acid positions with a minimum identity of 0% and a maximum identity of 100% but omitting insertions or deletions (indels) due to ambiguous positional homology. Phylogenetic analyses were performed with ARB and the Phylip software package version 3.6a2.1 (12). Trees were constructed with the distance matrix-based methods, neighbor joining (ARB and PHYLIP), and FITCH (PHYLIP), parsimony PROTPARS (PHYLIP), and maximum likelihood MOLPHY (Institute Pasteur, Paris, France; http://bioweb.pasteur.fr/seqanal/interfaces/prot_nucml.html). Statistical evaluation of tree topologies was performed by bootstrap analysis (PHYLIP) with 1,000 resamplings for neighbor joining (Jones-Taylor-Thornton amino acid replacement model) and parsimony. After comparison of trees generated with different methods, a consensus tree was constructed by introducing multifurcations where the topology was not resolved.
Nucleotide sequence accession numbers.
norB gene sequences from pure cultures and sediment samples have been deposited in the EMBL nucleotide sequence database under accession numbers AJ507329 through AJ507380.
RESULTS
Primer design.
Alignment and phylogenetic analysis of norB sequences that were available from the databases revealed two distantly related clusters of norB sequences. The first cluster comprised five norB genes of the denitrifier Ralstonia eutropha and nondenitrifying strains of Synechocystis sp. and Neisseria meningitis spp. which exhibited an N-terminal extension and for which quinol served as an electron donor for the protein. The norB genes of the denitrifiers Pseudomonas aeruginosa, Pseudomonas stutzeri, Paracoccus denitrificans, Halomonas halodenitrificans, Rhodobacter sphaeroides, Bradyrhizobium japonicum, Pseudomonas sp., and Alcaligenes faecalis S-6 grouped in a second cluster of norB genes with an adjacent norC gene and with cytochrome c as an electron donor. The sequence identities of all pairwise compared norB sequences (100 amino acids) that were available from the database ranged from 22.1 to 100%. Within clusters, sequence identity was higher, from 41.3 to 98.3% for genes with the N-terminal extension and from 53.0 to 100% for genes with an adjacent norC gene. Therefore, primers were designed for each cluster separately and designated qnorB and cnorB (Table 1). Degeneracies were introduced representing all wobble positions observed among the aligned sequences. Comparison of the chosen primer sequences to all sequences stored in the sequence databases with the FastA program (http://www.ebi.ac.uk/fasta33/) indicated significant sequence similarity only to norB genes.
TABLE 1.
Selected primers used for amplification of norB genes
| Primera | Positionb (nt) | Primer sequencec (5′-3′) |
|---|---|---|
| qnorB2F | 1204-1220 | GGN CAY CAR GGN TAY GA |
| qnorB5R | 1466-1444 | ACC CAN AGR TGN ACN ACC CAC CA |
| qnorB7R | 1841-1822 | GGN GGR TTD ATC ADG AAN CC |
| cnorB1F | 364-380 | GAR TTY CTN GAR CAR CC |
| cnorB2F | 553-571 | GAC AAG NNN TAC TGG TGG T |
| cnorB6R | 942-925 | GAA NCC CCA NAC NCC NGC |
| cnorB7R | 1007-991 | TGN CCR TGN GCN GCN GT |
Primers are named by qnorB-targeting genes for quinol-oxidizing nitric oxide reductase and by cnorB-targeting genes for cytochrome c-oxidizing nitric oxide reductase; forward and reverse primers are indicated by F and R as the last letter, respectively.
Positions correspond to the qnorB gene of Ralstonia eutropha H16 (AF002661) and the cnorB gene of Paracoccus denitrificans Pd1222 (U28078). nt, nucleotide.
N = A, C, G, or T; Y = C or T; R = A or G; D = G, A, or T.
Amplification of norB genes from pure cultures and environmental samples.
Primers were evaluated with all possible combinations, i.e., eight combinations for qnorB and 17 combinations for cnorB in PCR amplification of norB from a variety of pure culture DNA extracts from denitrifying and nondenitrifying strains (Table 2). Generally, for a given denitrifying strain, amplification was accomplished with primer sets specific for either the qnorB or the cnorB cluster. Two combinations, qnorB2F-5R and qnorB2F-7R, yielded the expected amplification products (262 and 637 bp) for qnorB from the nondenitrifying Synechocystis sp. strain PCC6803 and denitrifying Ralstonia eutropha H16 and additionally detected the qnorB type from three denitrifying Alcaligenes strains.
All other denitrifying strains representing different genera within the Proteobacteria were targeted by primer combinations cnorB2F-6R (389 bp) and cnorB2F-7R (454 bp) specific for cnorB. Amplification with primer combination cnorB1F-6R (578 bp) was also successful for the majority of these denitrifying strains except for Alcaligenes faecalis A15, Pseudomonas aeruginosa, and Rhodobacter sphaeroides. Other primer sets targeting qnorB and cnorB yielded specific PCR products only from a subset of the strains tested (data not shown). The gene specificity of the primers was confirmed by amplification of a PCR product of the expected size and by the failure of these primers to amplify any DNA fragment from nondenitrifying bacteria. Whenever a PCR product of unexpected size occurred, the PCR conditions were adjusted to higher stringency by applying higher annealing temperatures until amplification yielded a single band of the expected size.
When these primer sets were applied to amplify norB gene fragments from total DNA extracts from sediments of the Red Cedar River and the Washington margin, both primer combinations for qnorB yielded a distinct band of the expected size. For cnorB, however, PCR products of the expected size were obtained from primer set cnorB2F-6R, but primer combination cnorB2F-7R yielded additional bands of unexpected sizes and the combination cnorB1F-6R failed to amplify norB genes from these environmental samples.
RFLP analysis.
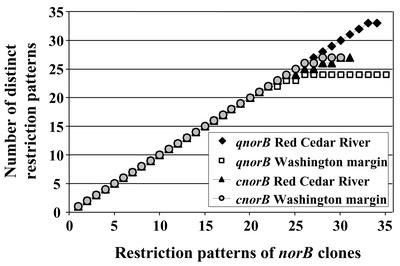
Cloned norB PCR products (130 clones) from sediment samples generated with primer combinations qnorB2F-7R and cnorB2F-6R were screened by restriction fragment polymorphism (RFLP) analysis (Fig. 1). For both norB types, a large number of restriction patterns of the clones screened for each clone library (34 and 35 qnorB clones and 31 and 30 cnorB clones from Red Cedar River and the Washington margin, respectively) were unique for each sediment sample, meaning that 63 to 94% of the clones were nonredundant. One very abundant group of qnorB clones from the Washington margin represented 28% of all clones, whereas qnorB clones from the Red Cedar River sediment showed the highest level of diversity, with only a single redundant clone. One qnorB clone was redundant between both environmental samples. Communities from both habitats based on cnorB genes had a similar intermediate level of diversity.
FIG. 1.
Diversity of nitric oxide-reducing bacteria in environmental samples as evaluated by RFLP analysis of cloned nitric oxide (norB) genes from marine and freshwater sediments. qnorB clones were hydrolyzed with restriction enzymes MspI and RsaI, and cnorB clones were hydrolyzed with HhaI and MspI.
Sequence analysis.
Analyses of primary sequences and deduced amino acids confirmed that PCR products of the expected sizes from denitrifying strains were indeed norB fragments, based on sequence similarity and the presence of heme and iron binding sites. Furthermore, all PCR products amplified from total environmental DNA extracts with the qnorB primer set were specific norB genes. All cnorB clones from the Red Cedar River sediments were identified as norB gene fragments, but two clones from the Washington margin sediment were not identified as norB sequences. One of these clones contained a PCR product which was slightly larger than expected.
Pairwise comparison of qnorB sequences showed that genes from four denitrifying strains (Ralstonia eutropha and three Alcaligenes strains) were significantly more similar (t test, P < 0.05) to each other, with an average identity of 81.6% (70.6 to 97.4%) than to nondenitrifying strains (x̄ = 53.9%; range, 47.9 to 62.0%) or to environmental clones (x̄ = 56.7%; range, 47.9 to 87.6%). The average pairwise similarity of cloned qnorB genes was 62.1% (47.6 to 92.8%), and clones from the two sampling sites were not significantly different (t test, P < 0.05) from each other, with an average pairwise identity of 63.6% and 60.8% for clones from the Red Cedar River and the Washington margin, respectively.
cnorB genes, based on a broader variety of denitrifying strains, were less identical by pairwise comparison, with an average identity of 73.9%, ranging from 26.6% identity (Pyrobaculum aerophilum compared to Achromobacter cycloclastes, Pseudomonas sp. strain G-179, and Sinorhizobium meliloti) to 100% (Ochrobactrum anthropi compared to “Corynebacterium nephridii”). Genes from pure cultures were significantly different from clones, with an average pairwise similarity of 65.8% (t test, P < 0.05), but clones from both habitats showed similar levels of identity, 62.3% and 62.8% for Red Cedar River and the Washington margin, respectively, and thus they were not statistically different.
Phylogenetic analysis.
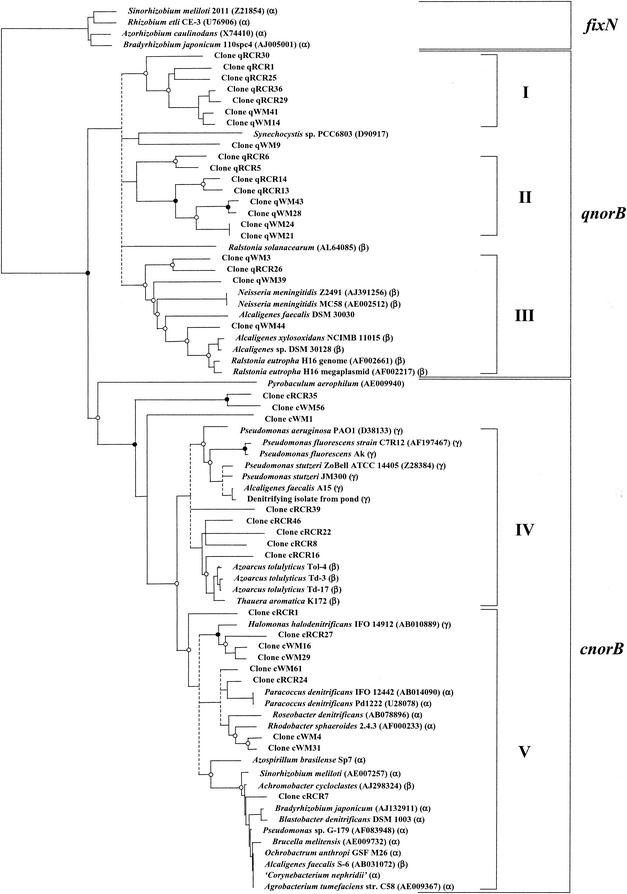
Phylogenetic analyses consistently separated the norB genes into two major clusters, qnorB and cnorB genes (Fig. 2). The qnorB cluster consisted of sequences from denitrifying strains of the genus Alcaligenes and Ralstonia eutropha and several nondenitrifying strains. All denitrifying strains were found within one subcluster (subcluster III), but genes from two nondenitrifying strains of Neisseria meningitidis were closely related, belonging to the same subcluster. Genes from described species within this cluster originated from members of the β-proteobacteria. The majority of genes from environmental samples fell into subcluster I and subcluster II, which consisted of environmental clones exclusively. Clones from the Red Cedar River and the Washington margin sediments were found in both subclusters, thus not showing any habitat-specific clustering. A minority of clones belonged to subcluster III, which was dominated by the described species.
FIG.2.
Phylogenetic analysis of norB genes. Neighbor joining tree (Jones-Taylor-Thornton model of amino acid exchange) based on partial norB amplicons (100 amino acids; accession numbers in parentheses). A consensus tree was constructed from distance methods (neighbor joining and FITCH), parsimony, and maximum likelihood by introducing multifurcations (dashed lines) where tree topology was not consistently resolved. Bootstrap values were generated from 1,000 replicates of neighbor joining and parsimony analysis. •, bootstrap values >90%; ○, bootstrap values 50 to 90%; bootstrap values of <50% are omitted. Clones obtained from the Washington margin and Red Cedar River are designated WM and RCR, respectively, plus q and c for qnorB and cnorB, respectively. The phylogenetic positions of isolates are indicated by α, β, and γ for the subgroups of the Proteobacteria. Roman numbers indicate clusters of norB genes.
For cnorB, genes were grouped into two major subclusters (IV and V), with four distantly related sequences from environmental clones cRCR5, cRCR8, and cWM1 and Pyrobaculum aerophilum branching deeply outside these subclusters. Cluster IV consisted of cnorB sequences from the genera Pseudomonas, Azoarcus, and Thauera, and cluster V consisted of genes from a variety of denitrifying species belonging to the α-proteobacteria except for Halomonas halodenitrificans (γ-proteobacterium) and Achromobacter cycloclastes and Alcaligenes faecalis S-6 (β-proteobacteria), respectively. Environmental clones from both habitats clustered on separate branches within the radiation of norB sequences from denitrifying isolates belonging to the α-, β-, and γ-proteobacteria.
DISCUSSION
NO production occurs during respiratory denitrification but also during nitrate respiration of fungi (36, 37). However, nitric oxide reductase (cytochrome P450nor) from denitrifying fungi differs structurally and functionally from prokaryotic nitric oxide reductases (25) and is restricted to fungi (39, 40). Therefore, primer sets developed to detect each of the two classes of norB genes (qnorB and cnorB) in our PCR assays are specific for prokaryotic NO reductase genes. Furthermore, the primer sets were designed to exclude amplification of FixN, subunit I of cytochrome oxidases, which was proposed to be a NorB homologue (32, 41).
With these primers, a norB gene of either type was found within all of the denitrifiers tested. Genes of the Ralstonia type were detected in other denitrifying strains (Alcaligenes faecalis DSM 30030, Alcaligenes xylosoxidans NCIMB11015, and Alcaligenes sp. strain DSM 30128), indicating that this norB type is found in denitrifiers other than Ralstonia eutropha. qnorB genes from these denitrifiers and Ralstonia eutropha which are phylogenetically affiliated to the β-proteobacteria are more closely related to each other than to any norB gene from nondenitrifying species. However, in Thauera aromatica and Azoarcus tolulyticus, also β-proteobacteria, norB genes of the cnorB type were detected instead. The presence of cnorB genes in a variety of denitrifying strains was confirmed by amplification and sequencing of these genes. Additionally, we demonstrated the cnorB type for a variety of denitrifying strains, including Azospirillum brasilense, Blastobacter denitrificans, “Corynebacterium nephridii,” and Ochrobactrum anthropi. These organisms belong to the α-, β-, and γ-proteobacteria, respectively.
Primer specificity was also confirmed by cloning norB genes from a freshwater sediment and a marine sediment. Clones chosen for sequencing by screening through RFLP revealed a large number of unique norB genotypes in both habitats at a level similar to our observations of nirK and nirS gene diversity in marine sediment samples (4). Sequence analysis of two redundant and representative clones (qWM21 and qWM24) confirmed the specificity of the predominant group of qnorB genes from the Washington margin sample. Generally, the qnorB primer combination was highly specific because all clones sequenced were indeed norB gene fragments. For cnorB, two clones from the Washington margin sample were not identified as norB sequences. A third clone was a chimera and was excluded from further analysis. All deduced amino acid sequences of specific norB amplicons exhibited the three conserved histidine residues corresponding to positions 194, 245, and 246 of the Paracoccus denitrificans amino acid sequence, which are required for binding of two hemes and nonheme iron (FeB) (1, 10, 44). These residues are conserved in both types of NorB, which supports the fact that even the very unrelated norB sequences from environmental samples are indeed fragments of norB genes. Additionally, a glutamate residue (E198 in Paracoccus denitrificans) which is essential for the activity but not for the assembly of nitric oxide reductases (6) was recovered in all norB sequences. Conservation of structural motifs provides strong evidence for proper functionality of the predicted norB gene products, although they are rather dissimilar in their primary structure.
Phylogenetic analysis placed environmental qnorB genes in subclusters separate from those from cultivated organisms and environmental cnorB genes on separate branches within clusters, demonstrating the now typically observed discrepancy of genes from cultured organisms versus environmental sources (4, 35). Generally, the topology of the trees was stable with respect to the two main clusters of qnorB and cnorB genes and for the subclustering when fixN genes were included as an outgroup in tree calculation. However, the positions of subclusters within the main clusters depended on the method of tree calculation. Therefore, multifurcations were introduced when the topology was not resolved. Thus, three main clusters of qnorB genes were found, two of which consisted exclusively of environmental clones, suggesting that these genes originated from as yet uncultivated species with unknown physiological features, whereas the third contained mainly genes from described species.
The newly detected qnorB genes from Alcaligenes spp. also grouped into the latter subcluster with qnorB from Ralstonia eutropha and nondenitrifying strains such as the Neisseria sp. Other than in the photosynthetic cyanobacterium Synechocystis and Ralstonia solanacearum, qnorB was mainly detected in nondenitrifying pathogens, e.g., Mycobacterium avium, Staphylococcus aureus, and Corynebacterium diphtheriae (18), although these sequences were not included in our analysis due to uncertainties in the alignment. The recruitment of this single gene from the denitrification pathway by pathogens may provide a detoxification mechanism for NO produced by macrophages from the host defense system, e.g., qNorB of Neisseria gonorrhoeae was found to be active in its host (19). Nitric oxide has cytotoxic effects and inhibits a number of metalloproteins found in bacterial respiratory chains (44). Under environmental conditions, organisms face NO produced by bacteria such as nitrifiers (7) and actinomycetes (11), by cyanobacteria, green algae (22), and even higher plants (45). The function of a norB gene in the nondenitrifying Synechocystis sp. is probably for NO removal because it was demonstrated to be potentially functional (5).
The majority of known denitrifiers harbor norB genes of the norCB type, based on analysis of the genes from pure cultures obtained in this study and on database sequences. Environmental norB clones of this class with the exception of three deeply branching clones (cRCR35, cWM1, and cWM56) are found in subclusters with known denitrifiers belonging to the Proteobacteria. The distribution of norB sequences among known denitrifiers suggests that for the cnorB branch, most of the environmental sequences might be derived from Proteobacteria, which is consistent with our findings from a previous study exploring the diversity of nitrite reductase genes in environmental samples (4). More obscure is the origin of the very unrelated cnorB genes and genes of the qnorB subclusters I and II. Interestingly, norB genes from known denitrifiers did not cluster strictly according to the phylogeny of the organisms and their type of nitrite reductase genes (4), suggesting that denitrification genes were acquired through horizontal gene transfer. However, these inconsistencies in gene phylogeny were not surprising, considering that in P. stutzeri, denitrification genes are organized in gene clusters (51), in Ralstonia eutropha they exist on megaplasmids (10), and they may be dispersed over the chromosome, as in Bradyrhizobium japonicum (23). Further evidence for horizontal gene transfer is the fact that the norB genes from both marine and freshwater sediments were almost evenly distributed throughout all norB subclusters, which was in contrast to our previous study, which found nitrite reductase gene clusters to be strongly habitat specific (4, 27).
In summary, the development of these primer sets to target norB genes allows broad detection of the last denitrification gene for which a detection assay was lacking, nitric oxide reductase. Thus, molecular tools are available to explore all key steps in the denitrification process independently, for example, to study horizontal gene transfer or expression of denitrification genes.
Acknowledgments
We gratefully acknowledge Ralf Conrad for allowing us to complete this work in his laboratory and Michael W. Friedrich for advice for constructing phylogenetic trees. Valérie Michotey and Rainer Cramm are acknowledged for kindly providing Pseudomonas fluorescens strain AK and Synechocystis sp. strain PCC6803; Sabine A. Rech and John K. Davis are acknowledged for kindly providing DNA extracts from Azoarcus sp. and Thauera aromatica, respectively; and Allan H. Devol is acknowledged for providing the Washington margin sediment core.
This research was funded by the Biotechnological Investigations-Ocean Margins Program, Biological and Environmental Research, U.S. Department of Energy (grant DE-FG02-98ER 62.535).
REFERENCES
- 1.Arai, H., Y. Igarashi, and T. Kodama. 1995. The structural genes for nitric oxide reductase from Pseudomonas aeruginosa. Biochim. Biophys. Acta 1261:279-284. [DOI] [PubMed] [Google Scholar]
- 2.Bartnikas, T. B., I. E. Tosques, W. P. Laratta, J. Shi, and J. P. Shapleigh. 1997. Characterization of the nitric oxide reductase-encoding region in Rhodobacter sphaeroides 2.4. J. Bacteriol. 179:3534-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braker, G., A. Fesefeldt, and K.-P. Witzel. 1998. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 64:3769-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braker, G., J. Zhou, L. Wu, A. H. Devol, and J. M. Tiedje. 2000. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific Northwest marine sediment communities. Appl. Environ. Microbiol. 66:2096-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Büsch, A., B. Friedrich, and R. Cramm. 2002. Characterization of the norB gene, encoding nitric oxide reductase, in the nondenitrifying cyanobacterium Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 68:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butland, G., S. Spiro, N. J. Watmough, and D. J. Richardson. 2001. Two conserved glutamates in the bacterial nitric oxide reductase are essential for activity but not assembly of the enzyme. J. Bacteriol. 183:189-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad, R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60:609-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyne, M. S., A. Arunakumari, B. A. Averill, and J. M. Tiedje. 1989. Immunological identification and distribution of dissimilatory heme cd1 and nonheme copper nitrite reductases in denitrifying bacteria. Appl. Environ. Microbiol. 55:2924-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramm, R., A. Pohlmann, and B. Friedrich. 1999. Purification and characterization of the single-component nitric oxide reductase from Ralstonia eutropha H16. FEBS Lett. 460:6-10. [DOI] [PubMed] [Google Scholar]
- 10.Cramm, R., R. A. Siddiqui, and B. Friedrich. 1997. Two isofunctional nitric oxide reductases in Alcaligenes eutrophus H16. J. Bacteriol. 179:6769-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutruzzolà, F. 1999. Bacterial nitric oxide synthesis. Biochim. Biophys. Acta 1411:231-249. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1993. PHYLIP: phylogeny inference package (version 3.5c). Department of Genetics, University of Washington, Seattle, Wash.
- 13.Fries, M. R., J. Zhou, J. Chee-Sanford, and J. M. Tiedje. 1994. Isolation, characterization, and distribution of denitrifying toluene degraders from a variety of habitats. Appl. Environ. Microbiol. 60:2802-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gliesche, C. G., and P. Hirsch. 1992. Mutagenesis and chromosome mobilization in Hyphomicrobium facilis B-522. Can. J. Microbiol. 38:1167-1174. [DOI] [PubMed] [Google Scholar]
- 15.Gliesche, C. G., M. Menzel, and A. Fesefeldt. 1997. A rapid method for creating species-specific gene probes for methylotrophic bacteria. J. Microbiol. Methods 28:25-34. [Google Scholar]
- 16.Gregory, L. G., A. Karakas-Sen, D. J. Richardson, and S. Spiro. 2000. Detection of genes for membrane-bound nitrate reductase in nitrate-respiring bacteria and in community DNA. FEMS Microbiol. Lett. 183:275-279. [DOI] [PubMed] [Google Scholar]
- 17.Hallin, S., and P.-E. Lindgren. 1999. PCR detection of genes encoding nitrite reductases in denitrifying bacteria. Appl. Environ. Microbiol. 65:1652-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks, J., A. Oubrie, J. Castresana, A. Urbani, S. Gemeinhardt, and M. Saraste. 2000. Nitric oxide reductases in bacteria. Biochim. Biophys. Acta 1459:266-273. [DOI] [PubMed] [Google Scholar]
- 19.Householder, T. C., E. M. Fozo, J. A. Cardinale, and V. L. Clark. 2000. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 68:5241-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kukimoto, M., M. Nishiyama, M. Tanokura, and S. Horinouchi. 2000. Gene organization for nitric oxide reduction in Alcaligenes faecalis S-6. Biosci. Biotechnol. Biochem. 64:852-857. [DOI] [PubMed] [Google Scholar]
- 21.Lyman, J., and R. H. Fleming. 1940. Composition of sea water. J. Mar. Res. 3:134-167. [Google Scholar]
- 22.Mallick, N., L. C. Rai, F. H. Mohn, and C. J. Soeder. 1999. Studies on nitric oxide (NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum. Chemosphere 39:1601-1610. [DOI] [PubMed] [Google Scholar]
- 23.Mesa, S., M. Göttfert, and E. J. Bedmar. 2001. The nir, nor, and nos denitrification genes are dispersed over the Bradyrhizobium japonicum chromosome. Arch. Microbiol. 176:136-142. [DOI] [PubMed] [Google Scholar]
- 24.Michotey, V., V. Méjean, and P. Bonin. 2001. Comparison of methods for quantification of cytochrome cd1-denitrifying bacteria in environmental marine samples. Appl. Environ. Microbiol. 66:1564-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto, N., Y. Imai, H. Shoun, and Y. Shiro. 1998. Site-directed mutagenesis of the conserved threonine (Thr243) of the distal helix of fungal cytochrome P450nor. Biochemistry 37:8839-8847. [DOI] [PubMed] [Google Scholar]
- 26.Petri, R., and J. Imhoff. 2000. The relationship of nitrate reducing bacteria on the basis of narH gene sequences and comparison of narH and 16S rDNA based phylogeny. Syst. Appl. Microbiol. 23:47-57. [DOI] [PubMed] [Google Scholar]
- 27.Priemé, A., G. Braker, and J. M. Tiedje. 2002. Diversity of nitrite reductase (nirK and nirS) gene fragments in forested upland and wetland soils. Appl. Environ. Microbiol. 68:1893-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purdy, K. J., T. M. Embley, S. Takii, and D. B. Nedwell. 1996. Rapid extraction of DNA and rRNA from sediments by a novel hydroxyapatite spin-column method. Appl. Environ. Microbiol. 62:3905-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson, D. J. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology 146:551-571. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai, N., and T. Sakurai. 1998. Genomic DNA cloning of the region encoding nitric oxide reductase in Paracoccus halodenitrificans and a structural model relevant to cytochrome oxidase. Biochem. Biophys. Res. Commun. 243:400-406. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J. E., F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor. N.Y.
- 32.Saraste, M., and J. Castresana. 1994. Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 341:1-4. [DOI] [PubMed] [Google Scholar]
- 33.Satoh, T., Y. Hoshino, and H. Kitamura. 1976. Rhodopseudomonas sphaeroides forma sp. denitrificans, a denitrifying strain as a subspecies of Rhodopseudomonas sphaeroides. Arch. Microbiol. 108:265-269. [DOI] [PubMed] [Google Scholar]
- 34.Scala, D. J., and L. J. Kerkhof. 1998. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol. Lett. 162:61-68. [DOI] [PubMed] [Google Scholar]
- 35.Scala, D. J., and L. J. Kerkhof. 1999. Diversity of nitrous oxide reductase (nosZ) genes in continental shelf sediments. Appl. Environ. Microbiol. 65:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoun, H., D.-H. Kim, H. Uchiyama, and J. Sugiyama. 1992. Denitrification by fungi. FEMS Microbiol. Lett. 94:277-283. [DOI] [PubMed] [Google Scholar]
- 37.Shoun, H., and T. Tanimoto. 1991. Denitrification by the fungus Fusarium oxysporum and involvement of cytochrome P-450 in the respiratory nitrite reduction. J. Biol. Chem. 17:1078-1082. [PubMed] [Google Scholar]
- 38.Staley, J. T. 1968. Prosthecomicrobium and Ancalomicrobium, new prosthecate freshwater bacteria. J. Bacteriol. 95:1921-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaya, N., S. Suzuki, S. Kuwazaki, H. Shoun, F. Maruo, M. Yamaguchi, and K. Takeo. 1999. Cytochrome P450nor, a novel class of mitochondrial cytochrome P450 involved in nitrate respiration in the fungus Fusarium oxysporum. Arch. Biochem. Biophys. 372:340-346. [DOI] [PubMed] [Google Scholar]
- 40.Tsuruta, S., N. Takaya, L. Zhang, H. Shoun, K. Kimura, M. Hamamoto, and T. Nakase. 1998. Denitrification by yeasts and occurrence of cytochrome P450nor in Trichosporon cutaneum. FEMS Microbiol. Lett. 168:105-110. [DOI] [PubMed] [Google Scholar]
- 41.van der Oost, J., A. P. de Boer, J. W. de Gier, W. G. Zumft, A. H. Stouthamer, and R. J. van Spanning. 1994. The heme-copper oxidase family consists of three distinct types of terminal oxidases and is related to nitric oxide reductase. FEMS Microbiol. Lett. 121:1-9. [DOI] [PubMed] [Google Scholar]
- 42.van Elsas, J. D., and K. Smalla. 1995. Extraction of microbial community DNA from soils, p. 1.3.3/1-1.3.3/11. In A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 43.Vincent, J. M. 1971. A manual for the practical study of root-nodule bacteria. Burgess and Son LTB, Oxford, United Kingdom.
- 44.Watmough, N. J., G. Butland, M. R. Cheesman, J. W. B. Moir, D. J. Richardson, and S. Spiro. 1999. Nitric oxide in bacteria: synthesis and consumption. Biochim. Biophys. Acta 1411:456-474. [DOI] [PubMed] [Google Scholar]
- 45.Yamasaki, H., and Y. Sakihama. 2000. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 468:89-92. [DOI] [PubMed] [Google Scholar]
- 46.Ye, R. W., B. A. Averill, and J. M. Tiedje. 1992. Characterization of Tn5 mutants deficient in dissimilatory nitrite reduction in Pseudomonas sp. strain G-179, which contains a copper nitrite reductase. J. Bacteriol. 174:6653-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye, R. W., M. R. Fries, S. G. Bezborodnikov, B. A. Averill, and J. M. Tiedje. 1993. Characterization of the structural gene encoding a copper-containing nitrite reductase and homology of this gene to DNA of other denitrifiers. Appl. Environ. Microbiol. 59:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, J., M. R. Fries, J. C. Chee-Sanford, and J. M. Tiedje. 1995. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth of toluene and description of Azoarcus tolulyticus sp. nov. Int. J. Syst. Bacteriol. 45:500-506. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, J., M. E. Davey, J. B. Figueras, E. Rivkina, D. Gilichinsky, and J. M. Tiedje. 1997. Phylogenetic diversity of a bacterial commmunity determined from Siberian tundra soil DNA. Microbiology 143:3913-3919. [DOI] [PubMed] [Google Scholar]
- 50.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zumft, W. G. 1997. Enzyme diversity and mosaic gene organization in denitrification. Antonie van Leeuwenhoek 71:43-58. [DOI] [PubMed] [Google Scholar]
- 52.Zumft, W. G., C. Braun, and H. Cypers. 1994. Nitric oxide reductase from Pseudomonas stutzeri. Eur. J. Biochem. 219:481-490. [DOI] [PubMed] [Google Scholar]
- 53.Zumft, W. G., H. Körner, S. Löchelt, A. Viebrock, and K. Frunzke. 1988. Defects in cytochrome cd1-dependent nitrite respiration of transposon Tn5-induced mutants from Pseudomonas stutzeri. Arch. Microbiol. 149:492-498. [DOI] [PubMed] [Google Scholar]