Abstract
Increased resistance to apoptosis promotes lymphomagenesis with aberrant expression of cell survival proteins such as BCL-2 and c-MYC occurring in distinct lymphoma subtypes. Galectin-3 is an anti-apoptotic protein that protects T cells, macrophages, and breast carcinoma cells from death triggered by a variety of agents. We have found high levels of galectin-3 protein expression in a subset of B-cell neoplasms including diffuse large B-cell lymphoma (DLBCL), primary effusion lymphoma (PEL), and multiple myeloma (MM), in both cell lines and patient samples. However, we failed to detect galectin-3 in Burkitt lymphoma (BL), follicular lymphoma (FL), marginal zone lymphoma (MZL), MALT lymphoma or B-small lymphocytic lymphoma (B-SLL) cell lines or patient samples. To determine whether galectin-3 expression protects B cells from apoptosis, galectin-3-negative BL cells were transfected with a galectin-3 expressing plasmid, which resulted in markedly increased resistance to anti-Fas-induced cell death. In contrast, galectin-3-positive PEL cells transfected with an amino-terminal truncated galectin-3 vector showed increased sensitivity to anti-Fas induced apoptosis. During normal B-cell development, galectin-3 expression was lowest in germinal center and plasma B cells, from which DLBCL, PEL, and MM derive, and highest in long-lived naïve and memory B cells. This pattern of expression suggests that aberrantly increased galectin-3 levels in specific B-cell populations may yield a protective advantage during transformation and/or progression of certain B-cell neoplasms.
Non-Hodgkin lymphoma (NHL) is the fifth most common cancer in the United States. Ten to twenty percent of immune-compromised individuals, such as those with AIDS, develop NHL, usually of B-cell origin (B-NHL). While B-NHL is a common malignancy, transformation of peripheral B cells at different stages of development results in biologically and morphologically distinct B-NHL subtypes. In addition, tumor heterogeneity in B-NHL is further enhanced by accumulation of genetic and epigenetic alterations during malignant progression.1–3 Successful treatment of B-NHL varies widely, depending on the subtype of lymphoma and stage of tumor progression. For example, only about 40% of patients with diffuse large B-cell lymphoma (DLBCL) respond well to current therapy, while 50% to 85% of patients with follicular lymphoma (FL) are disease-free 5 years following treatment.4 The variable response to therapy in B-NHL likely reflects the distinct etiology and genetic heterogeneity of the different subtypes of lymphomas that comprise the disorder.
Heterogeneous responses to lymphoma therapy occur since B-NHL is typically classified according to morphology alone, and lymphomas that are morphologically similar may contain distinct molecular abnormalities. Recent advances in gene expression profiling have allowed an improved classification of lymphomas beyond morphological appearance; this approach may more closely reflect the distinct biological characteristics and treatment outcomes observed within and between categories. For example, gene expression profiling has resulted in the subdivision of morphologically indistinguishable DLBCL into germinal center (GC) B-like DLBCL and activated B-like DLBCL.5 These newly identified subtypes of DLBCL have different cellular origins, different responses to therapy, and distinct clinical outcomes and prognoses. Alternatively, the gene grouping strategy for DLBCL by Shipp et al, 6 correlated gene expression profiles with disease outcome, independent of morphological categorization. These and other gene profiling strategies have identified genes involved in distinct biological processes that promote lymphomagenesis, including genes involved in cellular proliferation, metastasis, and resistance to apoptosis.
Increased resistance of cells to apoptotic stimuli appears to be essential to the transformation process. One of the earliest examples that increased cell survival plays an important role in lymphomagenesis was the discovery of the anti-apoptotic protein BCL-2 and the increased expression of BCL-2 in FL (reviewed in7–10). Virtually all cases of Burkitt lymphoma (BL) contain translocations of c-MYC; the c-MYC protein normally regulates proliferation and cell survival, but when aberrantly expressed causes increased cell cycle progression.11,12 In addition, 60% to 80% of BL contain mutations in the p53 tumor suppressor gene, thereby inactivating p53-dependent apoptosis.13 It is becoming apparent that specific lymphoma subtypes are associated with alterations of specific regulators of apoptosis. However, factors that contribute to apoptotic resistance in B-lymphoma subtypes other than FL and BL are mainly lacking.
The galectins are a family of multifunctional proteins that regulate apoptosis (reviewed in14,15). Galectins bind oligosaccharide ligands on cell surface glycoproteins and glycolipids and are defined by a canonical carbohydrate recognition domain. Galectins have a number of extracellular (signaling, adhesion) and intracellular (signaling, transcription) functions.16,17 Several galectin family members, including 1, 7, 8, 9, and 12, promote apoptosis.18–22 In contrast, galectin-3 is the only member of the galectin family that has an anti-apoptotic function. Over-expression of galectin-3 in Jurkat T cells protected the cells from Fas- and staurosporine-induced cell death23 and galectin-3 overexpression in breast carcinoma cells protected the cells from apoptosis induced by cis-platin, cyclohexamide, nitric oxide, and UV treatments.24–28 In addition, decreased survival of peritoneal macrophages in galectin-3 null mice has been observed.29
Studies from several laboratories have suggested a role for galectin-3 in epithelial cell proliferation, malignant transformation and metastases.30–32 Galectin-3 also has diverse roles in T-cell, macrophage, and eosinophil activities, but it is not clear what role(s) galectin-3 plays in B-cell biology or transformation.14 Using a gene array approach, Shipp et al6 detected higher galectin-3 mRNA expression in DLBCL compared to low-grade FL. However, the increased expression of galectin-3 protein in DLBCL that would correlate with increased galectin-3 gene expression has not been demonstrated,33,34 nor has a functional role for galectin-3 in B-lymphoma cell survival been addressed. To determine a potential role for galectin-3 in B-cell transformation, we surveyed broad panels of transformed B-cell lines and primary B-NHL, along with normal B-cell subsets, for galectin-3 expression and evaluated the ability of galectin-3 to protect B cells from apoptosis.
Materials and Methods
Cell Lines
Cell lines were maintained at 37°C in 5% CO2 and grown in RPMI 1640 supplemented with 2 mmol/L L-glutamine and 15% fetal bovine serum. BL and DLBCL cell lines 2F7, E, R, and RRBL were kind gifts from B. Bonavida35,36; BL cell line P3HR-1 was from O. Martinez-Maza; BL cell lines BL41, BL41/B95-8, BJAB and BJAB-B1, along with primary effusion lymphoma (PEL) lines BCBL-1, BC-1, BC-3 and KS-1 were provided by R. Sun; and DLBCL cell line KS-2 was from J. Said (UCLA School of Medicine, Los Angeles, CA). BL cell line Akata A1.5 was kindly provided by J. T. Sample (University of Tennessee Health Sciences Center, Memphis, TN). The BL cell lines Ramos and Raji were purchased from ATCC. The following DLBCL cell lines were purchased from A. Epstein (University of Southern California, Norris Cancer Center, Los Angeles, CA): NU-DHL-1, SU-DHL-6, SU-DHL-8, SU-DHL-9. Multiple myeloma (MM) cell lines AF-10 and H929 were gifts from M. Kuehl (National Cancer Institute, Genetics Branch, Bethesda, MD).
Transfection of B-Cell Lines
Purified plasmids pMH-galectin-3-neo (containing a full-length galectin-3 cDNA) and pMH-neo were linearized using NdeI.23 Plasmids pEF1-galectin-3C-neo (containing an amino-terminus truncated version of galectin-3) and pEF1-neo were linearized with HindIII.23 Linearized DNA was used to transfect B-cell lines by electroporation.37,38 Transfected clones were generated by limiting dilution and selected by culturing with 1 mg/ml of G418. Expression of galectin-3 and galectin-3C was verified by Western blot analysis (data not shown).
Patient Tumor Samples and Immunohistochemistry
B-NHL were characterized by histology and immunophenotype, grouped according to the World Health Organization classification system39 and retrieved from the files of the Surgical Pathology Division of the Anatomical Pathology Department at UCLA. Paraffin sections were examined for galectin-3 expression using the biotin-streptavidin method (DAKO, Carpinteria, CA) as previously described.33,40 Briefly, formalin-fixed tissue sections were deparaffinized, blocked, and incubated overnight at 4°C with affinity-purified polyclonal rabbit anti-galectin-3 antiserum at 8.6 μg/ml. Galectin-3 antiserum was generated as previously described.41 Evaluation of each immunostained histological section was performed independently by two pathologists.
B-Cell Isolation from Human Tonsil
Hyperplastic tonsils were obtained from the Human Tissue Resource Center at UCLA with adherence to established guidelines for the protection of human subjects. B-cell subsets were isolated as previously described.42,43 Briefly, tonsils were minced in PBS, passed through a 40-μm filter, and lymphocytes were isolated by centrifugation on a Ficoll-Paque Plus cushion (Amersham Pharmacia Biotech, Piscataway, NJ). B cells were fractionated using a magnetic activated cell sorting (MACS) system (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Isolated populations were analyzed by flow cytometry to determine percentage purity. Naïve B cells (CD3−/CD27−/IgD+) were >90% pure. GC B cells (CD3−/CD10+/IgD−) were >85% pure. Memory B cells (CD3-/CD10−/CD27+/IgD−) were >90% pure, and verified by additional staining and flow cytometry with anti-CD19 antibody (FITC-CD19, leu-12; BD PharMingen, San Diego, CA) and anti-CD20 antibody (FITC-CD20, leu-16; BD PharMingen). Plasma cells (CD3−/CD138+) were isolated as previously described.44
Western Blot Analysis
Cells were washed twice with PBS then lysed as previously described in NP-40 lysis buffer containing protease inhibitors.43 Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Ten micrograms of total protein for each sample was separated by SDS-PAGE and electroblotted to a nitrocellulose membrane (Hybond; Amersham Pharmacia Biotech). Membranes were blocked in Tris-buffered saline containing 0.1% Tween (TBST) and 5% dry milk for 1 hour, followed by an incubation with polyclonal rabbit anti-galectin-3 serum (diluted 1:15,000) in TBST overnight at 4°C. Membranes were then washed 4 times in TBST then incubated in anti-rabbit IgG HRP-linked antiserum (diluted 1:10,000; New England Biolabs, Beverly, MA) in TBST for 1 hour, followed by 6 washes in TBST. Immuno-positive bands were visualized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech). Densitometric analysis was performed using the ChemiImager 5500 with AlphaEaseFC software (Alpha Innotech, San Leandro, CA).
Apoptosis Induction and Analysis
For apoptosis assays, pMH-galectin-3-neo, pMH-neo, pEF1-galectin-3C-neo, and pEF1-neo transfected Raji (galectin-3-negative) or BCBL-1 (galectin-3-positive) B cells (1 × 106 cells/ml) were treated with 50 ng/ml of mouse monoclonal anti-human Fas (clone CH-11, Upstate, Lake Placid, NY) for 24 hours at 37°C in 5% CO2. Cell death was measured by Annexin V binding and propidium iodide permeability according to the manufacturer’s instructions (BD PharMingen). Flow cytometry was performed on a Coulter Elite (Beckman Coulter, Miami, FL) and the data analyzed using FCS Express v2.0 (DeNovo Software, Thornhill, ON, Canada).
Results
Galectin-3 Expression in Primary B-NHL Samples
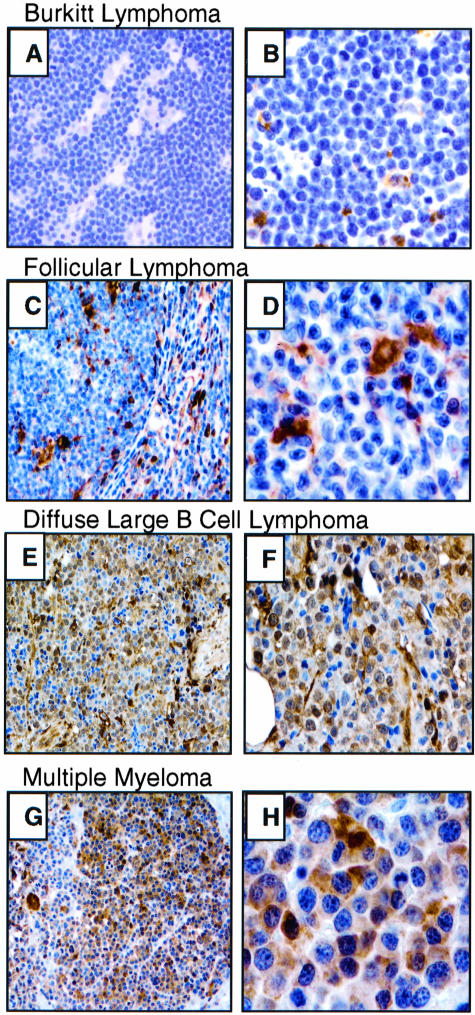
We assessed galectin-3 expression in a total of 64 GC-derived FL, BL, and DLBCL by immunohistochemistry (Table 1 and Figure 1). We observed moderate to strong cytoplasmic and/or nuclear galectin-3 expression in 21 of 32 (66%) DLBCL patient samples (Figure 1). DLBCL samples lacking galectin-3 expression in tumor cells nevertheless contained moderate to abundant galectin-3 expression in surrounding macrophage and dendritic cells (Figure 1). None of the FL (0 of 17) or BL (0 of 15) samples tested showed expression of galectin-3 in tumor cells, but interspersed macrophage and dendritic cells were galectin-3 positive (data not shown). These results demonstrate abundant galectin-3 protein in tumor cells from a majority of primary DLBCL, but not in the other GC-derived lymphomas (BL and FL) surveyed.
Table 1.
Galectin-3 Expression in Primary B-NHL
| Lymphoma type | Histogenesis | Gal-3+ tumor/total |
|---|---|---|
| Follicular lymphoma | GC B | 0/17 |
| Burkitt lymphoma | GC B | 0/15 |
| Diffuse large B-cell lymphoma | GC (or post-GC) B | 21/32 |
| Marginal zone lymphoma | Post-GC B | 0/8 |
| MALT/BALT lymphoma | Post-GC B | 0/9 |
| B-small lymphocytic lymphoma | Memory B | 0/8 |
| Multiple myeloma | Plasma B | 4/16 |
Formalin-fixed, paraffin-embedded tissues were immunostained with galectin-3 antiserum. Lymphomas were grouped according to the WHO classification.39
MALT, mucosa-associated lymphoid tissue; BALT, bronchus-associated lymphoid tissue; GC, germinal center; Gal-3+, galectin-3 positive, as determined by immunohistochemistry.
Figure 1.
Galectin-3 immunostaining of representative B-NHL. BL (A and B) and FL (C and D) tumor cells are negative for galectin-3. DLBCL (E and F) and MM (G and H) tumor cells express high levels of galectin-3. Macrophages and dendritic cells, when present within the lymphoma (D), are positive for galectin-3, serving as an internal control for staining.49 Hematoxylin counterstain. Original magnification: A, C, E, G, ×200; B, D, F, ×400; H, ×1000.
We also examined lymphoid neoplasms that arise specifically from post-GC and terminal stages of B-cell differentiation for galectin-3 expression. Galectin-3 was detected in tumor cells in 4 of 16 (25%) MM samples (Table 1 and Figure 1). Twenty-five additional post-GC-derived B-cell lymphomas, including nodal and extranodal marginal zone lymphomas (MZL), MALT/BALT lymphomas and B-small lymphocytic lymphomas (B-SLL), were all negative for tumor cell immunostaining with galectin-3 (Table 1). Overall, we observed significant galectin-3 protein expression in tumor cells from 66% of DLBCL and 25% of MM patient samples and no expression from 57 additional mature B-cell tumor subtypes. However, interspersed nonmalignant macrophage and dendritic cells contained within all tumor samples expressed moderate to abundant galectin-3, as expected,14 providing a positive control to assess expression in tumor cells and a potential extracellular source of galectin-3.
Galectin-3 Expression in Transformed B-Cell Lines
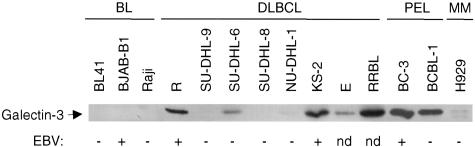
To examine the consistency of galectin-3 expression between patient samples and cell lines, we next evaluated galectin-3 protein content in multiple B-cell lines. Galectin-3 expression was detected in 6 of 8 DLBCL cell lines by Western blot analyses (Table 2 and Figure 2). Five lines demonstrated moderate to high levels of galectin-3 protein, while one line (NU-DHL-1) expressed very low levels of galectin-3. Gene expression profiling demonstrated that moderate galectin-3-expressing SU-DHL-6 is a BCL-6-positive, GC B-like DLBCL,45 while the cellular origins of the other DLBCL are from GC or possibly post-GC B cells. The expression of galectin-3 in 6 of 8 (75%) DLBCL cell lines parallels the expression of galectin-3 in 21 of 32 (66%) primary DLBCL (Table 1). This pattern contrasts sharply with the lack of detectable galectin-3 expression in 9 of 9 GC-derived BL cell lines assessed by Northern analysis (data not shown) and Western blotting (Table 2 and Figure 2). The lack of galectin-3 expression in all nine BL cell lines tested is consistent with the lack of detectable galectin-3 expression in 15 primary BL samples (Table 1).
Table 2.
Galectin-3 Expression and EBV Status in B-NHL Cell Lines
| Cell line | Type | Galectin-3 | EBV | Cell line | Type | Galectin-3 | EBV |
|---|---|---|---|---|---|---|---|
| Akata A1.5 | BL | − | + | R | DLBCL | + | + |
| BJAB | BL | − | − | RRBL | DLBCL | + | nd |
| BJAB-B1 | BL | − | + | SU-DHL-6 | DLBCL | + | − |
| BL41 | BL | − | − | SU-DHL-8 | DLBCL | − | − |
| BL41-B98/8 | BL | − | + | SU-DHL-9 | DLBCL | − | − |
| P3HR-1 | BL | − | + | AF10 | MM | (+) | − |
| Raji | BL | − | − | H929 | MM | (+) | − |
| Ramos | BL | − | − | BC-1 | PEL | + | + |
| 2F7 | BL | − | + | BC-3 | PEL | + | − |
| E | DLBCL | + | nd | BCBL-1 | PEL | + | − |
| KS-2 | DLBCL | + | + | KS-1 | PEL | + | − |
| NU-DHL-1 | DLBCL | (+) | − |
B-cell lines examined for galectin-3 expression by Western blot, including EBV status.
(+), low-level expression detected; +, moderate- to high-level expression; nd, not determined.
Figure 2.
Galectin-3 protein expression in B-cell lines. A total of 23 B-NHL cell lines were examined. All 8 DLBCL examined are shown, along with representative lines for BL, PEL, and MM. The EBV status for each line is noted below the Western blot, with the lymphoma subtype listed above. No correlation between EBV status and galectin-3 expression level was observed.
We next examined cell lines derived from B-NHL corresponding to post-GC stages of B-cell development. Galectin-3 was expressed, at low levels, in 2 of 2 plasma cell-derived MM cell lines (Figure 2 and Table 2). Also, 4 of 4 PEL cell lines, derived from liquid suspension B-cell tumors that occur primarily in AIDS patients, were strongly galectin-3 positive (Figure 2 and Table 2). Similar to MM, PEL is also likely derived from a plasma cell or plasma cell-like stage of B cell differentiation.46,47 Overall, these data show that most DLBCL cell lines tested expressed moderate to high levels of galectin-3, in contrast to the observed lack of expression in BL lines, despite the typically common origin of both tumor types from GC B cells. This pattern is in agreement with the expression pattern seen in DLBCL and BL patient samples. Furthermore, galectin-3 was expressed in cell lines from two types of plasma cell-derived B lymphomas, PEL and MM, with levels in PEL as high as that observed for certain DLBCL lines.
c-MYC and Epstein-Barr Virus Do Not Affect Galectin-3 Expression
As BL are strongly associated with c-MYC dysregulation, we examined whether c-MYC expression levels correlated with galectin-3 expression. One possibility was that elevated c-MYC expression could repress galectin-3 levels, potentially providing an explanation for the difference in galectin-3 expression between and within BL and DLBCL subtypes. All galectin-3-expressing and non-expressing B-cell lines tested expressed c-MYC mRNA at variable levels (data not shown). The different levels of c-MYC mRNA expression did not correlate with galectin-3 mRNA or protein levels, strongly suggesting that galectin-3 expression is not positively or negatively influenced by c-MYC dysregulation.
Several reports have indicated that galectin-3 is up-regulated by viral proteins in other cell types, such as in T cells and in breast carcinoma cells.24,34 We therefore compared galectin-3 expression with Epstein-Barr Virus (EBV) status since B cells and B-cell malignancies such as BL are frequently infected by EBV. We found that expression of galectin-3 in the B-cell lymphoma lines tested did not correlate with EBV infection status (Figure 2 and Table 2). In addition, galectin-3 expression did not vary with EBV status in the DLBCL cell lines. All endemic and sporadic BL cell lines failed to express galectin-3 regardless of EBV infection, including the EBV-negative cell lines BJAB and BL41, and their EBV-infected clones BJAB-B1 and BL41/B95-8. Overall, these data show that galectin-3 mRNA and protein levels do not correlate with EBV status.
Galectin-3 Expression During B-Cell Development
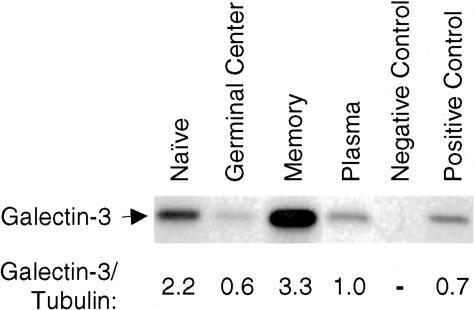
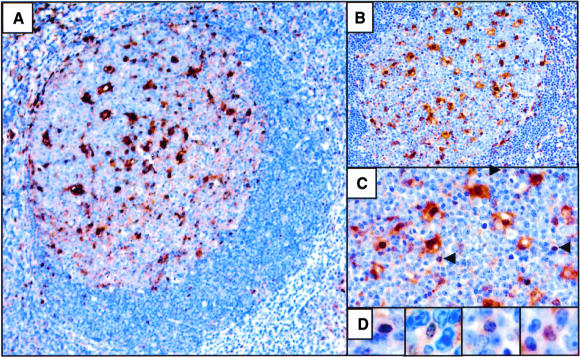
Since we detected moderate to high level galectin-3 expression in DLBCL, PEL, and MM cell lines and patient samples, but not in other B-NHL, we next determined if galectin-3 expression levels correlated with the stage of B-cell development from which these lymphomas arose. To define galectin-3 expression during normal B-cell development, B-cell populations from human tonsil were isolated by MACS and analyzed for galectin-3 protein expression by Western blot. Remarkably, galectin-3 expression levels varied widely among specific B-cell developmental stages (Figure 3). Notably, the lowest level of galectin-3 expression was observed in GC B cells (CD10+/IgD−). The GC is composed of dividing centroblasts and non-dividing centrocytes undergoing antigen-driven selection. Many of these GC B cells also undergo apoptotic death during this period. Therefore, the low level of galectin-3 expression in sorted GC B cells may be due either to a relatively low number of galectin-3 moderate to high-expressing B cells present in the GC, or because all or most GC B cells express a very low level of galectin-3. To distinguish between these two possibilities, we assessed galectin-3 expression in tonsil by immunostaining. The majority of lymphocytes in the light and dark zone of tonsil GC lacked detectable galectin-3 expression (Figure 4), although there appeared to be rare scattered lymphocytes within the GC that expressed variable levels of galectin-3.48 The largest population of galectin-3-positive cells within the GC was the dendritic reticular cells, as expected.49 Therefore, the low level of galectin-3 expression observed in the sorted GC B-cell population is probably due to galectin-3 expression in only a small subset of GC B cells, in agreement with Western blot analyses (Figure 3).
Figure 3.
Galectin-3 protein levels at distinct stages of B-cell development. Western blot analysis of MACS isolated B-cell populations from human tonsil probed with galectin-3 antiserum. The levels of galectin-3 expression were determined by densitometry and normalized to the densitometry-determined tubulin expression level (data not shown) for each population. Equal protein loading was confirmed with a Coomassie Brilliant Blue gel stain (data not shown). Negative and positive controls are galectin-3-negative Raji BL cells and a galectin-3 expressing Raji BL transfectant clone, respectively. Sorted phenotypes of the B-cell populations under study were naïve (CD3−/CD27−/IgD+), GC (CD10+/IgD−), memory (CD10−/CD27+/IgD−) and plasma (CD138+) B cells.
Figure 4.
Galectin-3 expression in tonsil. (A to C) GC follicle showing galectin-3 immunostaining with a hematoxylin counterstain. Positive cells within the GC consist primarily of dendritic reticular cells. The arrows in C indicate scattered, non-dendritic reticular cells staining positive for galectin-3. D: Rare lymphocytes within the GC stain positive for galectin-3. Magnifications: ×100 (A), ×200 (B), ×400 (C), ×600 (D).
In contrast to GC B cells, galectin-3 expression was high in naïve (CD27−/IgD+) and memory B cells (CD10−/CD27+/IgD−). Naïve B cells reside in the mantle zone of secondary follicles and in the interfollicular zone between follicles awaiting T-dependent or T-independent antigenic stimulation. Curiously, most mantle zone B cells appear negative for galectin-3 expression by immunohistochemistry (Figure 4). Naïve B cells of the mantle and non-recruited interfollicular B cells arriving in the tonsil, lymph node, or spleen (eg, T1, T2, and MZ B cells, for example) show scattered mantle zone and interfollicular staining for galectin-3. These cells likely account for the robust naïve B-cell galectin-3 expression seen on Western blot at this developmental stage. It must be noted that immunohistochemical and Western blot procedures are variably sensitive and dependent on the antibody or antiserum used. It is possible that the immunohistochemical detection of galectin-3 in mantle zone cells is not as sensitive as the detection of galectin-3 from these cells by Western blot. Also, long-lived, predominantly non-dividing memory B cells expressed the highest level of galectin-3 protein, from which galectin-3 negative B-chronic lymphocytic leukemia (and, by extension, B-SLL) originates (Table 1).50,51 Plasma cells (CD138+) expressed low levels of galectin-3, perhaps comparable to the low level of expression detected in the two MM cell lines tested. These data demonstrate a stage-specific regulation of galectin-3 expression during B-cell development, with the lowest galectin-3 expression levels in GC B cells undergoing antigenic selection and apoptosis, and in terminally differentiated, antibody-secreting plasma cells.
Galectin-3 Protects B Cells from Anti-Fas-Induced Apoptosis
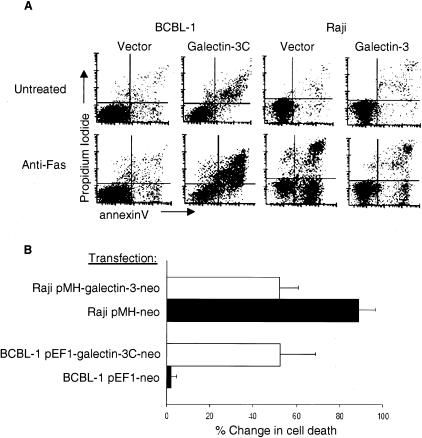
The robust expression of galectin-3 in long-lived B-cell subsets, such as memory B cells, suggested that galectin-3 expression might contribute to apoptotic resistance in B cells. We therefore directly examined whether galectin-3 protects B cells from apoptosis by treating these cells with anti-Fas antibody, a cell-surface initiator of cell death.52,53 Previous studies showed that galectin-3 protected Jurkat T cells from anti-Fas- and staurosporine-induced apoptosis, and also protected breast carcinoma cells from apoptotic stimuli.23,25 We expressed galectin-3 in galectin-3-negative Raji BL B cells (for example, see Figure 3, positive control lane). Raji cells expressing full-length galectin-3 demonstrated a 41% decrease in anti-Fas-induced apoptosis, compared to cells transfected with a control vector alone (Figure 5). In addition, galectin-3-expressing BCBL-1 B cells were transfected with a plasmid expressing an amino-terminal truncated version of galectin-3 containing only the carboxy-terminal domain (galectin-3C).37 The amino-terminal portion of galectin-3 is required for protection from apoptosis and has been shown to inhibit galectin-3 chemoattractant activity, suggesting that galectin-3C may act potentially as a dominant-negative form of galectin-3.54,55 In addition, galectin-3C inhibited breast cancer tumor growth and metastasis.56 Remarkably, we observed a 90% to 95% increase in anti-Fas-induced apoptosis of galectin-3 expressing PEL cells expressing transfected galectin-3C compared to cells transfected with a control neo-vector alone (Figure 5). Importantly, expression of galectin-3C in galectin-3-negative Raji BL cells did not alter the viability or sensitivity of these cells to anti-Fas treatments compared to vector control-transfected cells (data not shown), indicating that galectin-3C acts in a dominant-negative role to block galectin-3 B-cell survival without intrinsic pro-apoptotic activity. The cloned galectin-3 and galectin-3C transfectants were cultured in the presence of G418 throughout their selection and experimental use. Western blotting demonstrated that the clones expressed moderate levels of galectin-3 and galectin-3C (data not shown). The robust bio-responses seen in protecting or failing to protect B cells from Fas-induced cell death (Figure 5) would not be expected if only a minority of cells in the clone populations expressed galectin-3 or galectin-3C. Furthermore, the neomycin selection marker is linked to the cDNAs for galectin-3 and galectin-3C. The physical linkage and continued G418 selection argues for maintaining an open chromatin configuration and neomycin expression at the integrated locus expressing galectin-3 or galectin-3C. Combined with Western blotting and alterations in sensitivity to Fas-induced death, a majority, if not all, cells in the drug-selected clones co-express galectin-3 or galectin-3C at moderate levels. Together, these data demonstrate a strong role for galectin-3 in protecting B cells from Fas-induced cell death.
Figure 5.
Galectin-3 protects B cells from Fas-induced apoptosis. A: Flow cytometry analysis of annexin V and propidium iodide-stained galectin-3-positive BCBL-1 PEL cells transfected with a galectin-3C-expressing or vector control construct (left four panels) and galectin-3-negative Raji BL cells transfected with a galectin-3-expressing or vector control construct (right four panels) before or after treatment with anti-Fas antibody for 24 hours. B: Summary of cell death data, shown as the percent change in apoptosis between untreated and anti-Fas-treated cells when stably transfected with galectin-3, galectin-3C, and vector control expression constructs. Data are mean ± SD of triplicate samples and is representative of three independent experiments.
Discussion
Dysregulation of normal apoptotic mechanisms appears critical for tumorigenesis. In this study we have shown that galectin-3 behaves as an anti-apoptotic protein in B cells and is highly expressed in a majority of DLBCL samples and cell lines. Galectin-3 was also highly expressed in all PEL cell lines tested and was variably expressed in MM cell lines and patient samples. It is intriguing that GC-derived BL and FL have well-characterized mechanisms for protection from apoptosis, via c-MYC and BCL-2 respectively, while comparable mechanisms for protecting GC-derived DLBCL or plasma cell-derived MM and PEL from apoptosis have not yet been identified. In contrast, we did not detect galectin-3 expression in memory B-cell-derived B-SLL patient samples, despite the high level of galectin-3 expression in normal memory B cells. Down-regulation of galectin-3 during or before transformation and progression, or transformation of galectin-3-negative memory B cells, coupled to yet another distinct cell survival mechanism(s) may occur in B-SLL.
Galectin-3 expression in DLBCL (and in PEL and MM) may occur through several mechanisms. As shown in Figure 3, GC B cells had the lowest galectin-3 level of all B-cell populations. This suggests that galectin-3 may be up-regulated in DLBCL during or after transformation, independent of c-MYC expression levels. Alternatively, DLBCL may arise from rare galectin-3 high-expressing GC B cells that retain expression at this level during lymphoma progression. In the latter case, protection from apoptosis via abundant galectin-3 expression may be a critical pre-condition for the development of certain DLBCL.57–59 Increased galectin-3 expression has been shown to correlate with neoplastic transformation, metastatic potential, and with increased aggressiveness of hepatocellular carcinoma,60 astrocytomas,61 pancreatic cancer,62 and colon cancer.63 The mechanism of galectin-3 overexpression in these tumors is not yet understood. While T cells transfected with HIV Tat protein or infected with human T lymphotrophic virus increase expression of galectin-3,24,34,64 we observed that galectin-3 expression in transformed B-cell lines was not associated with, nor affected by, EBV infection. Thus, viral transformation is unlikely to be a general mechanism leading to galectin-3 up-regulation in B cells. However, we did observe high levels of galectin-3 expression in 4 of 4 PEL cell lines examined, suggesting that up-regulation of galectin-3 expression by KSHV/HHV-8 can possibly contribute to lymphomagenesis in AIDS patients.46
Although the mechanism(s) of transformation and protection from apoptosis in DLBCL, PEL and MM is largely unknown, galectin-3 may play a prominent role in protecting these B-NHL from cell death. Previous studies suggest that galectin-3 protects cells from apoptosis through interactions with BCL-2, by blocking alterations in mitochondrial membrane potential, and by reducing the formation of reactive oxygen species.23–25,54 In addition, transfection of T cells with galectin-3 increases proliferation of these cells,65 while transfection of breast carcinoma cells with galectin-3 anti-sense constructs decreases cellular proliferation.66 Both protection from apoptosis and increased proliferation, functions of galectin-3, could be linked to the lymphomagenesis of DLBCL, PEL, and MM. The cell protective role of galectin-3 could safeguard emerging DLBCL within the extensively apoptotic GC, while a proliferative advantage conferred by galectin-3 expression would allow the transformed cells to expand within and beyond the GC. A similar role could protect and stimulate senescent plasma cells during or after transformation to PEL and MM.
Supporting the notion that galectin-3 expression protects transformed B cells from apoptosis, we have shown that galectin-3 non-expressing BL cells transfected with a galectin-3 expression construct are significantly less sensitive to anti-Fas-induced apoptosis than control vector transfectants (Figure 5). Furthermore, galectin-3 expressing PEL cells transfected with the galectin-3 carboxy-terminal domain (galectin-3C) show a 90% to 95% increase in anti-Fas-induced apoptosis. It is known that galectin-3 can self-associate through interactions that are dependent on the amino- and carboxy-terminal domains,37 so that dimerization and multimerization may be important for the anti-apoptotic activity of galectin-3. Phosphorylation of serine-6, located at the amino-terminus of galectin-3, is required for the anti-apoptotic effects of galectin-3.54 Galectin-3C lacks the serine-6 residue in the amino terminus and therefore would not be phosphorylated. The increased sensitivity to Fas-induced apoptosis observed after galectin-3C transfection of PEL cells may result from the association of galectin-3C with endogenous galectin-3. This would reduce the availability of normal galectin-3 dimers by the creation of abnormal galectin-3/galectin-3C dimers, resulting in decreased serine-6 phosphorylation that is required for cell protection. Alternatively, galectin-3C may act alone as monomers or multimers to directly inhibit the protection from apoptosis afforded by intact galectin-3 as a competitive inhibitor. The lack of a measurable effect for galectin-3C in the absence of galetin-3 in Raji BL cells (data not shown) argues against a role for galectin-3C in directly promoting apoptosis in B cells.
Our galectin-3 immunohistochemical staining results, showing frequent moderate- to high-level galectin-3 protein expression in DLBCL patient samples is in agreement with studies of global RNA levels using gene expression profiling.6 Galectin-3 mRNA levels were shown to be among the best genetic discriminators between DLBCL and FL in a large panel of primary patient tumor samples. Importantly, galectin-3 is able to promote tumor cell survival by either intracellular or extracellular expression,23–26 suggesting that abundant galectin-3 in the surrounding stromal tissues may also have a more general role in promoting B-cell lymphomagenesis. In this regard, most if not all GC-based tumor samples examined here express high levels of galectin-3 from surrounding stromal cells (data not shown).
In contrast to DLBCL, both BL and FL have well-characterized means of protection from apoptosis. BL often harbor p53 mutations which, when combined with dysregulated c-MYC expression, can lead to inactivation of apoptosis and cell cycle signaling.13,67 Although BCL-2 is normally undetectable in GC B cells,68 BCL-2 is overexpressed by most FL, due primarily to a chromosomal translocation juxtaposing the BCL-2 gene to the immunoglobulin heavy-chain regulating elements.69,70 Although BCL-2 protects FL from apoptosis, expression of BCL-2 is usually absent in BL and has been observed in only a subset of DLBCL.71,72 This suggests that the three major types of GC-derived B-NHL, BL, FL, and DLBCL, each have unique molecular mechanisms to evade apoptosis. Galectin-3 may play this role for specific DLBCL.
Strikingly, in non-transformed lymphocytes, we found that GC B cells have the lowest level of galectin-3 expression. This population of B cells undergoes extensive apoptosis during selection. We suggest that weak or absent galectin-3 expression in the GC may facilitate apoptosis in those cells that fail to receive appropriate selection signals. In contrast, high expression of galectin-3 in naïve (pre-GC) and memory (post-GC) B cells serves to protect these cells for extended periods of time, while the reduction in galectin-3 expression in plasma cells may help limit the lifespan of these terminally differentiated effector cells.
In summary, we have shown that many DLBCL, PEL, and MM lines and patient samples express abundant galectin-3 protein. Galectin-3 expression is protective from Fas-induced death, a major source of B-cell elimination in vivo. Elucidation of the role that galectin-3 plays in evasion from apoptosis in these mature B-cell-derived lymphomas, as described for non-lymphoid malignancies, may improve clinical management and patient outcome in some types of lymphoma. Molecular therapies targeted to circumvent the anti-apoptotic effects of galectin-3 could ameliorate the progression of selected lymphomas and synergize with existing treatments.
Acknowledgments
We thank Mindy Miner and Girija Sulur for technical assistance, Drs. David Dawson, Samuel French, and Karen Pace for helpful discussions, and Brenda Mueller for assistance with manuscript preparation.
Footnotes
Address reprint requests to Michael A. Teitell, M.D., Ph.D., Department of Pathology and Laboratory Medicine, UCLA School of Medicine, 10833 Le Conte Avenue, Los Angeles, California 90095-1732. E-mail: mteitell@ucla.edu.
Supported by the Lymphoma Research Foundation (to J.W.S. and M.A.T.), the American Foundation for AIDS Research and CMISE, NASA URETI Institute (to M.A.T.), the National Neurological AIDS Bank (to J.W.S.) and by NIH Grants T32CA090056 (to K.K.H.), AI20958 and AI39620 (to F.T.L.), GM63281 (to L.G.B.) and CA90571 and CA107300 (to M.A.T.).
M.A.T. is a Scholar of the Leukemia and Lymphoma Society.
References
- Chow DA, Greenberg AH. The generation of tumor heterogeneity in vivo. Int J Cancer. 1980;25:261–265. doi: 10.1002/ijc.2910250214. [DOI] [PubMed] [Google Scholar]
- Torres J., Jr Increased sister-chromatid exchanges accompany serial transplantation of murine S49.1 lymphoma. Mutat Res. 1982;105:337–342. doi: 10.1016/0165-7992(82)90104-x. [DOI] [PubMed] [Google Scholar]
- French SW, Dawson DW, Miner MD, Doerr JR, Malone CS, Wall R, Teitell MA. DNA methylation profiling: a new tool for evaluating hematologic malignancies. Clin Immunol. 2002;103:217–230. doi: 10.1006/clim.2002.5186. [DOI] [PubMed] [Google Scholar]
- Vose JM. Current approaches to the management of non-Hodgkin’s lymphoma. Semin Oncol. 1998;25:483–491. [PubMed] [Google Scholar]
- Staudt LM. Gene expression profiling of lymphoid malignancies. Annu Rev Med. 2002;53:303–318. doi: 10.1146/annurev.med.53.082901.103941. [DOI] [PubMed] [Google Scholar]
- Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, Ray TS, Koval MA, Last KW, Norton A, Lister TA, Mesirov J, Neuberg DS, Lander ES, Aster JC, Golub TR. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693S–1700S. [PubMed] [Google Scholar]
- Wright DH. What is Burkitt’s lymphoma? J Pathol. 1997;182:125–127. doi: 10.1002/(SICI)1096-9896(199706)182:2<125::AID-PATH843>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Hecht JL, Aster JC. Molecular biology of Burkitt’s lymphoma. J Clin Oncol. 2000;18:3707–3721. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- Cherney BW, Bhatia KG, Sgadari C, Gutierrez MI, Mostowski H, Pike SE, Gupta G, Magrath IT, Tosato G. Role of the p53 tumor suppressor gene in the tumorigenicity of Burkitt’s lymphoma cells. Cancer Res. 1997;57:2508–2515. [PubMed] [Google Scholar]
- Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Liu F, Patterson R, Wang J. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, Zick Y. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci. 2000;113:2385–2397. doi: 10.1242/jcs.113.13.2385. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Kuwabara Y, Yang RY, Schuler M, Green DR, Zuraw BL, Hsu DK, Liu FT. Galectin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. J Biol Chem. 2002;277:3487–3497. doi: 10.1074/jbc.M109360200. [DOI] [PubMed] [Google Scholar]
- Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel β-galactoside-binding mammalian lectin. J Biol Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Matsukawa Y, Takahashi M, Nishizawa H, Kishida K, Matsuda M, Kuriyama H, Kihara S, Nakamura T, Tochino Y, Bodkin NL, Hansen BC, Matsuzawa Y. Galectin-12, an adipose-expressed galectin-like molecule possessing apoptosis-inducing activity. J Biol Chem. 2001;276:34089–34097. doi: 10.1074/jbc.M105097200. [DOI] [PubMed] [Google Scholar]
- Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- Matarrese P, Fusco O, Tinari N, Natoli C, Liu FT, Semeraro ML, Malorni W, Iacobelli S. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 2000;85:545–554. [PubMed] [Google Scholar]
- Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001;159:1055–1060. doi: 10.1016/S0002-9440(10)61780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria: a role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- Song YK, Billiar TR, Lee YJ. Role of galectin-3 in breast cancer metastasis: involvement of nitric oxide. Am J Pathol. 2002;160:1069–1075. doi: 10.1016/S0002-9440(10)64927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F. Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology. 1998;94:290–296. doi: 10.1046/j.1365-2567.1998.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Inohara H, Takenaka Y, Honjo Y, Akahani S, Nomura T, Raz A, Kubo T. Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol. 2001;18:787–792. doi: 10.3892/ijo.18.4.787. [DOI] [PubMed] [Google Scholar]
- Kuklinski S, Pesheva P, Heimann C, Urschel S, Gloor S, Graeber S, Herzog V, Pietsch T, Wiestler OD, Probstmeier R. Expression pattern of galectin-3 in neural tumor cell lines. J Neurosci Res. 2000;60:45–57. doi: 10.1002/(SICI)1097-4547(20000401)60:1<45::AID-JNR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285–293. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- Konstantinov KN, Robbins BA, Liu FT. Galectin-3, a β-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. Am J Pathol. 1996;148:25–30. [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Hammes SR, Kuwabara I, Greene WC, Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the β-galactoside-binding lectin, galectin-3. Am J Pathol. 1996;148:1661–1670. [PMC free article] [PubMed] [Google Scholar]
- Ng VL, Hurt MH, Fein CL, Khayam-Bashi F, Marsh J, Nunes WM, McPhaul LW, Feigal E, Nelson P, Herndier BG. IgMs produced by two acquired immune deficiency syndrome lymphoma cell lines: ig binding specificity and VH-gene putative somatic mutation analysis. Blood. 1994;83:1067–1078. [PubMed] [Google Scholar]
- Demidem A, Salahuddin Z, Lam T, Levine AM, Khan RS, Hober D, Bonavida B. Sensitization of AIDS related non-Hodgkin’s B lymphoma cell lines to cytotoxic drugs/toxins by interferon-γ. Int J Oncol. 1996;8:461–468. doi: 10.3892/ijo.8.3.461. [DOI] [PubMed] [Google Scholar]
- Yang RY, Hill PN, Hsu DK, Liu FT. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37:4086–4092. doi: 10.1021/bi971409c. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Menzin E, Saito T, Germain RN, Bierer BE. The complete sequences of plasmids pFNeo and pMH-Neo: convenient expression vectors for high-level expression of eukaryotic genes in hematopoietic cell lines. Gene. 1993;127:267–268. doi: 10.1016/0378-1119(93)90731-h. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- Teitell M, Damore MA, Sulur GG, Turner DE, Stern MH, Said JW, Denny CT, Wall R. TCL1 oncogene expression in AIDS-related lymphomas and lymphoid tissues. Proc Natl Acad Sci USA. 1999;96:9809–9814. doi: 10.1073/pnas.96.17.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992;267:14167–14174. [PubMed] [Google Scholar]
- Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci USA. 2000;97:12250–12255. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said JW, Hoyer KK, French SW, Rosenfelt L, Garcia-Lloret M, Koh PJ, Cheng TC, Sulur GG, Pinkus GS, Kuehl WM, Rawlings DJ, Wall R, Teitell MA. TCL1 oncogene expression in B cell subsets from lymphoid hyperplasia and distinct classes of B cell lymphoma. Lab Invest. 2001;81:555–564. doi: 10.1038/labinvest.3780264. [DOI] [PubMed] [Google Scholar]
- Jego G, Robillard N, Puthier D, Amiot M, Accard F, Pineau D, Harousseau JL, Bataille R, Pellat-Deceunynck C. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood. 1999;94:701–712. [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Carbone A. Emerging pathways in the development of AIDS-related lymphomas. Lancet Oncol. 2003;4:22–29. doi: 10.1016/s1470-2045(03)00957-4. [DOI] [PubMed] [Google Scholar]
- Jenner RG, Maillard K, Cattini N, Weiss RA, Boshoff C, Wooster R, Kellam P. Kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proc Natl Acad Sci USA. 2003;100:10399–10404. doi: 10.1073/pnas.1630810100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555–564. [PubMed] [Google Scholar]
- Nabarra B, Papiernik M. Phenotype of thymic stromal cells. An immunoelectron microscopic study with anti-IA, anti-MAC-1, and anti-MAC-2 antibodies. Lab Invest. 1988;58:524–531. [PubMed] [Google Scholar]
- Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ, Brown PO, Staudt LM. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L, Neri A, Califano A, Dalla-Favera R. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194:1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- John CM, Leffler H, Kahl-Knutsson B, Svensson I, Jarvis GA. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin Cancer Res. 2003;9:2374–2383. [PubMed] [Google Scholar]
- Raz A, Zhu DG, Hogan V, Shah N, Raz T, Karkash R, Pazerini G, Carmi P. Evidence for the role of 34-kDa galactoside-binding lectin in transformation and metastasis. Int J Cancer. 1990;46:871–877. doi: 10.1002/ijc.2910460520. [DOI] [PubMed] [Google Scholar]
- Hebert E, Monsigny M. Galectin-3 mRNA level depends on transformation phenotype in ras-transformed NIH 3T3 cells. Biol Cell. 1994;81:73–76. doi: 10.1016/0248-4900(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Hebert E, Roche AC, Nachtigal M, Monsigny M. Transformation but not ras-transfection increases the expression of galectin-3 in human HOS cells. C R Acad Sci III. 1996;319:871–877. [PubMed] [Google Scholar]
- Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. doi: 10.1002/(sici)1097-0215(19990517)81:4<519::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80:776–787. [PubMed] [Google Scholar]
- Berberat PO, Friess H, Wang L, Zhu Z, Bley T, Frigeri L, Zimmermann A, Buchler MW. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539–549. doi: 10.1177/002215540104900414. [DOI] [PubMed] [Google Scholar]
- Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995;75:2818–2826. doi: 10.1002/1097-0142(19950615)75:12<2818::aid-cncr2820751206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Fogel S, Guittaut M, Legrand A, Monsigny M, Hebert E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology. 1999;9:383–387. doi: 10.1093/glycob/9.4.383. [DOI] [PubMed] [Google Scholar]
- Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res. 1998;245:294–302. doi: 10.1006/excr.1998.4253. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- Lindstrom M, Wiman K. Role of genetic and epigenetic changes in Burkitt lymphoma. Semin Cancer Biol. 2002;12:381. doi: 10.1016/s1044-579x(02)00058-5. [DOI] [PubMed] [Google Scholar]
- Wang T, Lasota J, Hanau CA, Miettinen M. Bcl-2 oncoprotein is widespread in lymphoid tissue and lymphomas but its differential expression in benign versus malignant follicles and monocytoid B-cell proliferations is of diagnostic value. APMIS. 1995;103:655–662. doi: 10.1111/j.1699-0463.1995.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- Gaulard P, d’Agay MF, Peuchmaur M, Brousse N, Gisselbrecht C, Solal-Celigny P, Diebold J, Mason DY. Expression of the bcl-2 gene product in follicular lymphoma. Am J Pathol. 1992;140:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- Spina D, Leoncini L, Megha T, Gallorini M, Disanto A, Tosi P, Abinya O, Nyong OA, Pileri S, Kraft R, Laissue JA, Cottier H. Cellular kinetic and phenotypic heterogeneity in and among Burkitt’s and Burkitt-like lymphomas. J Pathol. 1997;182:145–150. doi: 10.1002/(SICI)1096-9896(199706)182:2<145::AID-PATH819>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Falini B, Mason DY. Proteins encoded by genes involved in chromosomal alterations in lymphoma and leukemia: clinical value of their detection by immunocytochemistry. Blood. 2002;99:409–426. doi: 10.1182/blood.v99.2.409. [DOI] [PubMed] [Google Scholar]