Abstract
In the setting of inactivated DNA damage-sensitive checkpoints, critically shortened telomeres promote chromosomal instability and the types of widespread cytogenetic alterations that characterize most human carcinomas. Using a direct telomere fluorescence in situ hybridization technique, we analyzed 114 invasive breast carcinomas, 29 carcinoma in situ lesions, 10 benign proliferative lesions, and different normal epithelial components of the male and female breast. We found marked telomere shortening in the majority (52.5%) of invasive carcinomas; smaller subsets of invasive carcinoma demonstrated moderate telomere shortening (17.5%) or normal telomere lengths (21%), while a small subgroup (5%) contained elongated telomeres. Strikingly, the majority (78%) of ductal carcinoma in situ demonstrated markedly or moderately shortened telomeres. Surprisingly, unlike all other normal epithelia studied to date, moderate telomere shortening was observed in benign secretory cells in approximately 50% of histologically-normal terminal duct lobular units (from which most breast cancer is thought to arise), while such shortening was not seen in myoepithelial cells or normal large lactiferous ducts of the female breast or male breast ducts (from which breast cancer infrequently arises). We postulate that such shortening is the result of hormonally driven, physiological proliferation, and may delineate a population of epithelial cells at risk for subsequent malignant transformation.
Telomeres cap the ends of each human chromosome, protecting against chromosome fusions and preventing chromosome termini from being recognized as double-stranded DNA breaks.1–7 Human telomeres are dynamic structures composed of 1000–2000 tandem repeats of the hexanucleotide TTAGGG complexed to a variety of proteins. With continued cell division (the “end-replication problem”) and/or oxidative damage,8 telomeres progressively shorten until one or more telomeres become dysfunctional, which normally triggers apoptosis or cell cycle arrest. In this fashion, telomeres are thought to function as tumor-suppressive biological clocks in normal somatic cells, placing an upper limit on cell division to avoid critically shortened telomeres that would otherwise lead to end-to-end chromosome fusions and structural alterations that are associated with malignancy. Germ cells and approximately 85% to 90% of human cancers circumvent this growth limitation because they express telomerase, a reverse transcriptase that adds single-stranded telomere repeats to chromosome ends. Interestingly, most human epithelial cancers (carcinomas) possess short telomere lengths despite their having telomerase activity.9–12 While telomere dysfunction may play a role in the genesis of the widespread genetic alterations and complex karyotypes typical of carcinomas, it is thought that the acquired telomerase activity of carcinoma cells allows stabilization of the genetic instability effected by short telomeres.1,2
Prior studies evaluating telomere lengths in human breast cancers have used bulk assessment techniques of whole tissues; such as Southern blot telomere restriction fragment (TRF) analysis or slot blot analysis.13–16 These methods are confounded by variable amounts of contaminating normal tissue, which itself is heterogeneous with respect to the proportion of fibrous and fatty stroma, lymphocytic infiltration, and epithelium that it contains. Not surprisingly, these studies have yielded conflicting results; while most have found that breast cancer samples contain shorter telomeres than normal breast tissue, studies disagree as to whether there is a correlation with grade and other predictive or prognostic factors.13,16 Additionally, no study has assessed telomere lengths in the precursors to breast carcinoma, so the timing of telomere length alterations in the evolution of breast carcinoma has not been determined.
Recently, we have used an in situ method that allows telomere length assessments in archival material, with single cell resolution in intact tissue architecture.17–19 This method correlates well with Southern blot TRF analysis. Using this novel technique, we now report the first in situ assessment of telomere length in human breast tissues.
Materials and Methods
Telomere-Immunostaining Fluorescence in Situ Hybridization (TELI-FISH)
Surgical specimens were routinely fixed in 10% neutral buffered formalin and subjected to standard processing and paraffin embedding. The protocol for combined staining of telomeric DNA (FISH probe) and immunostaining was performed without protease digestion, as previously described.17 Briefly, deparaffinized 4-μm thick slides underwent heat-induced antigen retrieval followed by hybridization with a Cy3-labeled, telomere-specific peptide nucleic acid (PNA) probe having the sequence (N terminus to C terminus) CCCTAACCCTAACCCTAA with an N-terminal covalently linked Cy3 fluorescent dye (Applied Biosystems, Framingham, MA). The slides were then processed for indirect immunofluorescence using a polyclonal rabbit anti-smooth-muscle actin primary antibody (Dako, Carpinteria, CA; catalog number m0851), followed by application of a 1:100 dilution of a goat anti-rabbit IgG fraction Alexa Fluor 488 (Molecular Probes, Eugene, OR; catalog number A-11034), primarily to help distinguish myoepithelial (ME) cells (actin-positive) from adjacent secretory cells or carcinoma cells (actin-negative). Slides were counterstained with DAPI (4′−6-diamidino-2-phenylindole; Sigma Chemical Co., St. Louis, MO).
Microscopy and Image Analysis
Serial adjacent hematoxylin and eosin (H&E)-stained reference slides were used as a guide during simultaneous examination of the TELI-FISH slides for telomere length assessment. Telomeric staining produced a speckled pattern of widely distributed nuclear signals in all cases examined, in keeping with results previously reported for mammalian somatic cells.17 Telomere lengths were evaluated by visual assessment of the fluorescent intensities of the telomeric signals, which are proportional to the length of telomeric TTAGGG DNA repeats.17 Telomeres were directly compared to those within normal-appearing epithelium within the same tissue section. When normal-appearing epithelium was lacking, comparisons were made using adjacent ME cells and normal stroma. Rare cases in which detectable telomere signals were not found in normal stromal cells (fibroblasts, endothelial cells), or in which excessive autofluoresence precluded assessment of telomere signals, were excluded from the study.
Digital fluorescent telomere signals in histologically normal breast terminal duct lobular units (TDLU) were quantitated using a semi-automated algorithm written with the image analysis software package IPLabs (Scanalytics, Inc., Fairfax, VA) and Microsoft Excel, as described previously.17 Ten to 20 representative secretory cell and ME cell nuclei per case were quantified. Nuclei were considered ME if their location was basal in the TDLU and their cytoplasm was diffusely labeled by actin. Nuclei were considered of secretory cell origin if their cytoplasm did not label at all with the actin antibody and they faced the lumen of the gland. Differences in mean telomere lengths between matched ME cells and luminal cells were tested for statistical significance by using t-test, with P values <0.01 considered significant.
Scoring System
Each lesion was scored by each of two authors (P.A., A.K.M.), and a consensus assessment was reached when there was a discrepancy. After discovering the variable intensity of normal secretory cells (see Results), we adopted the following semiquantitative scoring system. Lesions with normal telomere intensity had signals comparable to those of normal stromal fibroblasts, endothelial cells, or normal ME cells. Lesions with (moderately) short telomeres had telomere intensities appreciably dimmer than the normal stroma, but which were still readily detectable and within the range of the intensity seen in some histologically normal secretory cells. Lesions with very short telomeres demonstrated signals so dim that they were barely perceptible, and dimmer than that of virtually any normal epithelial cell we encountered in this study. Lesions with long telomeres demonstrated telomere signals that were appreciably brighter than that of the stromal cells, and close to that of lymphocytes, which gave consistently bright signals. Lesions in which the signal varied between cells from bright to short, or very short to normal (ie, lesions with telomere signals spanning two scoring categories) were classified as heterogeneous for telomere length.
Hybridization Probe Access Control
To rule out differences in probe penetration or target accessibility as potential sources of observed differences in fluorescent telomere signal intensities in fixed tissue samples, we used a second fluorescently labeled PNA probe with specificity for centromeric DNA repeats.20 These control hybridizations were performed on serial sections under identical conditions as described above using a 5′ rhodamine-labeled PNA probe having the sequence ATTCGTTGGAAACGGGA with specificity for centromeric CENP-B DNA repeats.20
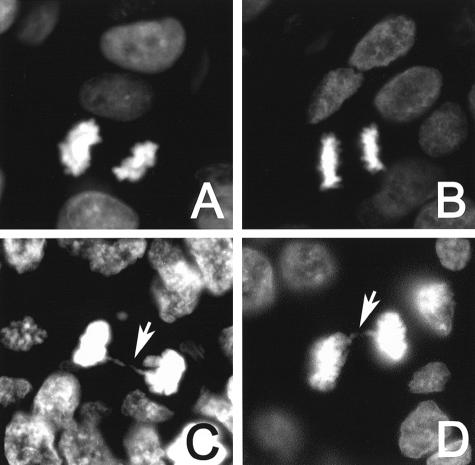
Anaphase Bridges
Anaphase bridges were scored with the DNA-specific DAPI stain; criteria for anaphase bridges required a well-separated parallel anaphase plate displaying a perpendicularly aligned amphophilic (“stretched”) connecting filament. Statistical comparison on the frequency of anaphase bridges between regions with either short or long telomeres was conducted by a test of proportions for two independent groups.
Results
Benign Breast Tissue
In all breast samples examined microscopically, normal stromal fibroblasts and endothelial cells uniformly demonstrated strong telomeric staining intensity, in agreement with previous results.17–19 This intensity was readily identifiable at low power (magnification, ×200), and was appreciably less than that of admixed stromal lymphocytes, which invariably displayed very strong telomere signals. Also in keeping with previous results, the intensity of the non-lymphocyte stromal labeling was then taken as the internal reference control to which other cells were compared. This approach has been taken by our group and by others using similar methodology.21
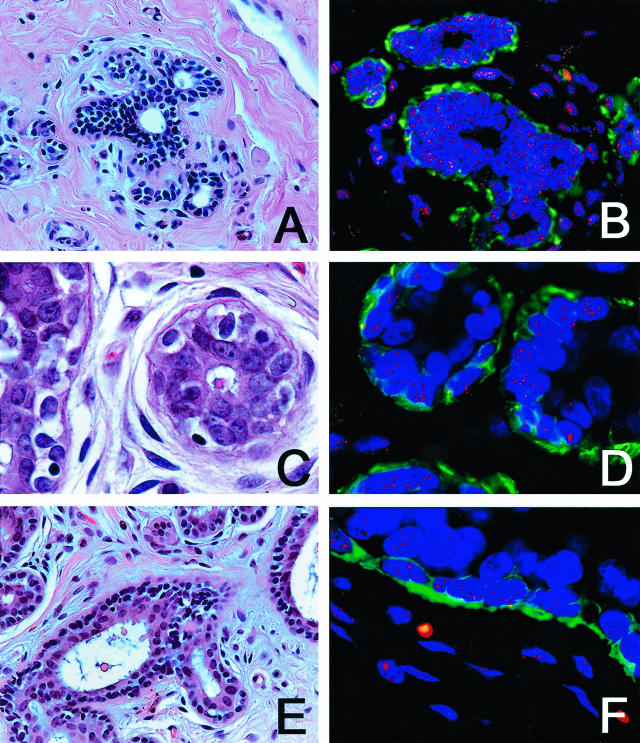
We first examined the TDLU, which are the milk-producing component of the breast and the postulated site of origin for most breast cancers,22,23 in 12 benign breast biopsies from women who had no evidence of cancer elsewhere in the breast. These biopsies were performed in most cases for macromastia, and histologically these samples showed no specific pathological changes. In all 12 cases, the normal outer ME cells, which were clearly delineated by the actin stain, demonstrated intense telomere signals, comparable to that of the normal stroma. In five of these cases, the inner secretory cells within these TDLU uniformly demonstrated comparable intensities to those of the adjacent ME cells (Figure 1, A and B). However, in three cases, the secretory cells demonstrated clearly dimmer signals than the adjacent ME cells as judged by visual inspection (Figure 1, C and D). In four other cases, there was marked variation in secretory cell telomere staining intensity, with some cells being comparable to the ME cells and other adjacent secretory cells in the same TDLU being appreciably dimmer (Figure 1, E and F).
Figure 1.
H&E staining (A, C, E) and TELI-FISH (B, D, F) analysis of telomere length in normal breast TDLU. A and B: Note that the secretory cells (negative with the green actin stain, facing the lumen) have comparable intensity of telomere signals as the ME cells (actin-positive). C and D: Note that the secretory cells in this TDLU have far less intense telomere signals that the ME cells. E and F: Note the variation in telomere signals among the secretory cells in this TDLU.
We then examined benign breast epithelium in patients with benign proliferative disorders and breast tissue from younger patients. Among 10 breast biopsies demonstrating proliferative fibrocystic changes (age range: 29 to 71 years), two showed foci of luminal cell telomere shortening within the fibrocystic changes. Of note, the three cases in this group which showed florid usual duct hyperplasia retained strong telomere signals in these areas. Examination of the normal TDLU found within 6 of these 10 biopsies revealed focal evidence of telomere shortening in all 6 cases. Two of seven breast biopsies from women less than 20 years of age demonstrated focal luminal cell shortening within histologically normal TDLU. Four of these patients had fibroadenomas, one of which demonstrated focal shortening of telomeres within luminal cells. The remaining three fibroadenomas demonstrated comparable signals in their ME and luminal cells.
We obtained similar results when examining secretory cells from histologically normal TDLU of women with cancer elsewhere in the breast. Among 12 such cases evaluated, three demonstrated normal (ME-like) telomeres, seven demonstrated shorter telomeres than those of the ME cells, while two cases showed marked variation in secretory cell telomere staining intensity.
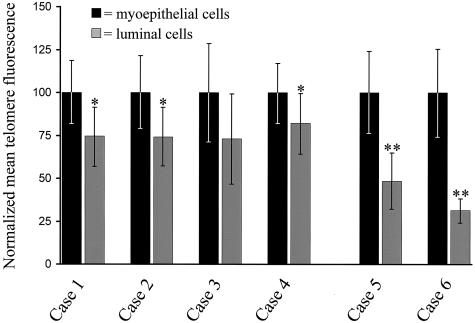
Using digital image analysis, we quantitated telomere lengths in TDLU from six representative cases. By visual inspection, two of these cases were judged to have clearly diminished signals in the luminal cells as compared to the ME cells. Image analysis confirmed this marked difference (Figure 2, cases 5 and 6) and indicated that it was highly statistically significant (P < 0.0000001). The remaining four cases were judged to have comparable telomere signals in both secretory and ME cells. Interestingly, in three of the four cases, image quantification indicated a small, statistically significant (P < 0.01) decrease in luminal telomeric signal intensity that was not detectable by eye (Figure 2, cases 1 to 4).
Figure 2.
Quantitation of telomere lengths in normal TDLU. Mean DAPI-normalized telomere signal intensities were determined by digital image analysis for 10 to 20 randomly selected luminal and ME cells. Cases 1 to 4 were judged by visual inspection to have comparable telomere lengths between the two cell types, while telomeres appeared markedly shorter in the luminal cells of cases 5 and 6. (* P value <0.01, ** P value <0.00000001).
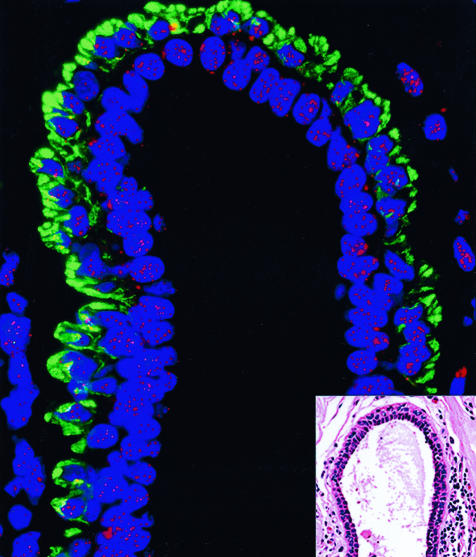
In contrast, we did not find luminal cell telomere shortening to be frequent in male ducts or female lactiferous ducts. First, 15 of 15 benign lactiferous ducts in women with cancer elsewhere (14 carcinoma, one angiosarcoma; patient age range: 31 to 86 years) in the breast demonstrated equally intense telomere signals in both luminal and ME cells (Figure 3). Similar results were obtained in the large ducts of 5 of 5 male breast tissues removed for gynecomastia (patient age range: 17 to 63 years). Interestingly, in the single male gynecomastia case in which true lobules were formed in the male breast, focal luminal cell telomere shortening was appreciated specifically within these lobules.
Figure 3.
TELI-FISH analysis of telomere length in normal lactiferous duct. Note that the luminal cells (negative with the green actin stain, facing the lumen) have comparable intensity of telomere signals as the ME cells (actin-positive). Inset: H&E-stained adjacent section.
Carcinoma in Situ
In all nine cases of pure ductal carcinoma in situ (DCIS) without invasive carcinoma examined, the ME cells retained their usual intense telomeric signals. In one of these cases, the telomere intensity of the DCIS cells was comparable to that of the ME cells. However, in four cases, the DCIS cells demonstrated very short telomeres that were barely detectable with the telomere-specific probe, while in the four remaining cases, the DCIS telomere signals were moderately short. In comparison to their respective surrounding normal breast TDLU secretory cells, the DCIS cells had shorter telomeres in 7 of 9 cases.
We next examined the DCIS components adjacent to 14 cases of invasive ductal carcinoma (IDC). Once again, in all of these cases, the retained normal ME cells contained robust telomere signals. In three cases, the DCIS cells had moderately short telomeres, while in seven cases the DCIS had very short (undetectable) telomeres (Figure 4). In one other case, the DCIS featured heterogeneous telomere lengths. Comparing the DCIS to their adjacent invasive ductal carcinomas, the DCIS was comparable to the IDC in telomere length in 11 of 13 cases. In the two other cases, the IDC featured shorter telomere lengths than the adjacent DCIS; these two DCIS lesions had normal-length telomeres.
Figure 4.
TELI-FISH analysis of telomere length in DCIS. Note that the luminal DCIS cells (negative with the green actin stain, facing the lumen) have virtually no telomere signal, in comparison to the ME cells (actin-positive). Inset: H&E-stained adjacent section.
There was a tendency for short telomeres to be associated with high nuclear grade among the DCIS. Of 11 DCIS with very short (virtually undetectable) telomeres, eight were grade 3, while three were grade 2. Among seven DCIS with short telomeres, five were grade 3, one was grade 2, while one was grade 1. Among four DCIS with telomere lengths comparable to those of the ME cells, two were grade 1, one was grade 2, while one was grade 3. The one case with heterogeneous telomere lengths was nuclear grade 3 (Table 1).
Table 1.
Telomere Lengths in DCIS as a Function of Nuclear Grade
| Telomere length | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| Normal | 2 | 1 | 1 | 4 |
| Short | 1 | 1 | 5 | 7 |
| Very short | 0 | 3 | 8 | 11 |
| Heterogeneous | 0 | 0 | 1 | 1 |
| Total | 3 | 5 | 15 | 23 |
Among six cases of lobular carcinoma in situ (LCIS) available for study, one featured very short telomeres, four featured short telomeres, while one had normal-length telomeres.
Invasive Carcinomas
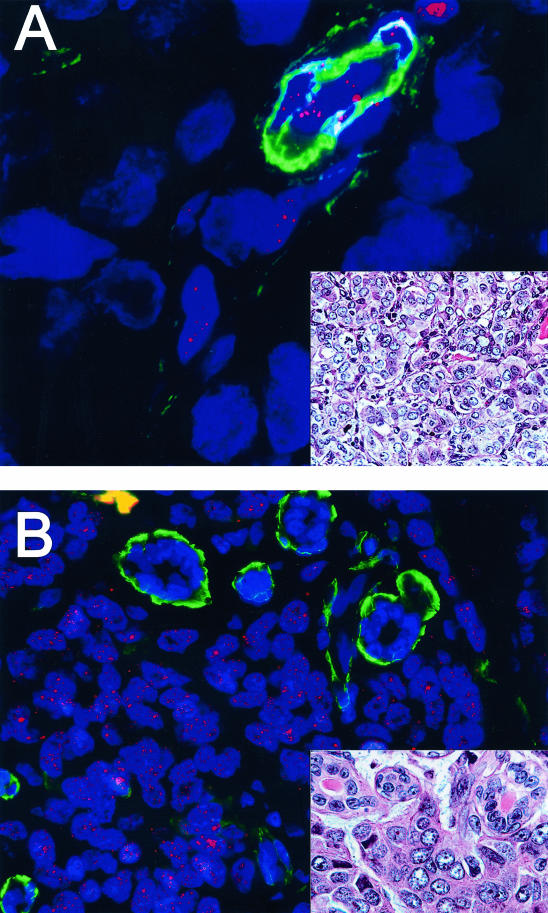
We next examined 114 cases of invasive mammary carcinoma. Among these cases, 60 (52.5%) had very short (barely detectable) telomere lengths, 20 (17.5%) had short telomere lengths, and 24 (21%) had normal (ME-like) telomere lengths. six (5%) cases demonstrated abnormally long telomeres, similar in intensity to that of adjacent lymphocytes (Figure 5). In four (4%) cases, marked intratumoral telomere length heterogeneity was observed. The results of telomere length assessment in these lesions are summarized in Table 2.
Figure 5.
TELI-FISH analysis of telomere length in invasive ductal carcinoma (IDC). Two examples of high-grade IDC are shown. A: This IDC has virtually undetectable telomere signals. Note the actin-positive pericytes of the capillary at the upper right of the figure, which, along with the benign endothelial cells, have intact telomere signals and hence serve as an internal control. B: This IDC has extremely bright telomere signals. Note the entrapped normal acini within the lesion, which are actin-positive. In this case, the tumor telomere signals are so strong that, at this setting, the normal acini and stromal telomere signals present are not visible in the image. Insets: adjacent H&E sections.
Table 2.
Summary of Telomere Length Results for Breast Luminal Cells
| Telomere length | Normal lobules (n = 24) | Normal lactiferous ducts (n = 15) | Male breast (n = 5) | Normal pediatric lobules (n = 7) | Fibrocystic changes (n = 10) | DCIS (n = 23) | LCIS (n = 6) | Invasive mammary carcinoma (n = 114) |
|---|---|---|---|---|---|---|---|---|
| Very short | 0 | 0 | 0 | 0 | 1 | 11 | 1 | 60 (52.5%) |
| Short | 10 | 0 | 0 | 2* | 1 | 7 | 4 | 20 (17.5%) |
| Normal | 8 | 15 | 5† | 5 | 8 | 4 | 1 | 24 (21%) |
| Long | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (5%) |
| Heterogeneous | 6 | 0 | 0 | 0 | 0 | 1 | 0 | 4 (4%) |
Luminal shortening was focal in these cases.
Focal luminal telomere shortening was seen in a lobule of a male breast with gynecomastia.
The relationship between telomere length and Elston grade of the invasive carcinoma is shown in Table 3. There appeared to be a tendency for high-grade tumors (Elston grade 3) to demonstrate non-normal (non-ME-like) telomere lengths; however, these differences were not statistically significant (data not shown). Of note, all of the cases with marked heterogeneity in telomere length and all cases with abnormally long telomeres were high-grade.
Table 3.
Telomere Length as a Function of Elston Grade in IDC
| Telomere length | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Very short | 3 (33%) | 20 (57%) | 37 (53%) |
| Short | 3 (33%) | 6 (17%) | 11 (16%) |
| Normal | 3 (33%) | 9 (26%) | 12 (17%) |
| Long | 0 | 0 | 6 (9%) |
| Heterogeneous | 0 | 0 | 4 (6%) |
Anaphase Bridges
When telomeres shorten to a critical length, telomeric fusions between chromosome ends may occur and lead to ring and dicentric chromosomes that form so-called “anaphase bridges” during mitosis. Anaphase bridges are therefore chromosomal bridges that are not resolved during anaphase,24 and are correlated with telomere dysfunction. Among tumors with telomeres scored as either very short or short, 18 of 26 anaphases demonstrated bridging (69%). In contrast, only seven anaphases among the 68 counted in tumors with either normal length or long telomeres demonstrated anaphase bridges (10%) (Figure 6, A and C). This difference was highly statistically significant (P < 0.0001). We then evaluated an invasive tumor with heterogeneous telomere lengths that contained 5 anaphases. Three anaphases were normal, and these three cells had normal telomere lengths. The two other anaphases demonstrated anaphase bridges; these two cells had short telomeres. Hence, the presence of anaphase bridges in invasive carcinomas correlated with the presence of shortened telomeres.
Figure 6.
Anaphase bridges in DAPI-stained IDC and DCIS. A: Normal anaphase in an IDC with normal length telomeres. B: Normal anaphase in a DCIS with normal length telomeres. C: Anaphase bridge in an IDC with abnormally short telomeres. D: Anaphase bridge in a DCIS with abnormally short telomeres.
We were unable to find adequate mitotic figures to formally study the effect of telomere lengths on anaphase bridges in DCIS. However, we noted no anaphase bridges in seven anaphases in one case of DCIS with normal telomere lengths. In the DCIS case that showed variable telomere lengths, anaphase bridges were noted, but only in the mitoses occurring in areas with shortened telomeres (Figure 6, B and D).
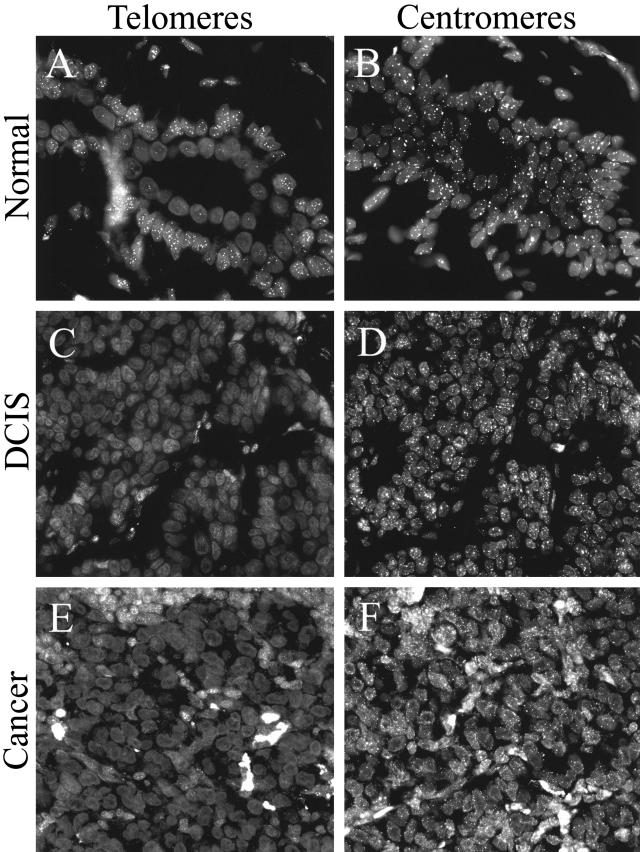
Confirmation of Hybridization Probe Access
The FISH-based telomere length assessment method used in this study was previously validated by comparing quantitative telomere length data obtained by TELI-FISH to data obtained by an independent method of telomere length measurement (Southern blotting), using a panel of formalin-fixed paraffin-embedded human cell lines. To rule out differences in either probe penetration or target accessibility as potential sources of the differences we observe in fluorescent telomeric signal intensities in fixed tissue samples, we used a second fluorescently labeled PNA probe with specificity for centromeric DNA repeats.20 Hybridizations were performed on serial sections of several tissue samples of normal breast, DCIS, and IDC used in this study, applying either the centromere-specific PNA probe, or the telomere-specific probe. In each case, the centromere-specific probe gave robust signals in all cell types, including those in which telomeric signals were dim or undetectable (Figure 7).
Figure 7.
PNA probe accessibility controls. Serial tissue sections were hybridized with either telomere-specific PNA probe (A,C,E) or centromere-specific PNA probe (B,D,F). A and B: Normal breast TDLU. C and D: DCIS. E and F: Invasive carcinoma. Note the diminished telomere signals in these examples of normal luminal cells, DCIS and IDC, as compared to the robust centromere signals in these same cells.
Discussion
We report the first in situ assessment of telomere length in human breast tissues. By permitting analysis at the single-cell level, the technique used overcomes the confounding influence that non-epithelial components have had on prior telomere length assessments reported in the literature. We report that marked telomere shortening is frequent in invasive mammary carcinoma, which is in agreement with prior studies.13–16 We note, however, that significant inter- and intratumoral heterogeneity in telomere length can occur in invasive carcinoma. Additionally, we demonstrate a correlation between telomere shortening in IDC and anaphase bridges, a morphological reflection of telomere dysfunction. For the first time, we document marked telomere shortening in DCIS, the recognized precursor to invasive ductal carcinoma. In fact, it appears that most telomere shortening in cases with concurrent DCIS and IDC occurs at the DCIS stage, as most of these cases demonstrated similarly shortened telomeres in both components compared to normal luminal epithelial cells. Hence, telomere shortening in IDC is not a result of continued cell proliferation during tumor expansion. Instead, it occurs at the pre-invasive level, and therefore may play a role in initiating tumorigenesis.
Our observation of marked telomere shortening in DCIS is concordant with prior morphological and molecular observations. Cytologically, low- and high-grade DCIS are highly similar to low- and high-grade IDC, respectively.22,23 At the molecular level, DCIS has been shown to harbor many of the same genetic alterations as IDC, both at the cytogenetic25–27 and gene expression levels.28,29 Most significantly, the majority of DCIS have previously been shown to harbor telomerase activity.30 Since telomerase reactivation is generally considered to be a mechanism of overcoming the growth-limiting effects of telomere shortening, it is not unexpected that DCIS would have short telomeres. Given that short telomeres have been associated with chromosome instability, it is tempting to speculate that those DCIS harboring very short telomeres are genetically unstable and predisposed to evolve toward invasive carcinoma.
We were initially surprised to find that normal secretory cells in TDLU often demonstrate moderate telomere shortening, since this finding is different from all other human epithelia studied to date.17–19 Moreover, on quantitative analysis, most of our cases in which the secretory cells appeared to have ME-like telomeres also appeared to be shorter than those of the ME cells, though this difference was small. However, consideration of the normal physiology of the breast and recently discovered distinctive biological properties of the TDLU provide a plausible mechanism. In normal TDLU, ME cells are essentially non-proliferative, while secretory cells proliferate cyclically with the menstrual cycle.22 Proliferation is most prominent late in the menstrual cycle, after which apoptosis becomes most prominent.31,32 Lactiferous ducts are less responsive to hormones and proliferate less, similar to male breast.32,33 Hence, our findings that lactiferous ducts, male breast, and ME cells tend to have normal telomere lengths is logical, since they are less mitotically active, while secretory cell telomeres in the TDLU should shorten with proliferation. The lobule-to-lobule variation in telomere length we observed in a given breast specimen is consistent with prior observations that different lobules vary in their degree of responsiveness to hormonal influences, and thus proliferate differentially.22 Secretory cells with sufficiently short telomeres are likely those normally destined to undergo apoptosis at the end of multiple menstrual cycles. TDLU secretory cells would then be repopulated from rare, yet-uncharacterized stem cells residing within the TDLU, which is consistent with the observations of Kolquist et al,34 who found the telomerase catalytic subunit mRNA in scattered cells in the TDLU but not in larger ducts of the breast.
Intriguingly, Romanov et al35 have recently shown that, in vitro, human mammary epithelial cells (HMECs) spontaneously inactivate p16 (part of the pRB mitotic checkpoint) by hypermethylation of the p16 gene, thus bypassing senescence that typically limits cell growth; these cells proliferate further until their telomeres become critically short and the cells enter telomere-based crisis. Interestingly, Holst et al36 have recently shown that p16 methylation also occurs in vivo in normal TDLU luminal cells but not ME cells. Since telomere shortening occurs frequently only in secretory cells of the TDLU and not in ME cells or luminal cells of lactiferous ducts, and given that the majority of breast carcinomas are thought to derive from and differentiate toward TDLU secretory cells, telomere shortening facilitated by p16 inactivation provides a plausible molecular mechanism that may predispose this specific cell population to breast carcinogenesis. These results may also help rationalize prior studies demonstrating genetic abnormalities in normal TDLU epithelium.37,38 It would be interesting to determine whether the proportion of TDLU with short secretory cell telomeres is increased in women at high risk for breast cancer, such as those with BRCA1 mutations, or decreased in populations at lower risk for breast cancer. Parenthetically, we suspect that most women of reproductive age harbor some TDLU with short secretory cell telomeres, given how frequently we observed focal shortening in the relatively small biopsy samples reviewed in this study. An autopsy study in which one could sample multiple different areas of benign breast would be useful to address this issue.
Taken together, the observations described above suggest a model for the involvement of telomere length changes in breast carcinogenesis, in which proliferating telomerase negative TDLU luminal epithelial cells bypass telomere length-dependent replicative senescence via genetic or epigenetic inactivation of the senescence checkpoint. Continued proliferation would then cause further loss of telomeric DNA until one or more telomeres become dysfunctional, thereby initiating chromosomal instability. This scenario is concordant with observations from in vitro immortalization studies in which replicative senescence is abrogated by expression of viral oncoproteins. Here, the period in which telomeres become dysfunctional is termed “crisis” and is characterized by marked chromosomal instability and cell death.39 In vitro, rare cells emerge from crisis by re-stabilizing their telomeres, either by activating telomerase, or by using an alternative recombinational pathway termed “ALT.” We believe that in vivo, crisis correlates with the appearance of DCIS, which frequently harbors chromosomal abnormalities, contains short telomeres, and has telomerase activity.
The intra- and interlesional heterogeneity of telomere lengths we observed in subsets of DCIS and IDC could stem from a number of sources. First, the extent of proliferation before stabilization by telomerase or ALT may affect the degree of overall telomere shortening. Second, the precise mechanism of stabilization may affect telomere lengths. In particular, heterogeneity of telomere length is a hallmark of the ALT phenotype.40 Since approximately 10% to 15% of breast cancers do not have detectable telomerase activity, it is possible that some of these are using the ALT pathway, although this remains to be shown. Third, different telomere lengths may arise due to heterogeneity in the level of telomerase expression. Notably, although a tumor may be scored as telomerase-positive by the standard PCR-based activity assay, the levels may be insufficient for maintenance of all telomeres in any given cell. Thus, dysfunctional telomeres and anaphase bridges may persist within a tumor despite evidence for telomerase activation. On the other hand, high levels of expression may lead to abnormally long telomeres. Indeed, intratumoral heterogeneity of telomerase activity has been previously demonstrated in microdissected samples of breast and prostate cancers.41–43 Lastly, there is accumulating evidence indicating that unrepaired oxidative DNA damage may contribute to telomere loss.44 Thus, variability in antioxidant defense capacity or exposure to oxidants, from either endogenous (eg, metabolic reactive oxygen species) or exogenous (eg, inflammation) sources could also affect telomere lengths.
We hypothesize that, while telomeres are stabilized to some extent during tumorigenesis, complete stabilization of every telomere in all cancer cells probably does not occur, thus some degree of chromosomal instability is retained, thereby fostering clonal evolution. In this model, the aggregate tumor genome is maintained in a metastable state, poised on the edge of chaos for rapid modulation of genetic instability as required for survival in response to new selective pressures, such as novel tissue microenvironments encountered during metastasis or to chemotherapy.
Finally, at a practical level, the variability in telomere length we observed among invasive mammary carcinomas has potential therapeutic implications. Since approximately 85% to 90% of human cancers, including invasive breast carcinomas, harbor telomerase activity, telomerase inhibitors are currently being developed for therapy.45 It is thought that such therapy should be most effective against tumors with short telomeres, since such tumor cells are dependent on telomerase to prevent further shortening of their telomeres, which would rapidly result in genetic catastrophe and cell death. On the other hand, tumors with long telomeres should be less responsive to such therapy, since multiple rounds of proliferation could occur before telomeres become critically short. Hence, one can postulate that the 5% of invasive carcinomas that we identified as having long telomeres may be resistant to telomerase-inhibition therapy. Additionally, the heterogeneity of telomere lengths we observed within a subset of tumors suggests that response to such therapy may be heterogeneous within a given lesion. Therefore, an in situ assay of telomere length in diagnostic breast tissue would be useful in attempting to correlate response to these agents with molecular features of the tumor. Finally, the fact that telomere shortening occurs in DCIS raises the possibility that chemo-preventative agents aimed at reducing telomere attrition or restoring telomere lengths may have promise in the chemoprevention of breast cancer.
Acknowledgments
We thank David Simon for his assistance with statistical testing.
Footnotes
Address reprint requests to Pedram Argani, M.D., The Johns Hopkins Hospital, Surgical Pathology, Weinberg Building, Room 2242, 401 North Broadway, Baltimore, MD 21231-2410. E-mail: pargani@jhmi.edu.
Supported by the National Cancer Institute Specialized Program of Research Excellence CA88843 (breast) and CA58236 (prostate) at The Johns Hopkins University, and NIH training grant T32DK07552 (to A.K.M.).
References
- Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–633. doi: 10.1038/nrc862. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kaminker P, Campisi J. Telomeres, aging and cancer: in search of a happy ending. Oncogene. 2002;21:503–511. doi: 10.1038/sj.onc.1205077. [DOI] [PubMed] [Google Scholar]
- Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21:619–626. doi: 10.1038/sj.onc.1205061. [DOI] [PubMed] [Google Scholar]
- Autexier C, Greider CW. Telomerase and cancer: revisiting the telomere hypothesis. Trends Biochem Sci. 1996;21:387–391. [PubMed] [Google Scholar]
- Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. doi: 10.1016/s0959-437x(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Gisselsson D, Jonson T, Petersen A, Strombeck B, Dal Cin P, Hoglund M, Mitelman F, Mertens F, Mandahl N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci USA. 2001;98:12683–12688. doi: 10.1073/pnas.211357798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Furugori E, Hirayama R, Nakamura KI, Kammori M, Esaki Y, Takubo K. Telomere shortening in gastric carcinoma with aging despite telomerase activation. J Cancer Res Clin Oncol. 2000;126:481–485. doi: 10.1007/s004320000137. [DOI] [PubMed] [Google Scholar]
- Mehle C, Ljungberg B, Roos G. Telomere shortening in renal cell carcinoma. Cancer Res. 1994;54:236–241. [PubMed] [Google Scholar]
- Takagi S, Kinouchi Y, Hiwatashi N, Chida M, Nagashima F, Takahashi S, Negoro K, Shimosegawa T, Toyota T. Telomere shortening and the clinicopathologic characteristics of human colorectal carcinomas. Cancer. 1999;86:1431–1436. [PubMed] [Google Scholar]
- Rogalla P, Rohen C, Bonk U, Bullerdiek J. Telomeric repeat fragment lengths are not correlated to histological grading in 85 breast cancers. Cancer Lett. 1996;106:155–161. doi: 10.1016/0304-3835(96)04304-2. [DOI] [PubMed] [Google Scholar]
- Rha SY, Park KH, Kim TS, Yoo NC, Yang WI, Roh JK, Min JS, Lee KS, Kim BS, Choi JH, Lim HY, Chung HC. Changes of telomerase and telomere lengths in paired normal and cancer tissues of breast. Int J Oncol. 1999;15:839–845. doi: 10.3892/ijo.15.4.839. [DOI] [PubMed] [Google Scholar]
- Griffith JK, Bryant JE, Fordyce CA, Gilliland FD, Joste NE, Moyzis RK. Reduced telomere DNA content is correlated with genomic instability and metastasis in invasive human breast carcinoma. Breast Cancer Res Treat. 1999;54:59–64. doi: 10.1023/a:1006128228761. [DOI] [PubMed] [Google Scholar]
- Odagiri E, Kanada N, Jibiki K, Demura R, Aikawa E, Demura H. Reduction of telomeric length and c-erbB-2 gene amplification in human breast cancer, fibroadenoma, and gynecomastia. Relationship to histologic grade and clinical parameters. Cancer. 1994;73:2978–2984. doi: 10.1002/1097-0142(19940615)73:12<2978::aid-cncr2820731215>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, De Marzo AM. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, Offerhaus GJ, Hicks JL, Wilentz RE, Goggins MG, De Marzo AM, Hruban RH, Maitra A. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Hong YK, Ontiveros SD, Egholm M, Strauss WM. Single base discrimination of CENP-B repeats on mouse and human Chromosomes with PNA-FISH. Mamm Genome. 1999;10:13–18. doi: 10.1007/s003359900934. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH, Crispin DA, Potter JD, Rabinovitch PS. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–284. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- Rosen PP. Rosen’s Breast Pathology. Philadelphia: Lippincott Williams and Wilkins,; 2001 [Google Scholar]
- Page DL, Anderson TJ. Edinburgh: Churchill Livingstone,; Diagnostic Histopathology of the Breast. 1987 [Google Scholar]
- Gisselsson D, Bjork J, Hoglund M, Mertens F, Dal Cin P, Akerman M, Mandahl N. Abnormal nuclear shape in solid tumors reflects mitotic instability. Am J Pathol. 2001;158:199–206. doi: 10.1016/S0002-9440(10)63958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger H, Simon R, Schafer KL, Diallo R, Littmann R, Poremba C, van Diest PJ, Dockhorn-Dworniczak B, Bocker W. Genetic relation of lobular carcinoma in situ, ductal carcinoma in situ, and associated invasive carcinoma of the breast. Mol Pathol. 2000;53:118–121. doi: 10.1136/mp.53.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger H, Otterbach F, Simon R, Schafer KL, Poremba C, Diallo R, Brinkschmidt C, Dockhorn-Dworniczak B, Boecker W. Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J Pathol. 1999;189:521–526. doi: 10.1002/(SICI)1096-9896(199912)189:4<521::AID-PATH472>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- O’Connell P, Pekkel V, Fuqua SA, Osborne CK, Clark GM, Allred DC. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst. 1998;90:697–703. doi: 10.1093/jnci/90.9.697. [DOI] [PubMed] [Google Scholar]
- Porter D, Lahti-Domenici J, Keshaviah A, Bae YK, Argani P, Marks J, Richardson A, Cooper A, Strausberg R, Riggins GJ, Schnitt S, Gabrielson E, Gelman R, Polyak K. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res. 2003;1:362–375. [PubMed] [Google Scholar]
- Lodato RF, Maguire HC, Jr, Greene MI, Weiner DB, LiVolsi VA. Immunohistochemical evaluation of c-erbB-2 oncogene expression in ductal carcinoma in situ and atypical ductal hyperplasia of the breast. Mod Pathol. 1990;3:449–454. [PubMed] [Google Scholar]
- Umbricht CB, Sherman ME, Dome J, Carey LA, Marks J, Kim N, Sukumar S. Telomerase activity in ductal carcinoma in situ and invasive breast cancer. Oncogene. 1999;18:3407–3414. doi: 10.1038/sj.onc.1202714. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R, Khan SA, Badve S. Morphological changes in breast tissue with menstrual cycle. Mod Pathol. 2002;15:1348–1356. doi: 10.1097/01.MP.0000039566.20817.46. [DOI] [PubMed] [Google Scholar]
- Joshi K, Smith JA, Perusinghe N, Monoghan P. Cell proliferation in the human mammary epithelium. Differential contribution by epithelial and myoepithelial cells. Am J Pathol. 1986;124:199–206. [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Connor RE. Cell proliferation in fibrocystic disease and postmenopause breast ducts measured by thymidine labeling. Cancer. 1982;50:746–751. doi: 10.1002/1097-0142(19820815)50:4<746::aid-cncr2820500420>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Kolquist KA, Ellisen LW, Counter CM, Meyerson M, Tan LK, Weinberg RA, Haber DA, Gerald WL. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998;19:182–186. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tlsty TD. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature. 2001;409:633–637. doi: 10.1038/35054579. [DOI] [PubMed] [Google Scholar]
- Holst CR, Nuovo GJ, Esteller M, Chew K, Baylin SB, Herman JG, Tlsty TD. Methylation of p16(INK4a) promoters occurs in vivo in histologically normal human mammary epithelia. Cancer Res. 2003;63:1596–1601. [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Larson PS, de las Morenas A, Bennett SR, Cupples LA, Rosenberg CL. Loss of heterozygosity or allele imbalance in histologically normal breast epithelium is distinct from loss of heterozygosity or allele imbalance in co-existing carcinomas. Am J Pathol. 2002;161:283–290. doi: 10.1016/S0002-9440(10)64180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Botelho FM, Wang P, Harley CB, Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994;68:3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel RR. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 2003;194:155–162. doi: 10.1016/s0304-3835(02)00702-4. [DOI] [PubMed] [Google Scholar]
- Tsao JI, Zhao YL, Lukas J, Yang XW, Shah A, Press M, Shibata D. Telomerase activity in normal and neoplastic breast. Clin Cancer Res. 1997;3:627–631. [PubMed] [Google Scholar]
- Mueller C, Riese U, Kosmehl H, Dahse R, Claussen U, Ernst G. Telomerase activity in microdissected human breast cancer tissues: association with p53, p21 and outcome. Int J Oncol. 2002;20:385–390. [PubMed] [Google Scholar]
- Wullich B, Rohde V, Oehlenschlager B, Bonkhoff H, Ketter R, Zwergel T, Sattler HP. Focal intratumoral heterogeneity for telomerase activity in human prostate cancer. J Urol. 1999;161:1997–2001. [PubMed] [Google Scholar]
- von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann NY Acad Sci. 2000;908:99–110. doi: 10.1111/j.1749-6632.2000.tb06639.x. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer Cell. 2002;2:257–265. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]