Abstract
Histamine, a biogenic amine with both neurotransmitter and vasoactive properties, is well recognized as an immunomodulatory agent in allergic and inflammatory reactions. It also plays a regulatory role in the development of antigen-specific immune responses. CD4+ T-cells from histamine H1 receptor (H1R)-deficient (H1RKO) mice produce significantly less interferon-γ and more interleukin (IL)-4 in in vitro recall assays compared to wild-type controls. H1RKO mice are also less susceptible to acute early-phase experimental allergic encephalomyelitis indicating that H1R signaling in CD4+ T cells plays a central role in regulating pathogenic T-cell responses. In this study, we show that mice lacking histamine H2 receptor (H2RKO) are similar to H1RKO mice in that they develop encephalitogen-specific T-cell responses as assessed by proliferation and IL-2 production and present with less severe acute early-phase experimental allergic encephalomyelitis. However, unlike T cells from H1RKO mice, which exhibit a strong Th2 bias, T cells from H2RKO mice do not. Rather, they are uniquely characterized by a significant inhibition of Th1 effector cell responses. Given that both histamine and adjuvants such as pertussis toxin modulate antigen-presenting cell (APC) maturation and function, including T-cell-polarizing activity, we analyzed the cytokines/chemokines secreted by APCs from wild-type, H1RKO, and H2RKO mice. Significant differences in cytokine/chemokine production by APCs from unimmunized and immunized mice were delineated. APCs from H2RKO mice produce significantly less IL-12 and IL-6 and markedly greater amounts of MCP-1 compared to wild-type and H1RKO mice. Because MCP-1 is known to inhibit IL-12 production, the failure of H2RKO mice to generate encephalitogenic Th1 effector cell responses is consistent with inhibition of negative regulation of MCP-1 secretion by H2R signaling in APCs.
A large number of immunopathological diseases, including allergic, infectious, and autoimmune, positively correlate with either CD4+ T helper-1 (Th1) or T helper-2 (Th2) effector cell activities.1–3 Th1 cells are characterized by the production of proinflammatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α whereas Th2 cells secrete interleukin (IL)-4, IL-5, and IL-13.4–6 This dichotomy is thought to be established early in T-cell antigen priming by contact with antigen-presenting cells (APCs).7,8 Signaling through histamine H1 and H2 receptors (Hrh1/H1R and Hrh2/H2R) is known to regulate Th1 and Th2 effector functions and the polarization of naïve CD4+ Th0 cells into either Th1 or Th2 cells. T cells from H1RKO mice stimulated in vitro with anti-CD3 and anti-CD28 antibodies produce lower intracytoplasmic levels of IFN-γ suggesting that H1R signaling in CD4+ T cells enhances Th1 responses. In contrast, T cells from H2RKO mice stimulated in the same way produce higher intracytoplasmic levels of both IFN-γ and the Th2-related cytokines IL-4 and IL-13, indicating that both Th1 and Th2 responses may be down-regulated by H2R signaling.9,10 Additionally, histamine acting primarily through H2R on APCs has been reported to strongly enhance the secretion of IL-10 and inhibit the production of IL-12, thereby influencing polarization of T-cell effector responses.11–14
Recently, we demonstrated that Bphs, an autoimmune disease gene controlling susceptibility to both experimental allergic encephalomyelitis (EAE) and autoimmune orchitis, is H1R.15 During the acute phase of disease, C57BL/6J H1RKO congenic mice exhibited a significant delay in the onset of EAE and a reduction in the severity of the clinical signs compared with C57BL/6J wild-type mice. This result was associated with immune deviation of the elicited CD4+ T-cell population from a highly encephalitogenic Th1 response to a less encephalitogenic Th2 response. In addition to the role of H1R in EAE, mRNA transcripts for molecules involved in the allergic arm of the immune response, including H1R and H2R, have been identified in central nervous system (CNS) lesions in EAE and multiple sclerosis (MS).16–18
Mast cells, the primary source of histamine in the body,19,20 have long been appreciated in EAE and MS. In 1890, Neuman21 reported their presence in MS plaques. Subsequent studies verified that the number and distribution of mast cells correlate with MS lesions and susceptibility to EAE,22,23 and have been shown to be essential for eliciting severe acute EAE.24 Histamine and histamine-releasing agents also have a dramatic effect on the blood-brain barrier, and tissue levels of histamine correlate with the onset of EAE.23,25 Inhibitors of mast cell degranulation and H1R selective antagonists have been shown to reduce the severity of EAE.26–29 Furthermore, mast cells activated in the periphery migrate to draining lymph nodes during the induction of an immune response suggesting that they have a regulatory influence on B- and T-cell responses.30–32 There is also bidirectional communication between histamine and cytokines in a variety of settings.33 Taken together, these studies suggest that histamine signaling through the H2, H3, and H4 receptors may also participate in the pathogenesis of autoimmune disease of the CNS.
In this study, we directly examined the role of H2R signaling in controlling susceptibility to myelin oligodendrocyte glycoprotein (MOG) peptide 35-55 (MOG35-55)-induced EAE using C57BL/6J H2RKO congenic mice.34 We report that H2RKO mice are significantly less susceptible to acute early-phase EAE compared to wild-type C57BL/6J mice even though no significant difference in their in vitro CD4+ T-cell proliferative response or secretion of IL-2 after stimulation with MOG35-55 was detected. However, CD4+ T cells from H2RKO exhibit significantly blunted Th1 effector cell responses. An analysis of the cytokines produced by APCs from H2RKO mice revealed profiles consistent with the effector T-cell responses observed, indicating that susceptibility to EAE may in part be controlled by H2R-mediated regulation of APC function.
Materials and Methods
Animals
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.129P-Hrh1tm1Wat (H1RKO) and B6.129P-Hrh2tm1Wat (H2RKO) mice were backcrossed to C57BL/6J mice for a total of 10 generations.15,34 Wild-type controls (designated as C57BL/6J), B6.129P-Hrh1tm1Wat, B6.129P-Hrh2tm1Wat, and (B6.129P-Hrh1tm1Wat × B6.129P-Hrh2tm1Wat) F1 hybrid mice were generated at Charles Rivers Laboratory (Wilmington, MA) and shipped to either the University of Oregon Health Sciences Center (Portland, OR) or the University of Vermont (Burlington, VT).
Flow Cytometric Analysis
Single cell suspensions of thymocytes and splenocytes were prepared and the contaminating red blood cells lysed with ammonium chloride. The cells were washed twice and incubated for 20 minutes on ice with the desired fluorochrome-conjugated monoclonal antibodies or isotype control Ig at 0.5 μg/106 cells. The cells were washed twice with phosphate-buffered saline/1% bovine serum albumin, fixed in 2% paraformaldehyde, and analyzed by FACScan (BD Biosciences, San Jose, CA) using the Cell Quest program (BD Biosciences).
Induction and Evaluation of EAE
Mice were injected subcutaneously in the flanks with 0.2 ml of an emulsion containing 200 μg of MOG35-55 (Beckman Institute, Palo Alto, CA) in saline and an equal volume of complete Freund’s adjuvant containing 200 μg of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI). On the day of immunization, each mouse received 25.0 ng of pertussis toxin (PTX) (List Biological Laboratories Inc., Campbell, CA) intravenously, and 67.0 ng 2 days later.15 The mice were assessed daily for clinical signs of EAE according to the following scale: 0, normal; 1, limp tail or mild hind limb weakness; 2, moderate hind limb weakness or mild ataxia; 3, moderately severe hind limb weakness; 4, severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5, paraplegia with no more than moderate forelimb weakness; 6, paraplegia with severe forelimb weakness or severe ataxia or moribund condition.
Brains and spinal cords were dissected from calvaria and vertebral columns, respectively, and fixed by immersion in 10% phosphate-buffered formalin (pH 7.2). After adequate fixation, brain and spinal cord were trimmed and representative transverse section embedded in paraffin, sectioned at 5 μm, and mounted on glass slides. Sections were stained with hematoxylin and eosin for routine evaluation and Luxol fast blue-periodic acid-Schiff for demyelination. Sections from representative areas of the brain and spinal cord (SC) were scored in a semiquantitative manner for the various histopathological parameters as previously described.35
Proliferation Assays
Draining lymph node cells were surgically removed from immunized mice at the peak of clinical disease (day 16 after immunization), and single cell suspensions were prepared.15,36 draining lymph node (DLN) cells (4 × 105/well) were plated on standard 96-well flat-bottom tissue culture plates for 72 hours at 37°C and 7% CO2 with and without antigen and in the presence of 0.5 μCi of 3H-thymidine during the last 18 hours. Cells were harvested onto glass fiber filters and thymidine uptake was determined by liquid scintillation.
Cytokine Assays
Spleens were surgically removed from immunized mice at the peak of clinical EAE (day 16 after immunization) and single cell suspensions were prepared. Splenocytes were suspended at 4 × 106 cells/ml in stimulation media with 20 μg/ml of MOG35-55. Cell culture supernatants were recovered at 48 hours and frozen at −70°C until needed for cytokine assays. Cytokine levels were measure by enzyme-linked immunosorbent assay (ELISA)15,36 using cytokine-specific capture and detection antibodies (PharMingen, San Diego, CA). Capture antibodies for IFN-γ were diluted to 2 μg/ml in bicarbonate coating buffer (0.1 mol/L NaHCO3, pH 8.2). IL-4 was quantified using an IL-4 ELISA (PharMingen). Standard curves for each assay were generated using recombinant mouse cytokines (PharMingen), and the concentration of cytokines in the cell supernatants was determined by interpolation from the appropriate standard curve. Mean and standard curves were determined using triplicate cultures from pooled splenocytes from each group.
IFN-γ, TNF-α, IL-2, and IL-5 were also simultaneously detected using the mouse Th1/Th2 cytokine CBA kit from BD Biosciences (San Jose, CA). Fifty μl of sample was mixed with 50 μl of the mixed capture beads and 50 μl of the mouse Th1/Th2 PE detection reagent. The tubes were incubated at room temperature for 2 hours in the dark, followed by a wash step. The samples were then resuspended in 400 μl of wash buffer before acquisition on the FACScan. The data were analyzed using the CBA software. Standard curves were generated for each cytokine using the mixed bead standard provided in the kit and the concentration of cytokine in the cell supernatant was determined by interpolation from the appropriate standard curve. Means and standard deviations were determined using data from individual animals.
In some experiments cytokine production was assessed using RayBio Mouse Cytokine Array 1 (RayBiotech, Inc., Norcross, GA). Cell culture supernatants were collected and assayed as per the manufacturer’s specifications. Complete media was used as the negative control in all assays. The results were normalized and the data presented as the relative abundance with respect to the cytokine/growth factor being produced in the highest concentration.
Assessment of Antibody Responses
Antibody reactivity to MOG35-55 was determined by indirect ELISA.15,36 Dilutions of mouse sera from MOG35-55-immunized C57BL/6J wild-type control and H2RKO mice were incubated in MOG35-55-coated wells, and bound antibody was detected spectrophotometrically with peroxidase-conjugated anti-mouse Ig, anti-mouse IgG1, and anti-mouse IgG2a and o-phenylene-diamine as a substrate.
Peritoneal Exudate (PE) Macrophages
PE cells were collected on days 7 to 8 after immunization by peritoneal lavage 3 days after the intraperitoneal injection of 1.5 ml of sterile thioglycolate (3%). The cells were suspended at a concentration of 1 × 106 macrophages/ml in media and the nonadherent cells removed after 3 hours of plating. The adherent PE macrophages (>95%) were subsequently cultured for 24 hours in media.
Real-Time Polymerase Chain Reaction (PCR) Analysis of Gene Expression
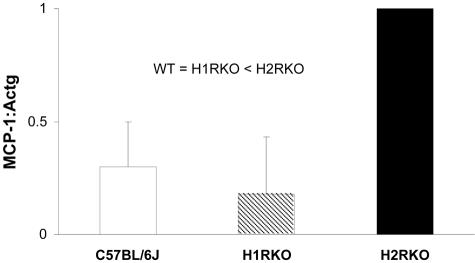
Total RNA was extracted from PE macrophages obtained after 3 hours of plating using Ultraspec RNA isolation reagent (Biotecx Laboratory, Houston, TX) as recommended by the manufacturer. Total RNA (1.0 μg) was used as a template to synthesize first strand cDNA using random primers (Promega, Madison, WI) with Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed using the TaqMan system (Applied Biosystems, Foster City, CA). PCRs were performed using the TaqMan Universal PCR Master Mix and the ABI PRISM 7700 sequence detection system. MCP-1 and γ-actin (Actg) (internal reference) were measured by real-time PCR using probes labeled with 6-carboxyfluorescein (FAM) and Black Hole Quencher (BHQ). The MCP-1 probes and primer set sequences used were as previously described.37 The Actg probe and primer sequences were: probe, 5′-6-FAM-d(CATTGCTCCCCCTGAGCGCAA)-BHQ-1-3′; forward primer, 5′-d(GCACCTAGCACGATGAAGATTAA GA)-3′; reverse primer, 5′-d(AGCCACCGATCCAGACTGAGT)-3′ (Biosearch Technologies, Novato, CA). MCP-1 and Actg mRNA levels were analyzed separately using the standard curve method. A no-reverse transcriptase control was also prepared for each sample and no genomic DNA was detected. Total RNA was isolated and reverse-transcribed into first strand cDNA. One μl of sample cDNA was used in the PCR reactions, three comparative threshold cycle (CT) values obtained and the average ratio of MCP-1:Actg calculated.
Results and Discussion
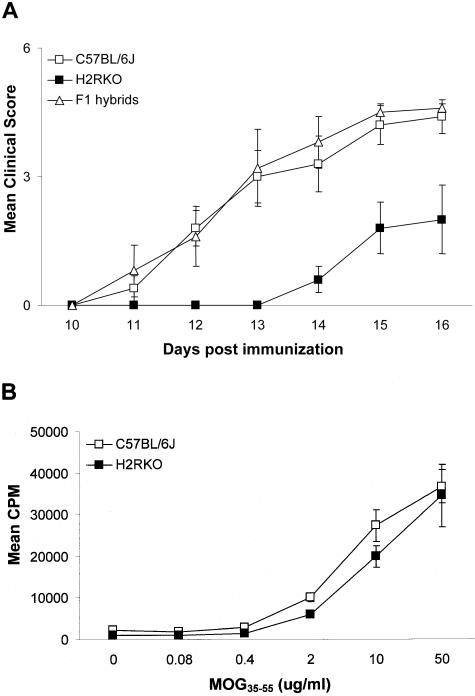
To assess the role of H2R in autoimmune disease, wild-type C57BL/6J and C57BL/6J H2RKO congenic mice were studied for susceptibility to MOG35-55-induced EAE. Both the day of disease onset and the severity of clinical signs were significantly different in H2RKO mice compared with C57BL/6J wild-type controls (Figure 1A). The day of onset and severity of clinical signs were restored to wild-type levels in (H1RKO × H2RKO) F1 hybrid mice indicating that a single wild-type Hrh2/H2R allele is dominant over the disrupted allele.
Figure 1.
A: Mean clinical disease course for C57BL/6J (n = 15), H2RKO (n = 15), and (H1RKO × H2RKO) F1 hybrid (n = 15) mice immunized with MOG35-.55. The data represent the combined results of three independent experiments (n = 5). The Wilcoxon-Gehan test for time to event and the Friedman test for correlated observations indicated that both the onset (P < 0.01) and severity of clinical signs (P < 0.0001) seen in the H2RKO animals is significantly different from C57BL/6J mice. Data are plotted as the mean clinical score ± SD. B: Proliferative response of T lymphocytes from C57BL/6J and H2RKO mice to MOG35-55 ex vivo. Mean cpm ± SD were calculated from triplicate wells.
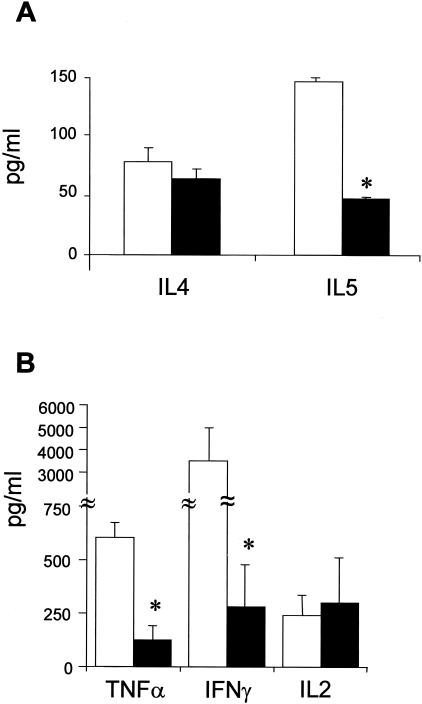
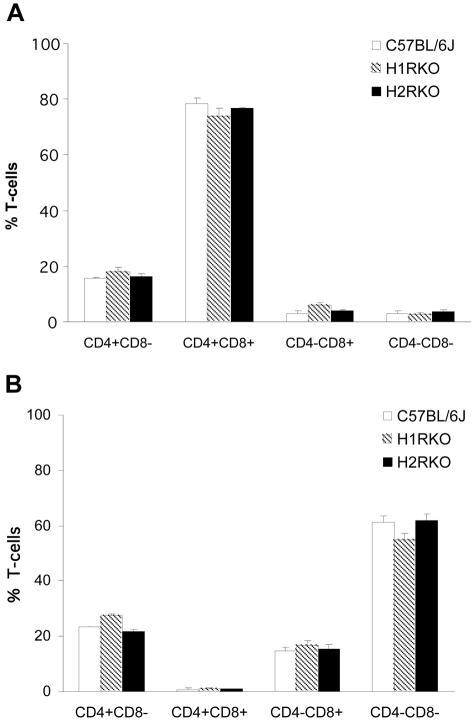
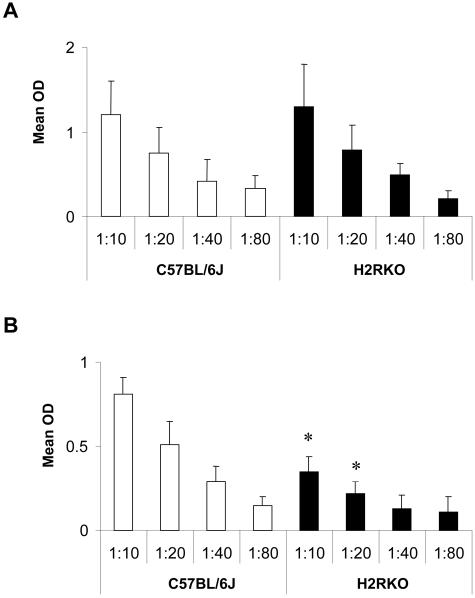
A variety of T-cell and APC parameters were examined to delineate the mechanism underlying the protection of H2RKO mice from EAE. No difference in the in vitro T-cell proliferative response to MOG35-55 was seen between wild-type and H2RKO mice (Figure 1B). In contrast, cytokine production differed significantly. The production of the Th1 cytokines IFN-γ and TNF-α and the Th2 cytokine IL-5 was decreased in H2RKO mice compared to wild-type mice (Figure 2, A and B). No difference in the production of the Th2 cytokine, IL-4, or IL-2 was detected. Importantly, no significant differences in either the total number (data not shown) or the composition of thymic (Figure 3A) or splenic T cells (Figure 3B) were observed between wild-type, H1RKO, and H2RKO mice. There was also no difference in the IgG1 anti-MOG35-55 antibody response between wild-type and H2RKO mice (Figure 4A) which is consistent with the equivalent levels of IL-4 produced by their T cells. However, there was a significant decrease in the titer of IgG2a antibodies in H2RKO mice correlating with the inhibition of IFN-γ production (Figure 4B). Additionally, the severity of the inflammatory infiltrates in the brain and spinal cord between H2RKO and wild-type mice were similar with the exception that H2RKO mice exhibited slightly more infiltrates of eosinophils (Figure 5). These results indicate that protection from EAE in H2RKO mice correlates with the induction of T-cell responses blunted in Th1 cytokine production, rather than a generalized suppression of T-cell responsiveness or strong polarization toward a less encephalitogenic Th2 response as is seen in H1RKO mice.15,38
Figure 2.
IL-4 and IL-5 (A) and IFN-γ, TNF-α, and IL-2 (B) production by MOG35-55 stimulated splenocytes from MOG35-55-immunized C57BL/6J (□) and H2RKO (▪) mice. Cytokine production was determined by either ELISA or cytometric bead assay. Significance of differences between wild-type and H2RKO mice were determined using the Student’s t-test (*, P < 0.05).
Figure 3.
Thymic (A) and splenic (B) T-cell composition of C57BL/6J, H1RKO, and H2RKO mice (n = 3).
Figure 4.
Anti-MOG35-55 IgG1 (A) and IgG2a (B) antibody response in C57BL/6J (□) and H2RKO (▪) mice immunized with MOG35-55. Differences between strains at the respective dilutions were determined using the Student’s t-test (*, P < 0.05).
Figure 5.
CNS lesions in C57BL/6J and H2RKO mice with EAE elicited by immunization with MOG35-55. Brain sections from H2RKO (A) and C57BL/6J mice (B) and spinal cord sections from H2RKO (C) and C57BL/6J mice (D) have principally a mononuclear inflammatory response associated with demyelination. In addition, the H2RKO mice (A, C) have numerous eosinophils (arrows) in the inflammatory response when compared to C57BL/6J mice (B, D). Brain and spinal cord sections from complete Freund’s adjuvant-immunized H2RKO control mice (E, F) and complete Freund’s adjuvant-immunized C57BL/6J control mice (G, H) have rare mononuclear inflammatory cell infiltrates perivascularly (E, arrow) and in the meninges and submeningeal white matter (H, arrow). H&E stains. Scale bar, 50 μm (A–H).
Because H2R signaling has been reported to dramatically influence the polarizing activity of human dendritic cells in culture by modulating cytokine production,11–14 the failure of the responding T cells in H2RKO mice to undergo Th1 polarization may be a reflection of APC activity. Similarly, PTX has been shown in vivo to modulate immune responses by influencing APC maturation and function in mice.39–42 Given the potential relevance of these effects in EAE, we undertook a series of experiments to assess whether inhibition of Th1 effector cell responses in MOG35-55-immunized H2RKO mice arise from dysregulation of polarizing cytokine production by their APCs.
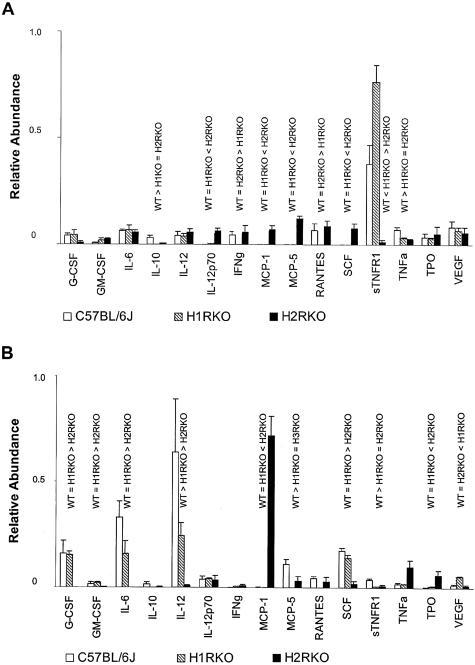
Cytokines, chemokines, and growth factor production by PE macrophages from unimmunized or immunized wild-type, H1RKO, and H2RKO mice was assessed using cytokine arrays. The results for 15 cytokines, chemokines, and growth factors on the arrays that are known to be expressed by macrophages43,44 are presented in Figure 6. Clearly, there are significant differences in the cytokine profiles of PE macrophages among the strains form both unimmunized (Figure 6A) and MOG35-55-immunized (Figure 6B) mice. The most dramatic difference seen in the unimmunized mice was the increased secretion of sTNFR1 by H1RKO PE macrophages compared to wild-type cells, and the decreased level of sTNFR1 expression by PE macrophages from H2RKO mice (Figure 6A). Significant differences were also seen in the levels of IL-10 (WT > H1RKO = H2RKO), IL-12p70 (WT = H1RKO < H2RKO), IFN-γ (WT = H2RKO > H1RKO), MCP-1 (WT = H1RKO < H2RKO), MCP-5 (WT = H1RKO < H2RKO), RANTES (WT = H2RKO > H1RKO), and SCF (WT = H1RKO < H2RKO) secretion by PE macrophages among the three strains.
Figure 6.
Cytokine production by PE macrophages from unimmunized (A) and MOG35-55-immunized (B) C57BL/6J, H1RKO, and H2RKO mice. Assays were performed using pooled cells from two to three mice per experiment. The results presented represent the average of four independent experiments and the data are expressed as the normalized average relative abundance ± SEM. Analysis of variance was used to evaluate the significance of differences in cytokine levels among the three strains followed by a Tukey’s multiple comparison test to determine the significance of differences between strains when warranted. For all analyses, significance was determined at P ≤ 0.05. Unimmunized: G-CSF (F = 1.80, P = 0.18); GM-CSF (F = 1.84, P = 0.18); IL-6 (F = 3.43, P = 0.05); IL-10 (F = 5.14, P = 0.02); IL-12 (F = 2.48, P = 0.11); IL-12p70 (F = 16.88, P < 0.001); IFN-γ (F = 6.71, P = 0.006); MCP-1 (F = 14.39, P < 0.001); MCP-5 (F = 83.6, P < 0.001); RANTES (F = 3.88, P = 0.03); SCF (F = 11.06, P = 0.001); sTNFR1 (F = 30.6, P < 0.001); TNF-α (F = 2.84, P = 0.08); TPO (F = 0.74, P = 0.49); VEGF (F = 0.82, P = 0.46). Immunized: G-CSF (F = 6.50, P = 0.006); GM-CSF (F = 4.31, P = 0.03); IL-6 (F = 8.81, P = 0.002); IL-10 (F = 3.52, P = 0.05); IL-12 (F = 4.64, P = 0.02); IL-12p70 (F = 0.05, P = 0.95); IFN-γ (F = 1.52, P = 0.24); MCP-1 (F = 8.17, P = 0.002); MCP-5 (F = 11.69, P < 0.001); RANTES (F = 3.12, P = 0.07); SCF (F = 6.45, P = 0.006); sTNFR1 (F = 7.26, P = 0.004); TNF-α (F = 4.05, P = 0.03); TPO (F = 5.39, P = 0.01); VEGF (F = 17.45, P < 0.001).
In MOG35-55-immunized animals, the most striking differences between the three strains were the decreased secretion of IL-12 and IL-6, and the marked increase in secretion of MCP-1 by H2RKO PE macrophages compared to wild-type and H1RKO cells (Figure 6B). The differences in MCP-1 production were verified at the transcriptional level by real-time PCR (Figure 7). These results provide a mechanistic basis underlying the delay in onset and reduction in severity of the acute clinical signs in EAE seen in H2RKO mice. IL-12 is one of the signature Th1-promoting cytokines, and its virtual absence of expression by H2RKO APCs explains the failure of the mice to develop strongly polarized encephalitogenic Th1 effector cell responses. Furthermore, IL-6 has been shown to play a crucial role in MOG-induced EAE.45,46 Monoclonal antibodies to IL-6 or selective IL-6 inhibitors can abrogate EAE induction,47,48 and reduced levels of IL-6 are seen in rats protected from EAE by lovastatin.49 Inhibition of positive regulation of IL-6 by disruption of H2R signaling would therefore be expected to lead to less severe EAE. In this regard, H2R signaling has been shown to enhance IL-6 secretion in a number of settings.33
Figure 7.
MCP-1 expression by PE macrophages from MOG35-55-immunized C57BL/6J, H1RKO, and H2RKO mice. MCP-1 expression was assessed by TaqMan PCR in 3-hour adherent PE macrophages collected at 7 days after immunization by peritoneal lavage 3 days after the intraperitoneal injection of 1.5 ml of sterile thioglycolate (3%). Expression levels were normalized to H2RKO levels and expressed as a mean ± SD (n = 3). Analysis of variance was used to assess the significance of differences in MCP-1 levels followed by a Tukey’s multiple comparison test to determine the significance of differences between strains. For both tests, significance was determined at P ≤ 0.05.
A full understanding of the mechanisms whereby H2R signaling regulates the Th1 polarizing activity of APCs is currently unknown. H2R agonists have been reported to inhibit IL-12 secretion by APCs in vitro and H2R antagonists have the expected opposite effect. Our finding that APCs from H2RKO mice immunized with MOG35-55 secrete very little IL-12 stands in contrast to these studies.11–14 However, our findings can readily be explained by the inhibition of negative regulation of MCP-1 secretion by H2R signaling. MCP-1-matured APCs exhibit a marked reduction in IL-12 production; and naïve T cells stimulated with MCP-1 primed APCs, such as T cells from H2RKO mice, produce significantly less IFN-γ.50 In addition, MCP-1 has been shown to selectively alter cytokine-driven APC differentiation and maturation without altering their T-cell-stimulating activity.46 Interestingly, MCP-1 has been shown to be an essential mediator during the effector phase of EAE,33 and PTX treatment of mice overexpressing MCP-1 exhibit a reversible encephalopathy.51 Clearly, the effect of MCP-1 depends on the context in which it is acting.52
Cimetidine, which was originally described as an H2R selective antagonist but is in reality an inverse agonist,53 does not prevent or inhibit EAE.26 Rather, it increases disease severity54 as well as Th1-mediated delayed type hypersensitivity reactions in general,55 and the production of IL-12 by human mononuclear cells.56 Dimaprit, an H2R agonist, has been reported to ameliorate EAE57 and inhibit the production of TNF-α and IL-12.58 The seemingly paradoxical results between our findings and pharmacological based approaches may be because of several factors. The phenotypic differences in knockout mice reflect both developmental and adult gene functions. Therefore, the differences seen in H2RKO mice not only reflect the effects of H2R signaling during adulthood but they also reflect the role that the absence of H2R signaling plays in the development of the normal innate and anticipatory components of the immune system. With respect to histamine, this paradox is exemplified by the mast cell abnormalities seen in histidine decarboxylase-deficient mice59 and the abnormalities in cytokine production seen in unimmunized PE macrophages form H1RKO and H2RKO mice in this study (Figure 6A). The life-long absence of histamine signaling through H2R may well show a different outcome than would be predicted using pharmacological agents in an intact mouse with a normal immune repertoire.
Additionally, it has been shown that tissue-specific expression of H2R in the mouse is under the control of histamine, and that exogenous histamine present in the diet modulates receptor expression.60 Differences in dietary histamine and in vitro culture conditions can therefore significantly impact the immunological read outs associated with pharmacological approaches. For example, the effects of histamine on T- and B-cell antigen-specific proliferative responses can only be observed by using histamine-free media and depleting c-Kit-positive cells from spleen and lymph node cell preparations.61
Regarding cytokine production by T cells, our results differ from those of Jutel and colleagues.9 They reported that splenic T cells from H2RKO mice stimulated with anti-CD3 and anti-CD28 antibodies exhibited increased intracytoplasmic staining for IFN-γ compared to wild-type mice. A slight increase in intracytoplasmic staining for IL-4 was also seen at 48 hours after stimulation. When histamine-pretreated spleen cells were stimulated with anti-CD3 antibody and supernatants assayed for cytokine production 72 hours later, they observed increased production of both Th1 and Th2 cytokines. The different results obtained in the present study may be because of histamine-signaling differences in T cells from mice that have been actively immunized with complete Freund’s adjuvant and PTX compared to naive T cells subjected to in vitro stimulation protocols. In this regard, the effect of histamine on IFN-γ production by Th1 cells has been shown to be dependent on the stimulating signals and mode of cellular activation.62 Similarly, there is increasing evidence indicating that microbial products drive the development of T-cell effector functions via their effects on APCs.63 Indeed, histamine-dependent changes in cytokine production by APCs is highly dependent on the presence or absence of LPS, a toll-like receptor (TLR) ligand with known adjuvant activity,13 and PTX as shown in this and other studies.39–42
Collectively, the results of our studies on Bphs/Hrh115 and the studies reported herein indicate that histamine signaling through H1R and H2R significantly influence the T-cell responses in EAE, particularly with respect to immune deviation and the nature of the cytokines they produce. Our results also suggest that Th1-dependent autoimmune diseases such as MS may be amenable to treatment with combinations of either H1R- and H2R-selective antagonist or agonists. Typically, H1R is coupled to the PLC/intracellular Ca2+ signaling pathway whereas H2R is considered to be coupled to the adenylate cyclase/cAMP pathway.53,64 However, an accumulating body of evidence indicates that signaling through H2R can result in the activation of the adenylyl cyclase/cAMP and PLC/intracellular Ca2+ second messenger systems. Multiple signaling is common among Gs-coupled receptors and has been suggested to be because of receptor density.65 In this regard, it was recently shown that H2R signaling through the PLC second messenger pathway is inhibited during cellular differentiation, and that this inhibition is correlated with decreased H2R expression.66 Thus, signaling through H2R may regulate immune deviation using different second messenger pathways as a function of the developmental state of either the T cells, APCs, or both. Although the details concerning the interplay between H1R- and H2R-signaling pathways and known signaling pathways regulating the polarization of T cells remains obscure, this interplay is nevertheless an avenue of investigation that has the potential to provide significant insight into the interactions between the environment, cells of the innate immune system, and the generation of effector T cells leading to disease.
Footnotes
Address reprint requests to Dr. Cory Teuscher, C317 Given Medical Bldg., University of Vermont School of Medicine, Burlington, VT 05405. E-mail: C.Teuscher@uvm.edu.
Supported by the National Institutes of Health (grants NS36526 to C.T. and E.P.B., AI4515 to C.T., AI41747 to C.T.) and the National Multiple Sclerosis Society (grants RG-3129 to C.T and E.P.B., AI42376 to H.O., NS23444 to H.O., and RG-3108 to H.O.).
References
- Kapsenberg ML, Jansen HM, Bos JD, Wierenga EA. Role of type 1 and type 2 T helper cells in allergic disease. Curr Opin Immunol. 1992;4:788–793. doi: 10.1016/0952-7915(92)90063-k. [DOI] [PubMed] [Google Scholar]
- Sher A, Coffman RL. Regulation of immunity to parasite by T-cells and T-cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Liblau RS, Springer SM, McDevitt HO. Th1 and Th2 CD4+ T-cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond WM, Giedlin MA, Coffman RL. Two types of murine helper T-cell clones. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mossman TT, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T-cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OAR, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–740. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–2593. [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Snijders A, Boeije LC, de Groot ER, Alewijnse AE, Leurs R, Aarden LA. Histamine inhibits production of interlerukin-12 through interaction with H2 receptors. J Clin Invest. 1998;102:1866–1873. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T-cell polarization. J Clin Invest. 2001;108:1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron G, Delneste Y, Roelandts E, Duez C, Bonnefoy J-Y, Pestel J, Jeannin P. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J Immunol. 2001;167:3682–3686. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KSK, Watanabe T, Zachary JF, Offner H, Blankenhorn EP, Teuscher C. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R, Garren H, Fontoura P, Tsai M, Galli SJ, Sobel RA, Steinman L. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA. 2003;100:1867–1872. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish MF, Austen FK. The diverse roles of mast cells. J Exp Med. 2001;194:1–5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie-Ryan M, Brown M. The role of mast cells in allergy and autoimmunity. Curr Opin Immunol. 2002;14:728–733. doi: 10.1016/s0952-7915(02)00394-1. [DOI] [PubMed] [Google Scholar]
- Neuman J. Ueber das Vorkommen der sogneannten “Mastzellen” bei pathologischen Veraenderungen der Gehirns. Arch Pathol Anat Physiol Virchows. 1890;122:378–380. [Google Scholar]
- Toms R, Weiner HL, Johnson D. Identification of IgE-positive cells and mast cells in frozen sections of multiple sclerosis brains. J Neuroimmunol. 1990;30:169–177. doi: 10.1016/0165-5728(90)90101-r. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast-cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–348. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–821. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babington R, Wedeking P. The influence of cinanserin and selected pharmacologic agents on experimental allergic encephalomyelitis (EAE). J Pharmacol Exp Ther. 1971;177:454–460. [PubMed] [Google Scholar]
- Linthicum DS, Munoz JJ, Blasket A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Pang X, Theoharides TC. Hydroxyzine inhibits experimental allergic encephalomyelitis (EAE) and associated brain mast cell activation. Int J Immunopharmacol. 2000;22:673–684. doi: 10.1016/s0192-0561(00)00029-1. [DOI] [PubMed] [Google Scholar]
- Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab EM, Tsai M, Galli SJ, Steinman L. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DS, Gurish MF, Austen KF, Hunt J, Stevens RL. Senescent jejunal mast cells and eosinophils in the mouse preferentially translocate to the spleen and draining lymph node, respectively, during the recovery phase of helminth infection. J Immunol. 2000;165:344–352. doi: 10.4049/jimmunol.165.1.344. [DOI] [PubMed] [Google Scholar]
- Karulin AY, Hesse MD, Yip HC, Lehmann PV. Indirect IL-4 pathway in type 1 immunity. J Immunol. 2002;168:545–553. doi: 10.4049/jimmunol.168.2.545. [DOI] [PubMed] [Google Scholar]
- Igaz P, Novak I, Lazaar E, Horvath B, Heninger E, Falus A. Bidirectional communication between histamine and cytokines. Inflamm Res. 2001;50:123–128. doi: 10.1007/s000110050735. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tonai S, Ishihara Y, Koga R, Okabe S, Watanabe T. Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J Clin Invest. 2000;105:1741–1749. doi: 10.1172/JCI9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105:1465–1472. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein-1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JF, Call SB, Fillmore PD, Watanabe T, Meeker ND, Teuscher C. Analysis of the role of Bphs/Hrh1 in the genetic control of responsiveness to pertussis toxin. Infect Immun. 2003;71:1281–1287. doi: 10.1128/IAI.71.3.1281-1287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, McCarthy L, Rappouli R, Mahon BP, Mills KHG. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7–1, B7–2 and CD28. Int Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- Shive CL, Hofstetter H, LaChelle A, Shaw C, Forsthuber TG. The enhanced antigen-specific production of cytokines induced by pertussis toxin is due to clonal expansion of T cells and not to altered effector functions of long-term memory cells. Eur J Immunol. 2000;30:2422–2431. doi: 10.1002/1521-4141(2000)30:8<2422::AID-IMMU2422>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: induction of Th1 cells and experimental allergic encephalomyelitis in the presence of high frequencies of Th2 cells. J Immunol. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- Hou W, Wu Y, Sun S, Shi M, Yang C, Pei G, Gu Y, Zhong C, Sun B. Pertussis toxin enhanced Th1 responses by stimulation of dendritic cells. J Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Sakoda OY, Fujimura S, Kishimoto SY, Yanagihara T. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glycoprotein 35-55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol. 1996;101:188–196. doi: 10.1016/s0165-5728(99)00139-3. [DOI] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- Gijbels K, Brocke S, Abrams JS, Steinman L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1:795–805. [PMC free article] [PubMed] [Google Scholar]
- Wang T, Nagai H, Bouda K, Matsuura S, Takaoka Y, Niwa S, Homma T, Tanaka H, Shudo K. Effect of selective IL-6 inhibitor Am-80 on experimental autoimmune encephalomyelitis in DA rats. Acta Pharmacol Sin. 2002;21:967–976. [PubMed] [Google Scholar]
- Stanislaus R, Gilg AG, Singh AK, Singh I. Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by Lovastatin. Neurosci Lett. 2002;333:167–170. doi: 10.1016/s0304-3940(02)00943-6. [DOI] [PubMed] [Google Scholar]
- Omata N, Yasutomi M, Yamada A, Iwasaki H, Mayumi M, Ohshima Y. Monocyte chemoattractant protein-1 selectively inhibits the acquisition of CD40 ligand-dependent IL-12-producing capacity of monocyte-derived dendritic cells and modulates Th1 response. J Immunol. 2002;169:4861–4866. doi: 10.4049/jimmunol.169.9.4861. [DOI] [PubMed] [Google Scholar]
- Huang DR, Tanji M, Wang J, Han Y, He TT, Weaver J, Charo IF, Tuohy VK, Rollins BJ, Ransohoff RM. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci. 2002;22:10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly D, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10:247–257. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- Bakker RA, Timmerman H, Leurs R. Histamine receptors: specific ligands, receptor biochemistry, and signal transduction. Clin Allergy Immunol. 2002;17:27–64. [PubMed] [Google Scholar]
- Staykova M, Kozovska M, Kirazin N, Goranoz I. Aggravation of experimental allergic encephalomyelitis by cimetidine. Ann Inst Pasteur Immunol. 1988;139:501–505. doi: 10.1016/0769-2625(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Snyman JR, Meyer EC, Schoeman HS. Cimetidine as modulator of the cell-mediated immune response in vivo using the tuberculin skin test as parameter. Br J Clin Pharmacol. 1990;29:257–260. doi: 10.1111/j.1365-2125.1990.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H, Fukui H, Takeyama N, Tanaka T. Cimetidine activates interleukine-12, which enhanced cellular immunity. Blood. 1999;93:1782–1783. [PubMed] [Google Scholar]
- Emerson MR, Orentas DM, Lynch SG, LeVine SM. Activation of histamine H2 receptors ameliorates experimental allergic encephalomyelitis. NeuroReport. 2002;13:1407–1410. doi: 10.1097/00001756-200208070-00012. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Shinohara M, Wang P-L, Hidaka A, Ohura K. Histamine inhibits chemotaxis, phagocytosis, superoxide anion production, and the production of TNFα and IL-12 by macrophages via H2-receptors. Int Immunopharmacol. 2001;1:1867–1875. doi: 10.1016/s1567-5769(01)00112-6. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- Fitzsimons CP, Lazar-Molnar E, Tomoskozi Z, Buzas E, Rivera ES, Falus A. Histamine deficiency induces tissue-specific down-regulation of histamine H2 receptor expression in histidine decarboxylase knockout mice. FEBS Lett. 2001;508:245–248. doi: 10.1016/s0014-5793(01)03070-8. [DOI] [PubMed] [Google Scholar]
- Banu Y, Watanabe T. Augmentation of antigen receptor-mediated responses by histamine H1 receptor signaling. J Exp Med. 1999;189:673–682. doi: 10.1084/jem.189.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna N, Elliott K, Khan MM. The effects of histamine on interferon gamma production are dependent on the stimulatory signals. Int Immunopharmacol. 2001;1:135–145. doi: 10.1016/s1567-5769(00)00005-9. [DOI] [PubMed] [Google Scholar]
- de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23:255–263. doi: 10.1016/s1471-4906(02)02215-9. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gilbert S, Birnbaumer M, Birnbaumer L. Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol. 1994;46:460–469. [PubMed] [Google Scholar]
- Fitzsimons C, Engel N, Policastro L, Duran H, Molinari B, Rivera E. Regulation of phospholipase C activation by the number of H2 receptors during Ca2+-induced differentiation of mouse keratinocytes. Biochem Pharmacol. 2002;63:1785–1796. doi: 10.1016/s0006-2952(02)00975-9. [DOI] [PubMed] [Google Scholar]