Abstract
Seven different strains of Saccharomyces cerevisiae were tested for the ability to maintain their fermentative capacity during 24 h of carbon or nitrogen starvation. Starvation was imposed by transferring cells, exponentially growing in anaerobic batch cultures, to a defined growth medium lacking either a carbon or a nitrogen source. After 24 h of starvation, fermentative capacity was determined by addition of glucose and measurement of the resulting ethanol production rate. The results showed that 24 h of nitrogen starvation reduced the fermentative capacity by 70 to 95%, depending on the strain. Carbon starvation, on the other hand, provoked an almost complete loss of fermentative capacity in all of the strains tested. The absence of ethanol production following carbon starvation occurred even though the cells possessed a substantial glucose transport capacity. In fact, similar uptake capacities were recorded irrespective of whether the cells had been subjected to carbon or nitrogen starvation. Instead, the loss of fermentative capacity observed in carbon-starved cells was almost surely a result of energy deprivation. Carbon starvation drastically reduced the ATP content of the cells to values well below 0.1 μmol/g, while nitrogen-starved cells still contained approximately 6 μmol/g after 24 h of treatment. Addition of a small amount of glucose (0.1 g/liter at a cell density of 1.0 g/liter) at the initiation of starvation or use of stationary-phase instead of log-phase cells enabled the cells to preserve their fermentative capacity also during carbon starvation. The prerequisites for successful adaptation to starvation conditions are probably gradual nutrient depletion and access to energy during the adaptation period.
Most studies concerning microbial processes have considered analysis of cell metabolism during growth of the organisms. In their natural environment, however, microorganisms such as the yeast Saccharomyces cerevisiae spend most of their time under nongrowing or very slow-growing conditions. Slow growth or stationary phase is usually due to severe limitation of one or several nutrients. Knowledge of the physiology of S. cerevisiae under such conditions is very limited and almost exclusively confined to aerobic conditions. Consequently, the response of S. cerevisiae to starvation under anaerobic conditions is an almost completely unexplored field. This is rather surprising because the anaerobic starvation response is of utmost importance for all biotechnological processes exploiting the natural ethanol-producing capacity of S. cerevisiae. Large-scale production of alcoholic beverages always includes stages of starvation affecting production efficiency and product quality. This investigation addresses the question of how the ethanol-producing capacity of anaerobically cultivated S. cerevisiae cells is affected by sudden removal of either their nitrogen source or their carbon source.
Aerobically, nitrogen starvation causes a much more severe reduction in catabolic capacity than carbon starvation in S. cerevisiae (2, 16). In part, this effect can be explained by inactivation of the glucose-transporting system during nitrogen starvation in the presence of a fermentable carbon source (2, 8). However, nitrogen starvation with ethanol instead of glucose still resulted in a severe reduction in catabolic capacity (16). Hence, additional factors, apart from glucose transport ability, have to be involved in the regulation of fermentative capacity after starvation. Attempts to correlate fermentative capacity with the level of one or several glycolytic enzymes has usually failed (9, 15, 16, 22), although there are examples in which such a correlation was observed (23). Another important feature during the production of baker's yeast is accumulation of trehalose (7, 21). This storage carbohydrate is required for the maintenance of good quality with a high fermentative capacity during its shelf life. However, the superiority of carbon-starved cells to nitrogen-starved cells in terms of catabolic capacity could not be attributed to trehalose levels (16). On the contrary, carbon-starved cells were virtually devoid of any storage carbohydrates while both trehalose and glycogen accumulated during nitrogen starvation (16). Other factors that are important for regulation of catabolic activity are the adenine nucleotides (4, 9, 11). For instance, it has been shown that ATP becomes limiting for glycolytic flux at concentrations below about 1 to 1.5 mM (11) while higher ATP levels have an inhibitory effect on the rate of glycolysis (9, 11). The results obtained after addition of glucose to S. cerevisiae cells subjected to carbon or nitrogen starvation suggested that a certain concentration of ATP is required to obtain a certain glycolytic flux (16). However, although ATP is an important player in the intricate regulatory network governing the starvation response in terms of fermentative capacity, it is certainly not the sole determinant.
The starvation response is not only due to the type of starvation, e.g., nitrogen or carbon, but the physiological state of the cells when challenged with this new condition is also of utmost importance. Cells originating from the stationary phase generally show high resistance to different types of stress (25). In fact, long-term survival without access to nutrients requires stationary-phase cells (27). However, the diauxic shift occurring when S. cerevisiae changes metabolism from respiro-fermentative growth on glucose to respiratory ethanol utilization in aerobic batch cultures has also proven to result in very stress-resistant cells (1, 14, 15). The most sensitive, or least tolerant, cells are usually obtained during respiro-fermentative growth on glucose (1, 14, 15). One reason why stationary-phase cells can withstand starvation is that they have faced a gradual nutrient depletion, and this is considered to be a prerequisite for proper establishment of stationary phase (6). The situation is, of course, very different when cells growing exponentially in a batch culture with an unlimited supply of nutrients are suddenly challenged by a starvation condition.
The aim of this investigation was to study the changes in fermentative capacity that occur when cells of S. cerevisiae growing exponentially in anaerobic batch cultures are suddenly subjected to nitrogen or carbon starvation. Cells were starved for 24 h, and their ethanol-producing capacity was subsequently assessed after the addition of glucose. The ethanol production rate was measured in the absence of a nitrogen source to avoid de novo protein synthesis during the test. Seven different strains of S. cerevisiae were compared to check whether the results were strain specific or not. Carbon-starved cells showed an almost complete lack of fermentative capacity, while some ethanol-producing activity remained after nitrogen starvation. In order to elucidate the mechanism(s) causing this difference in response, viability, glucose uptake capacity, glycolytic protein levels, storage carbohydrate accumulation, and ATP content were assessed. Furthermore, as a comparison, the response of stationary-phase cells, as well as the effect of a small addition of glucose during the onset of carbon starvation, was studied.
MATERIALS AND METHODS
Strains and media.
Six different commercially available strains (A to D, F, and G) and laboratory strain Y55 (strain E), kindly provided by Johan Thevelein (Katholieke Universiteit Leuven, Leuven, Belgium), were used. The strains were kept in 20% glycerol at −80°C or on YPD (10 g of yeast extract per liter, 20 g of peptone from meat per liter, 20 g of agar per liter, 20 g of glucose per liter) plates with 2% glucose at 4°C. A defined mineral medium was used and prepared as described by Verduyn et al. (24). The medium contained the following (per liter): glucose, 10 g; (NH4)2SO4, 5 g; KH2PO4, 3 g; MgSO4 · 0.7H2O, 0.5 g; EDTA, 15 mg; ZnSO4 · 0.7H2O, 4.5 mg; CoCl2 · 0.6H2O, 0.3 mg; MnCl2 · 0.4H2O, 1 mg; CuSO4 · 0.5H2O, 0.3 mg; CaCl2 · 0.2H2O, 4.5 mg; FeSO4 · 0.7H2O, 3 mg; NaMoO4 · 0.2H2O, 0.4 mg; H3BO3, 1 mg; KI, 0.1 mg; antifoam 289 (Sigma), 0.1 g; biotin, 0.05 mg; calcium pantothenate, 1 mg; nicotinic acid, 1 mg; inositol, 25 mg; thiamine HCl, 1 mg; pyridoxine HCl, 1 mg; para-aminobenzoic acid, 0.2 mg.
Growth conditions.
Cells were precultured on YPD plates for 48 h. Subsequently, inoculum cultures were prepared by using 100 ml of liquid medium in 300-ml shake flasks incubated with shaking at 150 rpm at 30°C for 24 h. In order to produce cells in a well-controlled and replicate manner, a Braun Biotech Biostat II fermentor (B. Braun Biotech, Melsungen, Germany) with a maximum working volume of 2.5 liters was used. Cultivations were initiated by adding 2 ml of the inoculum culture to 2 liters of medium. The temperature was kept constant at 30°C, the stirring rate was 500 rpm, and the pH was kept constant at 5.0 by automatic addition of 1 M NaOH. Anaerobic conditions were ensured by a flow of N2 gas controlled with a mass flow controller (Bronkhorst High-Tech B.V., Ruurlo, The Netherlands) to 500 ml/min. The exhaust concentration of CO2 in the off gas was measured online with a gas analyzer (Brüel & Kjaer, Naerum, Denmark).
Starvation procedure.
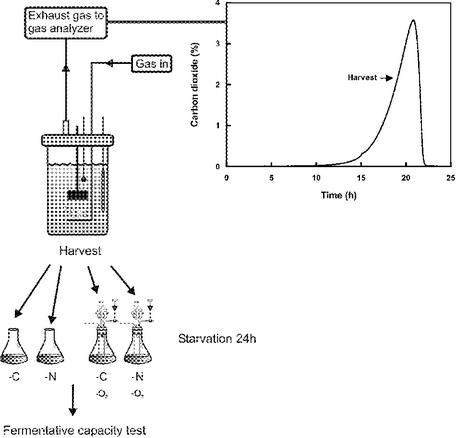
A schematic overview of the starvation tolerance test of the seven strains is seen in Fig. 1. The starvation medium was identical to the growth medium described above, except that nitrogen starvation was performed without addition of (NH4)2SO4 at a glucose concentration of 50 g/liter while glucose was omitted from the carbon starvation medium. Exponentially growing cells were harvested at a cell concentration of 0.5 g/liter (wt/vol) by centrifugation at 4,000 × g for 5 min and washed once with N or C starvation medium. Cells were resuspended in starvation medium to a final density of 1 g/liter (wt/vol) and incubated aerobically or anaerobically for 24 h at 30°C. Aerobically starved cells were incubated in 250-ml E flasks at 150 rpm, while anaerobic starvation was performed with an experimental setup described in reference 19. This consisted of sealed 100-ml flasks with thick rubber stoppers through which two steel capillaries for incoming gas and sampling, respectively, were inserted. A glass tube with a loop trap with 90% glycerol was also inserted through the rubber stopper to allow gas to flow out and to avoid diffusion of oxygen into the flask. Each flask was connected to a main tube with N2 gas. Stationary-phase cells were harvested 4 h after glucose exhaustion; otherwise, carbon and nitrogen starvation was performed as outlined above.
FIG. 1.
Schematic description of the experimental setup used in this study.
Fermentative capacity test.
Assessment of fermentative capacity was performed with a test medium identical to the growth medium, except that no nitrogen source was present. The reason for this was that we wanted to measure the fermentative capacity of the cells after 24 h of carbon or nitrogen starvation without involvement of de novo protein synthesis during the test. The cells were harvested by centrifugation at 4,000 × g for 5 min, washed once, and resuspended in test medium. Cells were incubated at 30°C, and glucose was added to a final concentration of 10 g/liter. Extracellular samples (2 × 1.5 ml) for measurement of ethanol production were collected every 10 min by centrifugation for 2 min at 16,000 × g or by sterile filtering (0.22 μm). The supernatant or filtrate was frozen in liquid nitrogen and stored at −20°C prior to ethanol analysis by high-performance liquid chromatography (HPLC) or enzyme combination kits (Biochemica Test Combination; Boehringer Mannheim GmbH, Mannheim, Germany) if indicated. The ethanol production of each strain was normalized to the dry weight of the culture at the initiation of starvation.
Sampling and HPLC analysis of extracellular metabolites.
Extracellular samples (2 × 1.5 ml) were obtained from the fermentor by filtration (0.22 μm). The filtrate was frozen in liquid nitrogen and stored at −20°C. The concentrations of glucose, ethanol, glycerol, and acetate were analyzed by HPLC with an Aminex HPX87H column (Bio-Rad Laboratories, Hercules, Calif.) at 60°C, preceded by a precolumn. The flow of eluent (5 mM H2SO4) was 0.6 ml/min, and the sample injection volume was 20 μl. For detection and quantification of the different metabolites, the chromatograms obtained by a refractive-index detector (Waters 410; Millipore, Milford, Mass.) were analyzed with the Millennium32 software. The ethanol measurements for determination of fermentative capacity were analyzed with a sample volume of 50 μl.
Biomass determination.
Samples of 2 × 5 or 2 × 10 ml were centrifuged in preweighed tubes for 5 min at 2,300 × g, washed twice with deionized water, dried for 24 h at 110°C, and stored in a desiccator before weighing.
The optical density of the cultures at 610 nm was monitored and used, together with CO2 analysis, for determination of the harvesting point, as well as estimation of the maximum growth rate (μmax).
Determination of glycogen and trehalose.
Two duplicate samples with a content of 20 to 30 mg of cells were centrifuged for 5 min at 4,000 × g and washed twice with cold 0.9% NaCl. The pellet was frozen in liquid nitrogen and stored at −20°C. For analysis, pellets were resuspended in 2 ml of 0.2 M citrate buffer (pH 4.8) and homogenized with 1 g of glass beads (diameter, 0.05 mm) for 30 min at 4°C in a water-cooled cell homogenizer (Vibrogen Zellmühle; Edmund Bühler, Tübingen, Germany). Samples were subsequently centrifuged for 5 min at 3,000 × g, and the supernatants were analyzed for glycogen and trehalose by incubation at 37°C overnight with amyloglucosidase (1.4 U/ml in 0.2 M citrate buffer [pH 4.8]) or trehalase (0.1 U/ml in 0.2 M citrate buffer [pH 5.7]), respectively. The resulting glucose was determined with enzyme combination kits (Biochemica Test Combination; Boehringer Mannheim GmbH).
CFU determination.
Two 0.5-ml volumes were sampled, and after proper dilution in 0.9% NaCl, 100-μl aliquots were spread on YPD plates and incubated at 30°C for 72 h.
Uptake capacity measurements.
Glucose uptake capacity was measured as described by Walsh et al. (26), with some minor modifications. Samples (50 ml from unstarved cultures or 25 ml from starved cultures) were harvested by centrifugation for 5 min at 4,000 × g and washed once in growth medium lacking a carbon and nitrogen source. The pellets were resuspended in 5 ml of medium (lacking a carbon and nitrogen source) and flushed with either air or nitrogen, depending on whether the cells were originating from aerobic or anaerobic conditions. An aliquot of cell suspension (50 μl) was mixed with 12.5 μl of a solution containing 100 mM potassium buffer (pH 6.5) and 14C-labeled glucose at a final concentration of 50 mM (20 μCi/ml) or 10 mM (10 μCi/ml). The mixture was incubated for 5 s at 30°C, and uptake was subsequently terminated by transfer of 50 μl of the mixture to 10 ml of quench buffer containing 100 mM potassium buffer (pH 6.5) and 500 mM unlabeled glucose maintained at a temperature below −5°C on salt-ice. Cells were collected and washed on glass fiber filters with 2 × 10 ml of quench buffer. Radioactivity was determined with a Beckman liquid scintillation counter. Five measurements at each of two glucose concentrations, 10 and 50 mM, were made. The rate of glucose uptake was normalized against the protein content, which was determined as described by Lowry.
2D-PAGE analysis of glycolytic protein patterns.
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) was performed as described by Norbeck and Blomberg (18). Silver staining and quantification were performed as described by Nilsson et al. (16). The protein map of S. cerevisiae can be seen on the World Wide Web server (http://yeast-2DPAGE.gmm.gu.se).
Determination of intracellular ATP.
Samples (2 × 1 ml) were extracted with trichloroacetic acid as previously described (5). Analyses were carried out with a CLSII ATP bioluminescence assay kit (Boehringer Mannheim GmbH) and a Packard Pico-Lite luminometer (Packard Instruments).
RESULTS
In order to study strain specificity, six industrial strains and one laboratory strain were compared for biomass and product formation in anaerobic batch cultures and their responses to sudden carbon or nitrogen starvation in mid-log-phase. Batch cultures were always performed under anaerobic conditions, while aerobic and anaerobic conditions were used for comparison during the subsequent 24-h starvation period (Fig. 1).
Strain performance during anaerobic growth in the fermentor.
The μmax, estimated on the basis of spectrophotometric measurements, varied between 0.34 and 0.42 h−1 (Table 1). The yields of biomass and the main extracellular products ethanol and glycerol were similar among the different strains, except strains D and E, which both showed a slightly higher yield of ethanol. The yield of acetate (data not shown) varied between 0.002 and 0.010 g/g of glucose. The intracellular amounts of the storage carbohydrates trehalose and glycogen at the time of harvesting were measured and were, at the most, 0.13 and 0.20% of the dry weight, respectively, but were, in general, so low that no strain differences could be distinguished. The fermentative capacity measured in harvested cells before starvation also showed no significant difference in performance among the strains (Table 1).
TABLE 1.
Strain comparison of growth data and fermentative capacity of unstarved cellsa
| Strain | μmax | Yx/s | Ye/s | Ygly/s | Glycogen | Trehalose | Fermentative capacity |
|---|---|---|---|---|---|---|---|
| A | 0.40 ± 0.03 | 0.10 ± 0.03 | 0.34 ± 0.00 | 0.11 ± 0.00 | 0.03 ± 0.03 | 0.10 ± 0.03 | 16.7 ± 6.3 |
| B | 0.34 ± 0.01 | 0.10 ± 0.00 | 0.35 ± 0.00 | 0.09 ± 0.00 | 0.15 ± 0.11 | 0.05 ± 0.04 | 20.8 ± 4.0 |
| C | 0.38 ± 0.00 | 0.11 ± 0.01 | 0.35 ± 0.01 | 0.10 ± 0.00 | 0.08 ± 0.04 | 0.06 ± 0.03 | 26.8 ± 2.4 |
| D | 0.38 ± 0.02 | 0.11 ± 0.01 | 0.37 ± 0.01 | 0.09 ± 0.00 | 0.20 ± 0.13 | 0.04 ± 0.02 | 22.8 ± 1.1 |
| E | 0.42 ± 0.02 | 0.11 ± 0.01 | 0.37 ± 0.01 | 0.11 ± 0.00 | 0.10 ± 0.04 | 0.13 ± 0.08 | 19.6 ± 6.0 |
| F | 0.35 ± 0.02 | 0.12 ± 0.01 | 0.33 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.07 ± 0.04 | 21.0 ± 1.1 |
| G | 0.39 ± 0.01 | 0.10 ± 0.01 | 0.34 ± 0.00 | 0.10 ± 0.00 | 0.01 ± 0.01 | 0.04 ± 0.04 | 20.4 ± 0.4 |
Minimum and maximum values originated from two independent experiments. Biomass yield (Yx/s [grams of biomass per gram of glucose]) ethanol yield (Ye/s [grams of ethanol per gram of glucose]) and glycerol yield (Ygly/s [grams of glycerol per gram of glucose]) were determined after glucose depletion. Trehalose and glycogen (percentage of dry weight) were measured at the time of harvesting and initiation of starvation. Fermentative capacity prior to starvation (millimoles of ethanol per gram of dry weight per hour) was determined at the time of harvesting and initiation of starvation.
Fermentative capacity after starvation. (i) Carbon starvation.
After 24 h of carbon starvation, the ethanol production of carbon-starved cells was severely decreased. Enzymatic assessment of ethanol concentrations during the fermentative capacity test following carbon starvation was performed for strains A, D, E, and F. Strains A and F exhibited very small increases, and 50 min after glucose addition, the ethanol concentrations had reached 0.04 ± 0.04 and 0.002 ± 0.000 g/liter, respectively, for aerobically and anaerobically starved cells of strain A. The corresponding concentrations for strain F were 0.02 ± 0.02 and 0.01 ± 0.01 g/liter. Strains D and E showed nonmeasurable amounts of ethanol. Hence, the fermentative capacity of all of the strains tested was virtually zero after carbon starvation.
(ii) Nitrogen starvation.
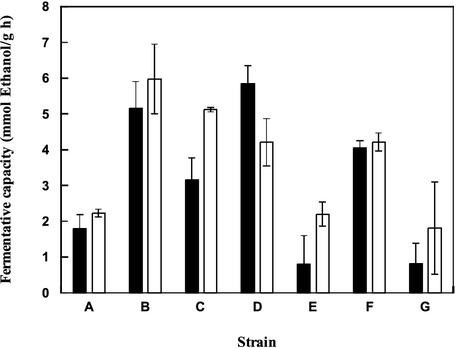
Nitrogen starvation also caused a decrease in fermentative capacity. Depending on the strain, starved cells retained between 5 and 30% of their fermentative capacity before starvation (Fig. 2). However, these rates are considerably higher than those of carbon-starved cells. There was no significant difference in fermentative capacity after aerobic or anaerobic starvation (Fig. 2). Among the different strains, it seemed that B, C, D, and F were fairly tolerant while A, E, and G were the most sensitive. In order to compare a tolerant and a sensitive strain, A and B were chosen for analysis of viability and glucose uptake capacity before and after starvation, respectively.
FIG. 2.
Comparison of the fermentative capacities of different strains of S. cerevisiae after aerobic and anaerobic nitrogen starvation. Cells were cultivated in anaerobic batch cultures, harvested in mid-log phase, and starved in the presence (black bars) or absence (white bars) of oxygen for 24 h. Error bars indicate minimum and maximum values from two independent experiments.
Viability of starved cells.
Because of the severely decreased ethanol production capacity after starvation for both carbon and nitrogen, the question of whether the cells were actually not viable anymore arose. However, during nitrogen starvation, there was, in fact, a small increase in the concentration of viable cells (Table 2). Carbon starvation, on the other hand, resulted in quite a substantial reduction in CFU. The most significant reduction was obtained for strain A, approximately 1% of whose cells survived this treatment, while the corresponding number for strain B was 4%. Nevertheless, it should be noted that even the samples subjected to carbon starvation contained a considerable amount of viable cells after a starvation period of 24 h (Table 2).
TABLE 2.
Effect of 24 h of carbon and nitrogen starvation on the viability of strains A and Ba
| Strain | Unstarved | C starvedb | N starvedb |
|---|---|---|---|
| A | 8.6 · 106 ± 4.4 · 106 | 6.5 · 104 ± 6.4 · 104 | 1.6 · 107 ± 3.5 · 106 |
| B | 5.2 · 106 ± 2.1 · 106 | 1.8 · 105 ± 8.8 · 104 | 1.0 · 107 ± 3.8 · 106 |
The results (CFU per milliliter) are mean values of two independent experiments for each strain, and the minimum and maximum values are indicated.
Starvation was performed anaerobically.
Glucose uptake capacity before and after starvation.
The glucose uptake capacity before and after carbon and nitrogen starvation was determined at two different glucose concentrations, 10 and 50 mM, of which the latter corresponds to the concentration chosen for the fermentative capacity tests. Both strains A and B showed a decreased but still substantial uptake capacity after the starvation period (Table 3). At a glucose concentration of 50 mM, a similar uptake capacity was measured, irrespective of the starvation conditions. In other words, the very different fermentative activities recorded following carbon and nitrogen starvation, respectively, were not reflected by a difference in the ability to transport glucose. Furthermore, the higher fermentative capacity of strain B than strain A after nitrogen starvation (Fig. 2) was not accompanied by a superior glucose uptake capacity of the former strain (Table 3). For both strains, it was found that cells subjected to carbon starvation responded by a stronger reduction in the uptake rate than that of nitrogen-starved cells when 10 mM glucose was used in the assay (Table 3).
TABLE 3.
Glucose uptake capacities before and after 24 h of carbon and nitrogen starvation of strains A and Ba
| Strain, glucose concn (mM) | Uptake (μmol/g of protein/min)
|
||
|---|---|---|---|
| Unstarved | C starved | N starved | |
| A, 50 | 305 ± 15 | 138 ± 6b | 120 ± 4b |
| 186 ± 13c | 152 ± 3c | ||
| B, 50 | 290 ± 7 | 122 ± 9b | 136 ± 30b |
| 118 ± 13c | 151 ± 9c | ||
| A, 10 | 122 ± 4 | 60 ± 4b | 101 ± 11b |
| 75 ± 5c | 105 ± 5c | ||
| B, 10 | 151 ± 6 | 64 ± 5b | 89 ± 5b |
| 54 ± 1c | 111 ± 12c | ||
The assay was performed with two different concentrations of glucose, 50 and 10 mM. The uptake rate was determined five times for each concentration, and means and minimum and maximum values are indicated.
Starvation was performed anaerobically.
Starvation was performed aerobically.
Glycolytic enzyme levels before and after starvation.
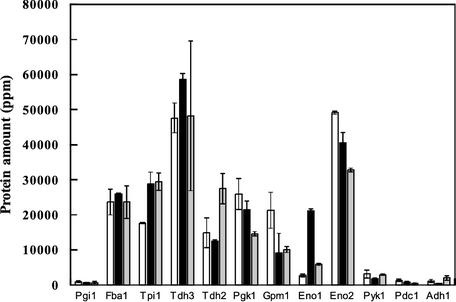
2D-PAGE analysis was used for determination of glycolytic enzyme levels in strain A before and after starvation. The levels of most enzymes, apart from some isoenzymes, are shown in Fig. 3. However, phosphofructokinase and hexokinase (Hxkp) levels could not be determined because the position is not identified (phosphofructokinase) or it could not be identified with certainty in this strain (Hxkp). Irrespective of the starvation condition, most glycolytic enzymes were present at levels similar to those of unstarved cells. The enzyme that was most affected by starvation was enolase 1 (Eno1p). Nitrogen starvation provoked an abundance of Eno1p eight times greater than that in unstarved cells, whereas in carbon-starved cells, a twofold increase was observed (Fig. 3).
FIG. 3.
Glycolytic protein levels of strain A analyzed by 2D-PAGE before starvation (white bars) and after nitrogen (black bars) or carbon (grey) starvation. Error bars indicate minimum and maximum values from two duplicated samples.
Carbon starvation response of log-phase cells compared to that of stationary-phase cells.
The severe loss of fermentative capacity observed when cells growing anaerobically were subjected to carbon starvation was rather surprising since investigations performed aerobically seemed to suggest the opposite result, i.e., that fermentative capacity is preserved to a larger extent during carbon starvation than during nitrogen starvation (2, 10, 16). Since it is known that stationary-phase cells often have a higher tolerance to stress than cells in other physiological states, the carbon starvation response of anaerobically cultivated stationary-phase cells of strain A was also tested. Indeed, the fermentative capacity of carbon-starved stationary-phase cells turned out to be almost identical to that of nonstarved stationary- and log-phase cells (Table 4). One explanation for the superior starvation tolerance of stationary-phase cells could be that they have faced a gradual nutrient depletion and thus managed to adapt successfully to a starvation condition (cf. references 6 and 27). Another important factor may be the accumulation of storage carbohydrates that could serve as energy sources required for proper adaptation to the starvation conditions. The cells in stationary phase had accumulated around 0.8 and 0.2% (grams per gram of dry weight) glycogen and trehalose, respectively, which is higher than the levels obtained in log-phase cells (Table 1).
TABLE 4.
Fermentative capacity of log-phase and stationary-phase cells of strain A after 24 h of carbon starvation
| Condition | Mean fermentative capacity (mmol/g h)a |
|---|---|
| Unstarved log-phase cells | 16.7 ± 6.3 |
| Carbon-starvedb log-phase cells | 1.41 ± 0.11 |
| Unstarved stationary-phase cells | 16.6 ± 3.3 |
| Carbon-starved stationary-phase cells | 13.8 ± 1.3 |
Fermentative capacity was determined in two independent experiments, and the minimum and maximum values are indicated.
The values for carbon-starved log-phase cells were obtained by addition of 0.1 g of glucose per liter at the initiation of starvation.
Effect of a small addition of glucose at the onset of carbon starvation.
Since the stationary-phase cells had a much higher tolerance to carbon starvation and also had a somewhat larger amount of accumulated carbohydrates, the question arose of whether the exponentially growing cells subjected to sudden carbon depletion also faced a loss of intracellular energy. An initial concentration of 0.1 g of glucose per liter was therefore added into the otherwise carbon-free starvation medium at the onset of starvation for strain A. This amount was chosen to reflect the amount accumulated after 24 h of nitrogen starvation of an aerobic batch culture sampled at the start of ethanol respiration (16). The glucose addition led to an increased carbon starvation tolerance, and the cells retained almost 10% of the fermentative capacity recorded before starvation (Table 4). In fact, these fermentative capacities, obtained after carbon starvation, were comparable to or even higher than the values recorded after nitrogen starvation.
Intracellular ATP concentrations.
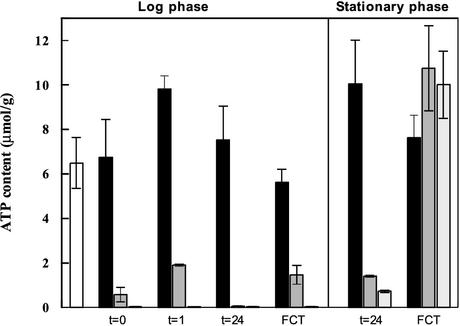
Exponentially growing cells already showed a very rapid drop in ATP content when challenged with carbon starvation conditions during harvesting (Fig. 4). It should be noted that washing of the cells during harvesting was performed with the respective starvation medium. Addition of 0.1 g of glucose per liter to the carbon starvation medium enabled the cells to maintain a slightly higher ATP level during harvesting. However, cells subjected to nitrogen starvation were really superior at maintaining a high ATP content, with a level indistinguishable from that recorded before starvation (Fig. 4). One hour of nitrogen or carbon starvation (in the presence of 0.1 g of glucose per liter) resulted in an elevated ATP content compared to the levels found during harvesting. Carbon-starved cells, on the other hand, reduced the already low ATP values even further. Prior to the fermentative capacity test, after 24 h of starvation, the carbon-starved cells showed very low ATP levels, with values of 0.05 ± 0.02 and 0.02 ± 0.01 μmol/g of dry weight with or without an initial addition of 0.1 g of glucose per liter, respectively. Nitrogen-starved cells maintained a high ATP content throughout the duration of the starvation treatment (Fig. 4). Despite the similar and very low ATP content during carbon starvation, cells that were provided with a small amount of glucose at the onset of starvation responded with a substantial increase in the ATP level when glucose was added during the fermentative capacity test. Carbon-starved cells that were not provided glucose at the onset of starvation did not manage to significantly increase the ATP content by addition of glucose. Stationary-phase cells showed elevated ATP levels compared to those of exponentially growing cells after 24 h of starvation, irrespective of the condition (Fig. 4). This was especially pronounced in cells subjected to carbon starvation. Furthermore, stationary-phase cells subjected to carbon starvation managed to increase the ATP content as a result of glucose addition during the fermentative capacity test to values similar to those recorded for nitrogen-starved cells. The effect of a small addition of glucose at the onset of carbon starvation seemed to be less important for stationary-phase cells than for exponentially growing cells (Fig. 4). These results were obtained by using strain A, but the experiments with exponentially growing cells were repeated with strain B with qualitatively the same results (data not shown).
FIG. 4.
Intracellular ATP contents of log- and stationary-phase cells of strain A before starvation (open column), during nitrogen starvation (black column) or carbon starvation with the addition of 0.1 g of glucose per liter (grey column), and during carbon starvation (light grey columns). t = 0 was measured immediately after harvest, t = 1 and t = 24 were measured after 1 and 24 h, respectively, of starvation, and the fermentative capacity was measured 1 h after addition of glucose with the fermentative capacity test (FCT). Error bars indicate minimum and maximum values from two independent experiments.
DISCUSSION
This study showed that cells of S. cerevisiae cultivated in anaerobic batch cultures and harvested in the exponential phase during growth on glucose were extremely sensitive to carbon starvation. The fermentative capacity dropped to virtually zero after 24 h of carbon starvation. Nitrogen starvation, on the other hand, did not provoke such a drastic reduction in fermentative capacity. This is in contrast to results obtained with cells originating from aerobic batch cultures, where nitrogen starvation induces a larger decrease in fermentative capacity compared to carbon starvation (2, 10, 16). The inactivation of glucose transporters that is known to occur during nitrogen starvation in the presence of a fermentable carbon source (2) is often considered responsible for the decrease in fermentative capacity that occurs under these conditions. However, nitrogen starvation in the presence of a nonfermentable carbon source such as ethanol still provoked a larger reduction in catabolic capacity in nitrogen-starved cells than in carbon-starved cells (16). Similarly, the inactivity observed during the fermentative capacity test following carbon starvation in the present study was not due to a lack of glucose transport capacity. In fact, very similar transport capacities were recorded irrespective of the starvation condition.
It is also striking that despite the large difference in fermentative capacity between carbon- and nitrogen-starved cells, the levels of glycolytic enzymes were remarkably similar, with the exception of Eno1p. This enzyme was extensively induced during nitrogen starvation but also to some extent during carbon starvation. It has been shown previously that Eno1p is induced under salt stress (17) and also when entering the stationary phase (3, 12). However, a similar induction of Eno1p has not been observed during starvation of cells originating from aerobic batch cultures (13, 15, 16). Furthermore, it is difficult to envisage that the fermentative capacity of carbon- and nitrogen-starved cells is connected to differences in the level of Eno1p.
The reason for the inactivity of carbon-starved cells recorded during the fermentative capacity test was almost surely energy deprivation and secondary effects thereof, such as an inability to adapt to starvation conditions. There was a very rapid drop in ATP content when anaerobic cells, growing exponentially, were suddenly subjected to carbon starvation. The ATP concentration after 24 h of carbon starvation could be estimated to only about 0.01 to 0.03 mM (by assuming an intracellular volume of 2 ml/g of dry weight [11]). Such levels are far below what has previously been reported to impose a limitation on glycolytic flux in S. cerevisiae (11). In fact, the concentrations are even well below the Km of 0.15 mM reported for ATP as a substrate of Hxkp (20) in the initial glycolysis reaction. Carbon starvation for 24 h resulted in similar ATP contents irrespective of whether glucose was added at the onset of starvation or not. However, addition of a small amount of glucose (0.1 g/liter) during initiation of carbon starvation enabled the cells to enhance the ATP level when glucose was added during the fermentative capacity test. Without this addition, the cells failed to increase the ATP level when glucose became available. This was also reflected in the ethanol production rates measured in the fermentative capacity test. Cells starved for carbon without the addition showed no ethanol production in the fermentative capacity test, whereas with the addition, a substantial ethanol formation rate was observed.
The beneficial effect of a small glucose addition at the onset of carbon starvation may occur because the necessary adaptations required for successful survival during starvation require energy. Exponentially growing cells are almost completely devoid of storage carbohydrates, and a sudden change to carbon (and energy source) starvation will render the cells incapable of making the necessary adaptations. It can be argued that the glucose addition will induce a short growth period and that the cells are, in fact, similar to stationary-phase cells. However, only 0.1 g of glucose per liter was added to a culture at a density of 1.0 g/liter, which is only enough to support the growth of a fraction of a new generation. The reason for the superior ability of stationary-phase cells to withstand starvation (25, 27) is perhaps that the gradual nutrient depletion ensures that the necessary energy-requiring adaptations for long-term survival during starvation are executed.
To summarize, this study showed that the sensitivity of S. cerevisiae to sudden carbon starvation when growing exponentially under anaerobic conditions is a general feature of this species. All of the strains tested showed that carbon-starved cells remained inactive when exposed to glucose after 24 h of starvation. This inactivity occurred even though the cells possessed a substantial glucose transport capacity. Instead, a lack of energy was identified as the most likely explanation for this behavior.
Acknowledgments
This work was supported by a grant from The Swedish Research Council (dnr 285-2000-656).
Anders Blomberg is gratefully acknowledged for constructive criticism concerning 2D-PAGE analysis.
REFERENCES
- 1.Blomberg, A., C. Larsson, and L. Gustafsson. 1988. Microcalorimetric monitoring of growth of Saccharomyces cerevisiae: osmotolerance in relation to physiological state. J. Bacteriol. 170:4562-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busturia, A., and R. Lagunas. 1986. Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J. Gen. Microbiol. 132:379-385. [DOI] [PubMed] [Google Scholar]
- 3.Carmen, A. A., P. K. Brindle, C. S. Park, and M. J. Holland. 1995. Transcriptional regulation by an upstream repression sequence from the yeast enolase gene ENO1. Yeast 11:1031-1043. [DOI] [PubMed] [Google Scholar]
- 4.Gancedo, C., and R. Serrano. 1989. Energy-yielding metabolism, p. 206-259. In A. H. Rose and J. S. Harrison (ed.), The yeasts, vol. 3. Academic Press Limited, London, England.
- 5.Gustafsson, L. 1979. The ATP pool in relation to the production of glycerol and heat during growth of the halotolerant yeast Debaryomyces hansenii. Arch. Microbiol. 120:15-23. [DOI] [PubMed] [Google Scholar]
- 6.Jona, G., M. Choder, and O. Gileadi. 2000. Glucose starvation induces a drastic reduction in the rates of both transcription and degradation of mRNA in yeast. Biochim. Biophys. Acta 1491:37-48. [DOI] [PubMed] [Google Scholar]
- 7.Kim, J., P. Alizadeh, T. Harding, A. Hefner-Gravink, and D.-J. Klionsky. 1996. Disruption of the yeast ATH gene confers better survival after dehydration, freezing and ethanol shock—potential commercial applications. Appl. Environ. Microbiol. 62:1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagunas, R., C. Dominguez, A. Busturia, and M. J. Saez. 1982. Mechanism of appearance of the Pasteur effect in Saccharomyces cerevisiae: inactivation of sugar transport systems. J. Bacteriol. 152:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson, C., A. Nilsson, A. Blomberg, and L. Gustafsson. 1997. Glycolytic flux is conditionally correlated with ATP concentration in Saccharomyces cerevisiae: a chemostat study under carbon- or nitrogen limiting conditions. J. Bacteriol. 179:7243-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson, C., A. Nilsson, and L. Gustafsson. 1994. Catabolic capacity of carbon- or nitrogen-starved cultures of Saccharomyces cerevisiae, p. 232-237. In E. Gnaiger, F. N. Gellerich, and M. Wyss (ed.), Modern trends in BioThermoKinetics, vol. 3. Innsbruck University Press, Innsbruck, Austria.
- 11.Larsson, C., I.-L. Påhlman, and L. Gustafsson. 2000. The importance of ATP as a regulator of glycolytic flux in Saccharomyces cerevisiae. Yeast 16:797-809. [DOI] [PubMed] [Google Scholar]
- 12.McAlister, L., and M. J. Holland. 1982. Targeted deletion of a yeast enolase structural gene: identification and isolation of yeast enolase isozymes. J. Biol. Chem. 257:7181-7188. [PubMed] [Google Scholar]
- 13.Nilsson, A. 2001. Fermentative capacity of the yeast Saccharomyces cerevisiae during growth and starvation. Gothenburg University, Gothenburg, Sweden.
- 14.Nilsson, A., C. Larsson, and L. Gustafsson. 1995. Catabolic capacity of Saccharomyces cerevisiae in relation to the physiological state and maintenance requirement. Thermochim. Acta 250:233-245. [Google Scholar]
- 15.Nilsson, A., J. Norbeck, R. Oelz, A. Blomberg, and L. Gustafsson. 2001. Fermentative capacity after cold storage of bakers' yeast is dependent on the initial physiological state but not correlated to the levels of glycolytic enzymes. Int. J. Food Microbiol. 71:111-124. [DOI] [PubMed]
- 16.Nilsson, A., I.-L. Påhlman, P.-Å. Jovall, A. Blomberg, C. Larsson, and L. Gustafsson. 2001. The catabolic capacity of Saccharomyces cerevisiae is preserved to a higher extent during carbon compared to nitrogen starvation. Yeast 18:1371-1381. [DOI] [PubMed] [Google Scholar]
- 17.Norbeck, J., and A. Blomberg. 1997. Metabolic and regulatory changes associated with growth of Saccharomyces cerevisiae in 1.4 M NaCl: evidence for osmotic induction of glycerol dissimilation via the dihydroxyacetone pathway. J. Biol. Chem. 272:5544-5554. [DOI] [PubMed] [Google Scholar]
- 18.Norbeck, J., and A. Blomberg. 1997. Two-dimensional electrophoretic separation of yeast proteins using a non-linear wide range (pH 3-10) immobilized pH gradient in the first dimension: reproducibility and evidence for isoelectric focusing of alkaline (pI >7) proteins. Yeast 13:1519-1534. [DOI] [PubMed] [Google Scholar]
- 19.Taherzadeh, M. J., R. Eklund, L. Gustafsson, C. Niklasson, and G. Lidén. 1997. Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind. Eng. Chem. Res. 36:4659-4665. [Google Scholar]
- 20.Teusink, B., J. Passarge, C. A. Reijenga, E. Esgalhado, C. C. van der Weijden, M. Schepper, M. C. Walsh, B. M. Bakker, K. van Dam, H. V. Westerhoff, and J. L. Snoep. 2000. Can yeast glycolysis be understood in terms of in vitro kinetics of the constituent enzymes? Testing biochemistry. Eur. J. Biochem. 267:5313-5329. [DOI] [PubMed] [Google Scholar]
- 21.van Hoek, P. 2000. Fermentative capacity in aerobic cultures of bakers' yeast. Delft University of Technology, Delft, The Netherlands.
- 22.van Hoek, P., J. P. van Dijken, and J. T. Pronk. 2000. Regulation of fermentative capacity and levels of glycolytic enzymes in chemostat cultures of Saccharomyces cerevisiae. Enzyme Microb. Technol. 26:724-736. [DOI] [PubMed] [Google Scholar]
- 23.van Hoek, P., J. P. van Dijken, and J. T. Pronk. 1998. Effect of specific growth rate on fermentative capacity of baker's yeast. Appl. Environ. Microbiol. 64:4226-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 25.Walker, G. M. (ed.). 1998. Yeast physiology and biotechnology. John Wiley & Sons, Chichester, England.
- 26.Walsh, M. C., H. P. Smits, M. Scholte, and K. van Dam. 1994. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J. Bacteriol. 176:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner-Washburne, M., E. Braun, G. Johnston, and R. Singer. 1993. Stationary phase in Saccharomyces cerevisiae. Microbiol. Rev. 57:384-410. [DOI] [PMC free article] [PubMed] [Google Scholar]