Abstract
Niemann-Pick type C disease (NPC) is characterized by neurodegeneration secondary to impaired cholesterol trafficking and excessive glycosphingolipid storage. Abnormal cholesterol and ganglioside metabolism may influence the generation and aggregation of amyloidogenic fragments (ie, C99 and Aβ) from amyloid-β precursor protein (APP), crucial factors causing neurodegeneration in Alzheimer’s disease. To reveal whether abnormal accumulation and aggregation of APP fragments also occurs in NPC, we studied their expression in cultured cortical neurons treated with U18666A, a compound widely used to induce NPC defects, and also in brain tissues from NPC patients. U18666A treatment resulted in increased intraneuronal levels of C99 and insoluble Aβ42, which were distributed among early and late endosomes, in compartments distinct from where endogenous cholesterol accumulates. Analyses of NPC brains revealed that C99 or other APP C-terminal fragments (APP-CTF), but not Aβ42, accumulated in Purkinje cells, mainly in early endosomes. In contrast, in hippocampal pyramidal neurons, the major accumulated species was Aβ42, in late endosomes. Similar to what has been shown in Alzheimer’s disease, cathepsin D, a lysosomal hydrolase, was redistributed to early endosomes in NPC Purkinje cells, where it co-localized with C99/APP-CTF. Our results suggest that endosomal abnormalities related to abnormal lipid trafficking in NPC may contribute to abnormal APP processing and Aβ42/C99/APP-CTF deposition.
Niemann-Pick type C disease (NPC) is a fatal autosomal-recessive lipid storage disease caused chiefly by mutations within the NPC1 gene or HE1 gene. Cells that harbor mutations in NPC1 accumulate low-density lipoprotein (LDL)-derived cholesterol in late endosomes/lysosomes and exhibit defects in glycolipid sorting.1,2 The major clinical manifestations are progressive neurological defects,3 characterized by cerebellar dysfunction, dystonia, dysarthria, mental retardation, and dementia. The mechanism of the profound neurodegeneration is not understood. Although toxicity from abnormal accumulations of cholesterol and gangliosides has been implicated, recent evidence has emphasized a defect in the trafficking of late endocytic cargo. This “lipid traffic jam”4,5 may result in membrane lipid redistribution, a change in membrane elasticity and fluidity, and aberrant protein sorting and processing.
Lipid metabolism and endocytic transport play essential roles in regulating the processing of amyloid-β precursor protein (APP), a transmembrane polypeptide. In the amyloidogenic pathway, APP first undergoes proteolytic cleavage by the β-secretase to generate a 99-residue COOH-terminal membrane fragment called C99 that is subsequently cleaved by γ-secretase to generate 40-residue Aβ40 or 42-residue Aβ42. The Aβ generated in the endocytic pathway makes a major contribution to the cellular Aβ pool.6,7 Aβ is the major constituent of amyloid deposits in the Alzheimer’s disease (AD) brain. The amyloidogenic C99, Aβ40, and Aβ42 are prone to aggregation and are implicated in neurodegeneration. Aβ42, compared to Aβ40, was more hydrophobic, more toxic, and particularly prone to aggregation.8 The dominant hypothesis regarding AD pathogenesis, the amyloid cascade hypothesis,9 states that accumulation and aggregation of Aβ triggers a pathological cascade that ultimately produces the complete pathological and clinical symptoms of AD.
Recently, we and others have demonstrated that diet-induced hypercholesterolemia enhances deposition of cerebral Aβ in APP transgenic mice.10,11 Many lines of evidence suggest that membrane lipids modulate Aβ production and its conformational changes. Cholesterol depletion inhibits the generation of Aβ in hippocampal neurons and increases the solubility of Aβ.12 Cholesterol-lowering reagents reduce Aβ levels in vitro and Aβ-related pathology in vivo.13,14 Molecules involved in cholesterol metabolism and trafficking, such as acyl-coenzyme A:cholesterol acyltransferase and ATP-binding cassette transporter A1 (ABCA1), affect the generation or secretion of Aβ.15–17 Aβ binds strongly and selectively to membranes containing gangliosides, and Aβ fibril formation is accelerated by ganglioside-containing vesicles.18 It is conceivable that disturbed membrane lipid distribution and the associated abnormal vesicular trafficking in NPC cells19 likely lead to abnormal APP processing, Aβ production, and its accumulation. In this study, we ask whether Aβ or other APP proteolytic fragments accumulate in cultured neurons with NPC defects and in the brains of NPC patients.
Materials and Methods
Chemicals and Antibodies
U18666A was purchased from Biomol Research Laboratories, Plymouth Meeting, PA. Aβ40 and Aβ42 synthetic peptides were obtained from Bachem Bioscience, King of Prussia, PA. The following primary antibodies have been described previously: polyclonal B994 reactive to the carboxyl 39 amino acids of APP and monoclonal 6C4 antibody to lysobisphosphatidic acid (LBPA).20,21 The following antibodies were from commercial sources: monoclonal 4G8 to Aβ17-24 and 6E10 to Aβ1-17 were from Senetek, Napa, CA; monoclonal 10D5 to Αβ1-16 was from Athena Neuroscience, South San Francisco, CA; monoclonal 22C11 to APP66-100 was from Chemicon, Temecula, CA; end-specific polyclonal anti-Aβ40 and anti-Aβ42 were from Biosource International, Camarillo, CA; monoclonal CDF4 to human Golgin-97 was from Molecular Probes, Eugene, OR; polyclonal anti-glucose regulated protein 94 (Grp94) was from StressGen Biotechnologies, Victoria, BC, Canada; polyclonal anti-Rab7 and anti-Rab5a were from CytoSignal, Irvine, CA; monoclonal anti-EEA1 (clone 14) was from BD Biosciences, San Jose, CA; polyclonal anti-cathepsin D was from Oncogene, Boston, MA; monoclonal anti-Lamp1 (clone 1D4B) was from Developmental Studies Hybridoma Bank, Iowa City, IA. Highly cross-adsorbed goat anti-rabbit IgG and goat anti-mouse (Alexa 488- or Alexa 568-conjugated) were obtained from Molecular Probes and used as secondary antibodies.
Primary Neuronal Cultures and Adenovirus Infection
The neuron cultures used for U18666A treatment experiments were derived from newborn C57BL6 mice. Cortical neurons were prepared as described.22 Briefly, individual cells were dissociated by trypsinization (0.125% in Hanks’ balanced salt solution, Ca2+- and Mg2+-free) for 25 minutes at 37°C and washed once with Hanks’ balanced salt solution containing Ca2+ and Mg2+ after inactivating the enzyme with trypsin inhibitor. Cells were dissociated further in serum-free Neurobasal medium plus B27 supplement (Life Technologies, Gaithersburg, MD) as previously described,23 by sequential mechanical dissociation, using a Pasteur pipette with the tip lightly fire-polished. Aliquots of cells were then mixed with an equal volume of trypan blue, and dye-excluding cells were counted in a hemocytometer. Cells were plated on poly-d-lysine-coated dishes (1 μg/ml) at 5.6 × 104 cells per cm2 in serum-free Neurobasal medium plus B27 supplement and maintained at 37°C in 5% CO2. Neurobasal medium and B27 supplement represent an optimized medium for sustaining the survival of central nervous system neurons.23 The medium supports long-term survival and suppresses glial growth to <2% of the total cell population.
The adenovirus driving the expression of APP695 (Adv-APP695) was a gift from Dr. Yoshito Kinoshita, Department of Neurological Surgery, University of Washington, Seattle, WA. The viruses (Adv-APP695 and vector control Adv-cont) were kept as stock concentrations of 2 to 9 × 1010 PFU/ml. Both viruses were added to 4-day-old neuron cultures at 300 PFU per cell, and U18666A was added on the following day. Cultures were homogenized after a further 24-hour incubation.
Preparation of Cell Extracts and Western Blot Analysis
Cultured neurons were homogenized in a buffer of 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1 mmol/L ethylenediaminetetraacetic acid, 0.32 mol/L sucrose, in the presence of a protease inhibitor cocktail (Sigma, St. Louis, MO). Aliquots were taken for protein determinations by using the Bio-Rad (Hercules, CA) protein assay dye reagent. Serial extraction of cellular proteins was performed as described.24 Cells were first lysed in RIPA [150 mmol/L NaCl, 1% Nonidet P-40, 0.5% deoxycholate (DOC), 0.1% sodium dodecyl sulfate (SDS), 50 mmol/L Tris, pH 7.5] and centrifuged for 20 minutes at 40,000 × g, 4°C, in a Beckman Optima TLX ultracentrifuge (Beckman, Fullerton, CA). Supernatants were saved and the pellets, after being washed once in RIPA, were resuspended in 70% formic acid and sonicated. The formic acid samples were then dried by speed vac and resuspended in 0.1 mol/L of Tris (pH 8.0) to neutralize the formic acid.
Cell extracts containing equivalent amounts of protein were boiled for 5 minutes in sample buffer containing 5% 2-mercaptoethanol/2% SDS and analyzed by SDS-polyacrylamide gel electrophoresis. Bicine gel electrophoresis for separation of Aβ40 and Aβ42 was performed as described.25 Proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA) and probed with antibodies as previously described.26 Visualization was performed using enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ).
Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) Quantification of Aβ40 and Aβ42
The sandwich ELISA assay was performed as previously described11 with some modifications; notably, 6E10 was used as capturing antibody and anti-Aβ40 and anti-Aβ42 (Signet Laboratories, Dedham, MA) as detecting antibodies. Briefly, each well of the 96-well plate was coated with 100 μl of 5 μg/ml 6E10 overnight, followed by incubation with 100 μl of 0.5% bovine serum albumin (Sigma) for 1 hour. After three washes, Aβ peptide standards (triplicates) and samples were then added at a volume of 100 μl. The plate was then incubated at 4°C overnight. Each well was washed three times and 100 μl of detecting antibody (0.5 μg/ml, diluted in bovine serum albumin-Tween 20 buffer) was added. This was incubated for 2 hours at room temperature with gentle rotation, followed by addition of 100 μl of horseradish peroxidase-avidin (1:4000 dilution in bovine serum albumin-Tween-20 buffer; Vector Laboratories, Burlingame, CA) and a further 1-hour incubation. After three washes, Slow 3,3′,5′,5′-tetramethylbenzidine liquid substrate (slow kinetic form) (Sigma) was added and the plate was read at 630 nm every 20 seconds for 15 minutes. The value obtained for each well is the Vmax (slope of OD versus time) in the linear range of the curve. The value was then converted to pg/ml according to a standard curve. The value was multiplied by the volume of each fraction to obtain the quantity of Aβ in each fraction.
Human Brain Tissue
Paraffin-embedded brain tissue sections from NPC patients used in this study were drawn from the same pool of material used in our previous study; these had been collected worldwide.27 The collection consists of 17 cases of clinically and neuropathologically confirmed NPC. The infantile case (age 7 months) could not be included because of depletion of tissue blocks. An additional adult case (age 23 years) was added. There thus were 7 juvenile cases from 4 to 17 years of age, and 10 adult cases from 19 to 55 years of age. There were six age-matched controls. 4G8 immunocytochemical staining was performed on five juvenile cases and five adult cases with similar results. For other studies, pilot experiments were first performed using sections from a 23-year-old case that provided abundant tissues. The results were then repeated using one to four additional NPC cases.
For immunoblotting analyses, temporal, hippocampal, or cerebellar tissues from 11- and 31-year-old NPC cases and one 23-year-old control were homogenized and stored as described.27 The same batch of homogenates with same protein concentration determinations was used in the previous and the present studies.
Immuncytochemistry
Immunofluorescence labeling and confocal microscopy for subcellular localization of antigens were performed as previously described.26,28 Neurons grown on Lab-Tek Chamber Slides (Nunc, Naperville, IL) were fixed in 4% paraformaldehyde for 10 minutes at 4°C. The cells were permeabilized with 0.03% saponin, followed by primary antibodies. After incubation with Alexa-conjugated secondary antibodies the cells were mounted in Vectorshield mounting media (Vector Laboratories) containing 4,6-diamidino-2-phenylindole for nuclear label. Images were examined with a Leica DM IRBE confocal microscope, captured using a Leica TCSSP camera and Leica confocal software.
Immunofluorescent detection of Aβ species was performed using biotinylated 4G8 (Senetek, Napa, CA) at a dilution of 1:200 followed by incubation with Alexa Fluor 488-conjugated streptavidin. Other antibodies used for Aβ staining were polyclonal end-specific antibodies, anti-Aβ40 and anti-Aβ42, followed by highly cross-adsorbed Alexa Fluor 488 goat anti-rabbit.
For immunocytochemical detection of Aβ species in human brains, the brain sections were treated with 89% formic acid (Sigma) followed by 3% H2O2 incubation. Primary antibodies and biotin-conjugated 4G8 was used at a dilution of 1:200 or as suggested by the manufacturers. After washes, sections were incubated with streptavidin or secondary antibodies conjugated with horseradish peroxidase (Zymed, South San Francisco, CA) at dilution of 1:200. Staining was visualized using chromogen 3-amino-9-ethyl carbazole (AEC, Zymed). Fluorescent labeling was performed as described above.
Negative controls were performed by omitting the primary antibodies and by using nonimmune serum on representative sections. Under these conditions, no specific immunostaining was observed.
For double labeling involving filipin, the fixed cells were washed three times with phosphate-buffered saline, permeabilized with filipin, 100 μg/ml, in dH2O for 2 hours at room temperature in the dark, before the second labeling.
Morphometric measurements of Rab5a-positive areas were performed with the MetaMorph software (version 4.6; Universal Imaging Corp., Downingtown, PA), using the integrated morphometry analysis tool under a consistent classifying standard for analyzed objects. When collecting images, care was taken to ensure that they were collected at levels below detector saturation by using a color look-up table.
Results
Increased Levels of Insoluble Aβ42 and C99 in Neurons Treated with U18666A
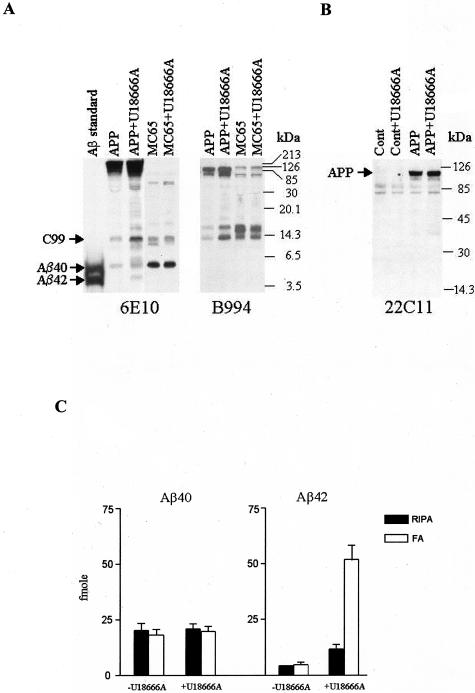
Yamazaki and colleagues29 demonstrated that Chinese hamster ovary cells or K295 cells, stably expressing APP751, accumulated significant amount of Triton-insoluble Aβ42 intracellularly after treatment with U18666A, a class 2 amphiphile that directly inhibits the function of NPC1 protein and induces an NPC-like phenotype.30 Because NPC is primarily a neurodegenerative disorder, we determined whether similar Aβ42 accumulation occurs in neurons treated with U18666A. Cultured primary mouse cortical neurons were infected with adenovirus that drove the expression of APP695 (Adv-APP695), the major isoform of APP in neurons, or with control virus (Adv-cont). As shown in Figure 1A, treatment of APP-expressing neurons with U18666A (3 μg/ml) resulted in an increased cellular level of Aβ42, which was barely recognizable in untreated cells. There was also an increase in the level of C99, which migrated as a 14-kd band,20,31 recognized by both 6E10 and B994, a polyclonal antibody reactive to the carboxyl terminal 39 amino acids of APP.20 This band also co-migrated with the overexpressed C99 in MC65 cells (see below). As expected, B994 did not recognize Aβ. A few 6E10-reactive bands with molecular masses between Aβ and C99 were not recognized by B994 either, possibly representing oligomeric or anomalously migrating forms of Aβ.32 Densitometric quantification of the intensities of the C99 bands in three repeated experiments revealed that C99 levels increased 8.6 ± 1.3-fold after U18666A treatment. The cellular level of Aβ40 remained unchanged after U18666A treatment. In addition, multiple bands with molecular masses between C99 and APP were present only in U18666A-treated neurons, recognized by 6E10 and/or by B994. These bands may represent aggregates of Aβ, C99, and other C-terminal fragments of APP (APP-CTF). Supporting this notion, these bands were diminished by formic acid extraction to disrupt the aggregates (not shown).
Figure 1.
U18666A treatment increases the level of C99 and insoluble Aβ42 in cultured cortical neurons but not in MC65 neuroblastoma cells expressing C99. A: Cortical neurons from newborn mice were infected with Adv-APP695 on day 4 in culture and treated with U18666A (3 μg/ml) on day 5 for 24 hours (APP+U18666A). MC65 cells were cultured in serum-free Opti-MEM medium without tetracycline to induce C99 expression for 24 hours, followed by U18666A (3 μg/ml) treatment (MC65+U18666A). For control cultures (labeled as APP and MC65), equal volumes of solvent were added. Equal amounts (10 μg from cortical neurons and 5 μg from MC65 cells) of proteins from cell homogenates were subjected to bicine SDS gel electrophoresis and Western blot analysis using 6E10 and B994 antibodies. The locations of C99, Aβ40, and Aβ42 bands are indicated. The Aβ standard contains a mixture of synthetic Aβ40 and Aβ42. Note that in bicine SDS-polyacrylamide gel electrophoresis, Aβ42 has a paradoxically lower apparent molecular mass than Aβ40.25 MC65 cells expressed relatively less endogenous APP; however, on longer exposure of the film, the APP bands recognized by 6E10 in MC65 samples became apparent. The experiment was performed three times with similar results. B: Same but independent experiment using cortical neurons was performed as described in A. The proteins were separated by 10 to 20% gradient Tris-HCL gel electrophoresis and the blot analyzed by 22C11 antibody. Shown are cultured neurons infected with Adv-cont (Cont) or Adv-APP695 (APP) without or with treatment of U18666A. C: Cortical neurons expressing APP695 with or without U18666A were sequentially extracted in RIPA followed by formic acid (FA), and the extracts were subjected to sandwich ELISA assay for quantification of Aβ40 and Aβ42. Depicted are mean values from two independent experiments, each done with duplicate samples. Error bars indicate 1 SD.
The possible change of APP level after U18666A treatment was separately evaluated using Tris-HCl gradient gel electrophoresis, which provides a better separation of higher molecular weight proteins. The Western blot probed with 22C11 (specific for N-terminal portion of APP, or APP66-100) in Figure 1B demonstrated the expected expression of APP after infection with Adv-APP695. The level of APP remained unchanged after U18666A treatment, which could not explain the increased cellular levels of C99 and Aβ42.
A β-cleavage is required to produce C99 and a subsequent γ-cleavage on C99 is required to produce Aβ. Therefore, the generation of Aβ depends on the levels of C99. In the next experiment, we used a neuroblastoma cell line MC65 that conditionally expresses C99 and accumulates Aβ40 throughout time,33,34 mainly in the late endosome/lysosome (L-W Jin and I Maezawa, unpublished observation). The use of this cell line enabled us to bypass the influence of U18666A on β-cleavage and assess its effect on γ-cleavage directly. Treatment of MC65 cells with U18666A did not result in any changes in the levels of Aβ40, indicating that γ-cleavage activity, at least the activity for Aβ40 generation, was not influenced by U18666A treatment (Figure 1A). Furthermore, C99 levels were also unchanged, implying that U18666A treatment did not alter the retention or degradation of C99. This result suggests that increased C99 and Aβ42 in U18666A-treated neurons was at least in part because of increased β-secretase activity that generates more C99 for subsequent Aβ production.
Because Aβ is found as insoluble deposits in amyloid plaques of the AD brain and, in particular, Aβ42 is more abundantly present in the insoluble intracellular pool in neurons than Aβ40,24 we considered the possibility of an altered solubility of neuronal Aβ species after U18666A treatment. In this analysis, neurons were sequentially extracted in detergent buffer (RIPA), and then in 70% formic acid, followed by sandwich ELISA to determine the levels of Aβ40 and Aβ42 in each extracted fraction.24 As shown in Figure 1C, U18666A treatment did not alter the levels of Aβ40, nor its distribution among RIPA- and formic acid-solubilized fractions. By contrast, U18666A treatment increased the total levels of Aβ42 ∼7.5-fold. Formic acid extraction revealed a much larger pool of Aβ42 in the U18666A-treated neurons. In the RIPA-solubilized pool, the average Aβ42/Aβ40 ratio was 0.58, whereas in formic acid-solubilized pool, 2.7. This result suggests that treatment with U18666A results in an expanded pool of insoluble Aβ, which tends to aggregate and become cytotoxic. Because our ELISA assay only detects Aβ species but not the C99, we do not know the extent of solubility of the expanded C99 pool in U18666A-treated neurons. A high propensity of C99 to aggregation has also been demonstrated.31,35,36 It is possible that the pool of insoluble APP fragments after the inclusion of C99 may be even larger than what we have detected via our measurements of Aβ.
Aβ-Positive Deposits Reside in Endosomal Compartments Distinct from Cholesterol-Rich Compartment in U18666A-Treated Neurons
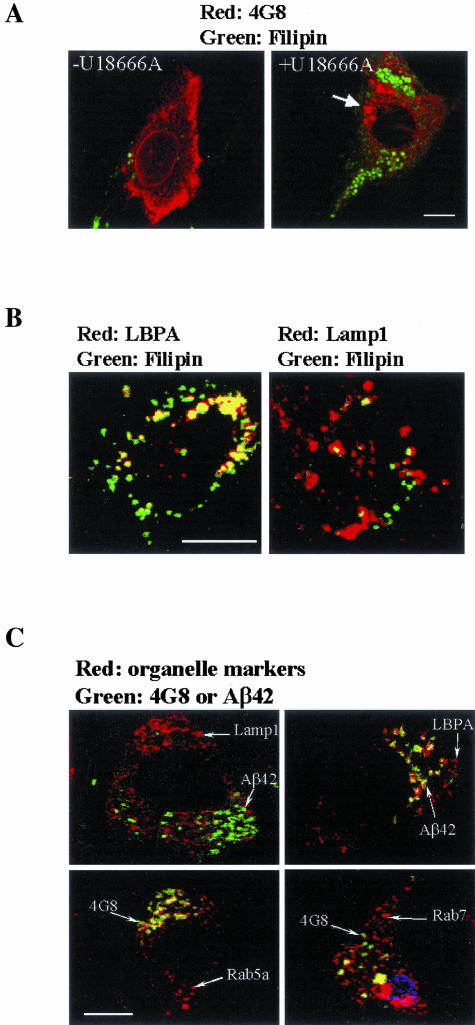
Because Aβ species in U18666A-treated neurons become less soluble and form higher molecular weight complexes, they may form intracellular aggregates that are readily stained by antibodies reactive to Aβ after formic acid pretreatment, as described in APP transgenic mice.26 In untreated cells, the Aβ immunoreactivities as detected by 4G8 appeared diffuse or finely granular. In contrast, the U18666A-treated neurons contained coarse granules or larger blotches, suggesting an intracellular aggregation (Figure 2A). The neurons infected with control adenovirus were entirely 4G8-negative. The same Aβ immunoreactivities were obtained using another Aβ antibody, 10D5 (data not shown). To determine whether there is a direct or close interaction between Aβ deposits and accumulated cholesterol, we performed confocal microscopy of neurons double labeled with antibodies to Aβ and filipin for free cholesterol. Filipin positivity was present in U18666A-treated cells as numerous punctate structures in the cell body and proximal portion of processes, indicative of cytoplasmic cholesterol-laden vesicles. Very few filipin-positive vesicles were present in untreated neurons (Figure 2A). Notably, since these neurons were established and cultivated in serum-free defined media for 6 days when assayed, the detected cholesterol was from endogenous synthesis. A similar staining pattern for endogenous cholesterol was demonstrated in cultured murine sympathetic neurons by Karten and colleagues.37 In contrast to what has been shown in cells enriched with LDL cholesterol,29,38 filipin-positive vesicles are entirely distinct from 4G8- or 10D5-positive granules (Figure 2A). Consistent with previous observations from nonneuronal cells, in U18666A-treated neurons, cholesterol accumulated in late endosomes that contained LBPA,21 and also in lysosomes positive for Lamp1 (Figure 2B).
Figure 2.
Aβ-positive deposits reside in endosomal compartments distinct from cholesterol-rich compartment in U18666A-treated neurons. Confocal microscopy was performed on double-fluorescence-labeled U18666A-treated neurons to determine the subcellular location of Aβ-positive deposits. Neurons were cultured and treated as described in Figure 1. Cells were then fixed and double labeled with filipin and 4G8 in LBPA (A) and Lamp1 (B). The arrow in A points to a large 4G8-positive granule. In C, neurons were double labeled with paired antibodies to Aβ (4G8 or anti-Aβ42) and to organelle markers (Lamp1, LBPA, Rab7, and Rab5a) in a manner that a monoclonal antibody was paired with a polyclonal antibody. Shown here are merged images. The color of fluorescence applied to each primary antibody is indicated. The yellow color in the merged images indicates co-localization. Scale bars, 4 μm.
To reveal the nature of the vesicular compartment(s) in which Aβ deposits reside, we performed double immunolabeling using 4G8 and specific antibodies for organelle markers. To exclude the possibility that the 4G8-positive deposits were entirely composed of APP or APP fragments but not Aβ, we also used polyclonal Aβ end-specific antibodies, anti-Aβ40 and anti-Aβ42, in some experiments. These two end-specific antibodies do not recognize APP fragments containing Aβ domain that is not at the C-terminal end.39 The specificity of these antibodies when used in immunocytochemistry has been tested by our laboratory.26 Using the same dilution (1:50) as was used to demonstrate the intraneuronal Αβ40 deposits in APP mice,26 we could not demonstrate Aβ40 deposits in U18666A-treated neurons. Instead, most 4G8-positive deposits are also Aβ42- and B994-positive, supporting a mixture of C99 (or other APP-CTF) and Aβ42. Figure 2C demonstrates that a small majority of Aβ-positive deposits resided in vesicles positive for Rab5a, whereas the rest are in vesicles positive for LBPA and Rab7. Clearly, areas of Aβ42 positivity are distinct from those positive for Lamp1, a lysosomal membrane marker, as well as the Golgi marker Golgin-97 and the endoplasmic reticulum marker Grp94 (not shown). These results indicate that Aβ42/C99/APP-CTF in U18666A-treated neurons accumulated in early endosomes and in a portion of late endosomes that do not accumulate cholesterol.
Accumulation of C99/APP-CTF in NPC Purkinje Cells and Its Localization in Early Endosomes
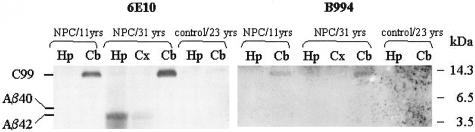
To examine the pathological relevance of the results obtained from U18666A treatment of cultured neurons, experiments were next performed on brain tissues from NPC patients and age-matched controls. Figure 3 shows Western blot analyses of brain homogenates from different brain regions. Because of the very limited availability of good quality frozen brain tissues from NPC and age-matched control patients, we could only obtain one juvenile (11 years old) and one adult (31 years old) NPC case and one 23-year-old control case27 for this experiment. In both NPC cases, the cerebellum contained significant amount of C99, recognized by both 6E10 and B994. Aβ42 was present in the hippocampus, and to a lesser extent, in the temporal cortex, of the adult NPC case, but not in the juvenile NPC case. Both C99 and Aβ42 were absent in the control.
Figure 3.
Increased levels of C99 in NPC cerebellum and Αβ42 in NPC hippocampus. Equal amounts of protein in homogenates of cerebral cortex (Cx), cerebellum (Cb), and hippocampus (Hp) from a juvenile NPC subject (11 years old), an adult NPC subject (31 years old), and a normal control (23 years old) were subjected to bicine SDS-polyacrylamide gel electrophoresis and Western blot analysis using 6E10 (left). Because of the limitation of available brain homogenates, the blot was then stripped by boiling in acidic pH buffer, neutralized, and reprobed with B994 antibody (right). The positions of the C99, Aβ40, and Aβ42 bands are indicated.
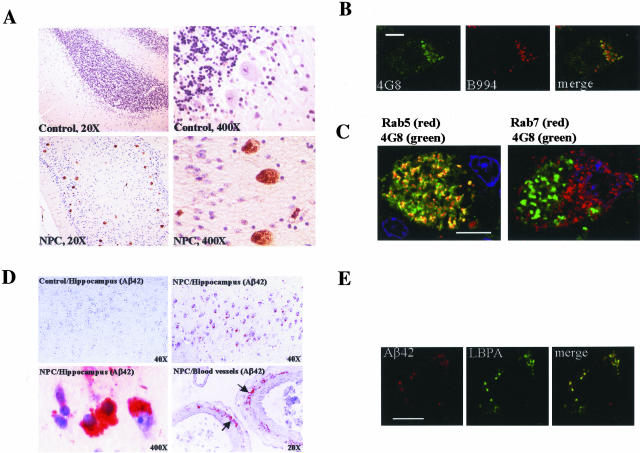
By immunohistochemistry, all juvenile (n = 5) and adult (n = 5) NPC cases show variable but significant Aβ immunoreactivities in neurons. In the NPC cerebellum, 4G8 strongly stained the surviving Purkinje cells. The 4G8 staining in the cerebellum from age-matched controls and cognitively normal aged individuals (up to 76 years old) was entirely negative by the immunoperoxidase method (Figure 4A). The same result was obtained using 10D5. Despite abundant 4G8 staining in NPC Purkinje cells, anti-Aβ40 and anti-Aβ42 yielded completely negative staining. On the other hand, B994-positive areas overlapped substantially with 4G8-positive areas (Figure 4B), suggesting that the accumulated APP fragments contained both the Aβ domain and the most C-terminal domain of APP. Considering our immunoblot finding that C99 accumulated, while Aβ was absent in the NPC cerebellar homogenates, it is likely that the accumulated 4G8 antigen was in fact C99, but not Aβ itself. However, the possible accumulation of other Aβ-containing fragments or APP-CTF cannot be excluded. 22C11 failed to stain the deposits (data not shown), suggesting the absence of N-terminal epitopes or full-length APP in the deposits.
Figure 4.
C99 accumulates in the early endosomes of NPC Purkinje cells and Aβ42 accumulates in NPC hippocampal pyramidal neurons, cerebral blood vessels, and co-localizes with LBPA. A: Paraffin-embedded sections from control and NPC cerebellums were immunostained with 4G8. Color development was then obtained using a DAB staining kit and Meyer’s hematoxylin counterstain. Presented are cerebellar sections from a 23-year-old NPC subject and an age-matched control. Original magnifications are indicated. B: Confocal microscopic images of a representative Purkinje cell in NPC cerebellar sections double labeled with 4G8 and B994. C: Confocal microscopic images of a representative Purkinje cell in NPC cerebellar sections double labeled with 4G8 and endosomal markers Rab5a and Rab7. Shown are merged images. The color of fluorescence applied to each primary antibody is indicated. The yellow color in the merged images indicates co-localization. D: Paraffin-embedded sections from control and NPC hippocampus were immunostained with anti-Aβ42. Color development was then obtained using red chromogen 3-amino-9-ethyl carbazole (AEC) and Meyer’s hematoxylin counterstain. Arrows point to Aβ42 deposits in the blood vessel wall. Original magnifications are indicated. E: Confocal microscopic images of a representative pyramidal neuron in NPC CA1 double labeled with antibodies to Aβ42 and LBPA. The yellow color in the merged images indicates co-localization. Scale bars, 4 μm.
Double-immunolabeling experiments demonstrated that in NPC Purkinje cells, a large majority of 4G8-positive deposits resided in Rab5a-positive early endosomes. They are entirely absent in Rab7-positive late endosomes (Figure 4C). In addition, the 4G8-positive early endosomes appear enlarged in size, compared to Rab7-positive late endosomes. Thus, there is a close concordance between cultured neurons treated with U18666A and NPC Purkinje cells in that they both accumulate amyloidogenic fragments of APP, mainly in their early endosomes.
Accumulation of Aβ42 in Cortical and Hippocampal Neurons and in Blood Vessels in the NPC Brain
Our immunoblot experiment demonstrated an accumulation of Aβ42 in the hippocampus, and to a lesser degree, in the cerebral cortex, in an adult NPC case. Because hippocampus is the major site afflicted by AD and since late in the course of NPC, the patients also present dementia, we examined NPC hippocampus for possible Aβ42 deposits. In the hippocampus of adult NPC patients, numerous Αβ42-positve neurons were seen in CA1 (Figure 4D) and some in entorhinal cortex, a pattern similar to early AD.40 There was also Aβ42 immunoreactivity in or around the tunica media of the small arteries (Figure 4D). This Aβ42 angiopathy is rarely seen in normal young adults. The same staining pattern was also observed using antibodies 4G8 and 10D5, but not in age-matched controls or cognitively normal aged individuals. The Aβ42 immunopositivities in the hippocampus of juvenile NPC cases were quite variable. Thus, different from cerebellum, the NPC cortex and hippocampus have an accumulation of Aβ42, which seems to be age-dependent. Despite this, there was no extracellular amyloid plaque formation as seen in AD. Interestingly, Aβ42 was localized to LBPA-positive late endosomes in hippocampal pyramidal neurons (Figure 4E).
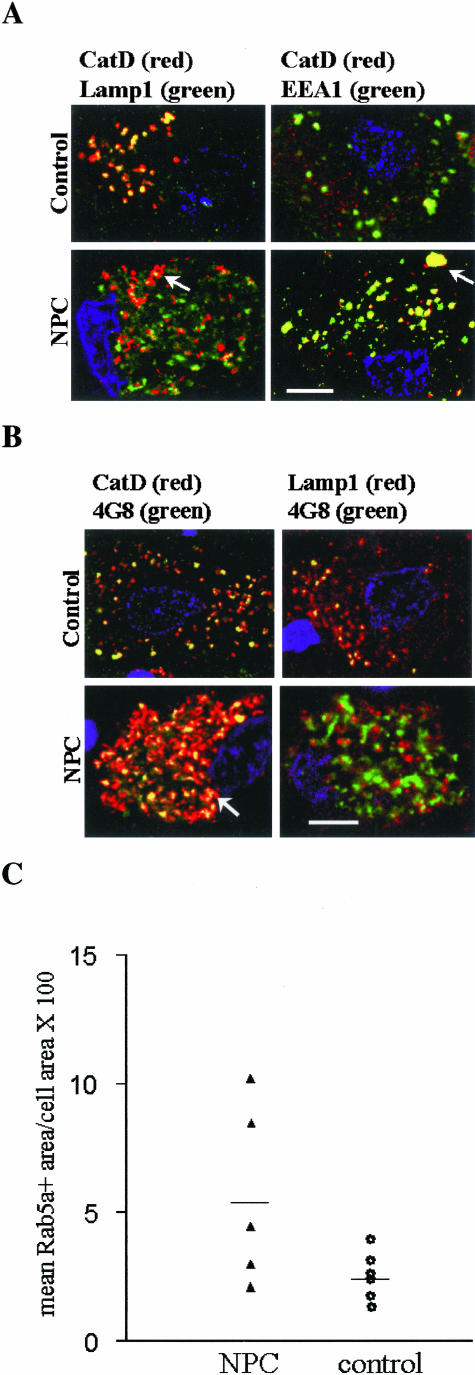
Redistribution of Cathepsin D (CatD) to Early Endosomes in NPC Purkinje Cells
Previously it was demonstrated that CatD, a major lysosomal aspartic protease, was redistributed to early endosomes in AD brains but rarely so in normal brains. This was considered as a potential mechanism of increased β-amyloidogenesis.41 Consistent with this notion, we demonstrated that in APP mice, intracellular accumulation of Aβ was associated with a redistribution of CatD.26 To determine whether the same phenomenon occurs in NPC, we performed double-immunolabeling experiments using a polyclonal antibody reactive to the mature form of CatD, paired with 4G8, Lamp1, and EEA1, a monoclonal marker for early endosomes. As shown in Figure 5A, there was an almost complete co-localization of Lamp1 and CatD in control Purkinje cells. Co-localization of CatD and EEA1 was seen in a small portion (13.9 ± 4.1%, mean ± SEM of three patients, each with 25 neurons counted) of neuronal early endosomes. However, in NPC Purkinje cells, only a small portion of CatD-positive granules were also Lamp1-positive; the majority (79.4 ± 6.6%, mean ± SEM of three patients, each with 25 neurons counted) of them co-localized with EEA1. Statistical analysis using Student’s t-test revealed a two-tailed P value <0.005 for comparison of above measurements between NPC and controls. Interestingly, similar to what was found in the at-risk neurons in AD brains,41 there were atypically large early endosomes highlighted by CatD and EEA1. We also determined whether CatD was co-localized with 4G8-positive deposits, which were localized to early endosomes. Figure 5B shows that in control Purkinje cells, the 4G8 staining was finely granular, and was co-localized with both Lamp1 and CatD, consistent with lysosomal localization of the Aβ epitope. Approximately 30% of the lysosomes were 4G8-positive. However, in NPC Purkinje cells, the 4G8 staining was coarsely granular, co-localized with CatD, but entirely distinct from Lamp1. These results indicate that in NPC Purkinje cells, there is a redistribution of CatD to early endosomes that tend to be enlarged. Morphometric measurements of the percentages of Purkinje cell volume occupied by Rab5a-positive early endosomes revealed a trend toward larger early endosomes in NPC Purkinje cells, but there was no statistically significant difference between NPC and control cases (Figure 5C). Because the intraneuronal accumulation of APP amyloidogenic fragments and CatD redistribution are common denominators in AD and NPC, the early endosomal abnormalities appear to be intimately associated with abnormal APP metabolism in both diseases.
Figure 5.
CatD is redistributed to early endosomes and co-localizes with C99. A: Confocal microscopic images of control and NPC Purkinje cells double labeled with antibodies to CatD and Lamp1 or EEA1. The arrow points to an enlarged CatD- and EEA1-positive vesicle. B: Confocal microscopic images of control and NPC Purkinje cells double labeled with 4G8 and antibodies to CatD and Lamp1. Shown are merged images. The color of fluorescence applied to each primary antibody is indicated. The yellow color in the merged images indicates co-localization. The arrow points to an enlarged CatD- and 4G8-positive vesicle. C: Control and NPC cerebellar sections were immunostained with Rab5a; high-power images of 25 randomly selected Purkinje cells were taken and the total area of immunopositive objects per cell was obtained using Metamorph software. Values presented are mean percentage of cell area occupied by Rab5a-positive early endosomes in each individual brain. The horizontal line indicates the mean value of patients in each group. Statistical analysis using Student’s t-test revealed a two-tailed P value of 0.06 for comparison between NPC and controls. Scale bars, 4 μm.
Discussion
Extensive effort has emphasized the pathological role of amyloidogenic fragments such as Aβ (especially Aβ42) and C99 in AD. Our in vitro and in vivo results suggest that Aβ-related pathways leading to neuronal degeneration in AD may also play a role in NPC. We demonstrated a substantial accumulation of Αβ42/C99/APP-CTF, likely in aggregated state, in neurons with NPC defects. A major portion of these deposits resided in early endosomes. In neuronal cultures, the endosomal compartments harboring these deposits were distinct from where endogenous cholesterol accumulated. The concordance of findings in cultured neurons and in NPC brains regarding these depositions suggests that they are more or less direct consequences of NPC defects, rather than secondary to neurodegeneration or glial abnormalities. These results also suggest that, at least in neurons, endosomal abnormalities, rather than accumulated cholesterol, make a more direct contribution to Aβ/C99/APP-CTF accumulation and aggregation. Yet another similarity between AD and NPC is that CatD, a lysosomal hydrolase, was redistributed to early endosomes where it co-localized with C99/APP-CTF in NPC Purkinje cells, further supporting the presence of endosomal abnormalities.
The aggregated state of Aβ42/C99 was suggested by the insolubility of accumulated Aβ42 and the detection of higher MW Aβ- and/or C99-immunoreactive species by Western blot analyses. This result is consistent with the reports of Yamazaki and colleagues29 and Runz and colleagues.38 However, some observations distinguish our results from theirs. First, the Aβ immunoreactivities were found in endosomes without accumulated cholesterol. This result does not support a direct or close interaction between Aβ deposits and accumulated cholesterol. Second, C99 levels were increased in both U18666A-treated neurons and in NPC Purkinje cells, suggesting an enhanced β-cleavage activity. In contrast, results from Runz and colleagues38 using neurons or neuroblastoma cells cultured in LDL-enriched medium suggest a reduction in β-cleavage after U18666A treatment.
We speculate that these discrepancies came from the different sources of cholesterol studied. The accumulated cholesterol in our study was from endogenous synthesis, whereas that in Yamazaki and colleagues29 and Runz and colleagues38 was from internalized LDL cholesterol. Because the major source of cholesterol in neurons is from endogenous synthesis rather than from exogenous uptake,42,43 it was suggested that the defect in endogenous cholesterol trafficking is a significant cause for NPC neurodegeneration.44 Karten and colleagues45 measured the antegrade transport of cholesterol in npc1−/− neurons and found that LDL cholesterol was normally transported into axons whereas endogenous cholesterol was not, implying a unique pathological role of endogenous cholesterol. Blocking the accumulation of cholesterol through the LDL receptor pathway does not alter the degenerative phenotype of NPC.46 Therefore, the well-documented NPC defect in LDL-derived cholesterol transport, the paradigm used by Yamazaki and colleagues29 and Runz and colleagues,38 may not lead to a complete understanding of neurodegeneration in NPC. In this respect, it is important to address neuron-specific economy of cholesterol in future experiments, to yield useful information. Karten and colleagues45 proposed that a reduction in the cholesterol content of NPC1-deficient axons because of impaired antegrade transport of endogenous cholesterol may be one of the fundamental mechanisms of neurodegeneration in NPC. Among many severe consequences, this deficiency may also affect the endosomal transport and processing of APP. APP undergoes fast axonal transport to nerve terminals,47 where full-length APP is present at the surface and then endocytosed and transported retrogradely in endosomal vesicles to the neuronal soma.48,49 The processing of APP and generation of Aβ/C99 in neurons is tightly coupled to its endosomal transport. Therefore, we hypothesize that in NPC, the lack of cholesterol in endosomal trafficking system contributes to abnormal APP processing and Aβ42/C99 deposition.
The effect of NPC defects on γ-secretase activity seems to depend on the type of neurons. Experiments using MC65 cells expressing C99 failed to support an effect of U18666A treatment on γ-cleavage, at least on the activity generating Aβ40. In NPC Purkinje cells, no Aβ was detected despite large amounts of C99; this lack of γ-cleavage of C99 may be a direct consequence of NPC defects, because cerebellar neurons are capable of producing Aβ.50 However, the γ-cleavage activity was robust in NPC hippocampal neurons, judging from their generation of Aβ42. Interestingly, the two isoforms of presenilins (membrane proteins required for γ-secretase activity) are differentially distributed among brain structures. Purkinje cells uniquely show high levels of presenilin 2 immunoreactivity compared to other human brain regions.51 A study of the differential regulation of presenilin 1 and presenilin 2 by altered cholesterol metabolism such as seen in NPC may yield useful insight to the differential display of γ-secretase activity between cerebellar and hippocampal neurons.
In addition to their effect on Αβ42/C99 production, NPC defects may also decrease its degradation, a result of abnormal sorting or decreased degradation enzyme activity. There are two notable possibilities, which may explain selective Aβ42 accumulation. First, Aβ42 may be retained in abnormal membrane domains. Tissues with NPC defects have been shown to have abnormal composition of membrane lipid rafts.52 Abnormal raft composition has been found associated with increased insoluble Aβ42.53 Second, Aβ42 may be bound to accumulated lipids such as glycosphingolipids, the major storage material in NPC brains.54 Sugimoto and colleagues55 reported accumulation of GM1 ganglioside in the early endosomes of NPC1-deficient cells. This raises the possibility that the early endosomal accumulation of Aβ42 in NPC neurons may be secondary to GM1. Membrane GM1 has been shown to selectively bind Aβ42 tightly, induce a change in Aβ conformation, and accelerate its aggregation.18,56 Whether GM1 plays a similar role in NPC remains to be determined.
There was an age-related increase in CatD-containing microglial cells in the thalamus and cerebellum of BALB/c npcnih mice.57 Revealed by our human study, CatD accumulates in both Purkinje cells and microglia (not shown). CatD was redistributed to early endosomes in Purkinje cells, but not in hippocampal neurons (not shown). By contrast, in AD, altered CatD distribution was seen in cortical and hippocampal neurons.41 Therefore, this altered distribution seems to occur in at-risk neurons or damaged neurons, indicating its association with neurodegeneration in both diseases. Early endosomal abnormalities were shown to be an early event in AD.58 The increased activity of CatD may affect the processing of APP. In vitro, C99 can be cleaved by CatD to produce Aβ42.59,60 However, despite the co-localization of C99/APP-CTF and CatD in NPC Purkinje cells, we could not detect Aβ in the NPC cerebellum. On the other hand, it was reported that hippocampal slices incubated with Aβ42 had 56% greater concentrations of CatD than controls,61 suggesting that accumulation of amyloidogenic Aβ may regulate CatD levels. In any event, the co-localization of C99/APP-CTF and CatD in early endosomes of NPC Purkinje cells constitutes a unique pathology reflecting the abnormal membrane properties of endosomal vesicles, resulting in abnormal endosomal transport and processing of APP and CatD.
The pathological consequence of intraneuronal Aβ42/C99/APP-CTF accumulation in NPC is unknown, although intraneuronal Aβ42 accumulation has been hypothesized to be an early and toxic event in AD.62,63 Aβ42 accumulates within vulnerable neurons in APP transgenic mice26 and in AD brains.40 Intracellular, rather than extracellular, Aβ may be the initial site of Aβ aggregation and neurotoxicity.64 Our results are consistent with the finding that intracellular Aβ42 is rather insoluble and resistant to degradation.24,65 Intracellular accumulation of C99 also causes various toxic effects and behavioral abnormalities in transgenic animals.66–70 In particular, Glabe71 proposed a mechanism that intracellular Aβ42 aggregates provide seeds for further assembly of amyloidogenic fragments of APP such as C99, as a possible starting point for neurodegeneration in AD. Our results are consistent with the presence of homo- or heteropolymeric aggregates of Aβ42/C99 in NPC neurons, implying the potential toxicity from these aggregates.
Footnotes
Address reprint requests to Lee-Way Jin, M.D., Ph.D., Department of Pathology, Box 359791, University of Washington School of Medicine, Seattle, WA 98104-2499. E-mail: lwjin@u.washington.edu.
Supported by grants from the Lambright Foundation (to L.W.J. and I.V.).
References
- Liscum L, Klansek JJ. Niemann-Pick disease type C. Curr Opin Lipidol. 1998;9:131–135. doi: 10.1097/00041433-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Strauss JF, Liu P, Christenson LK, Watari H. Sterols and intracellular vesicular trafficking: lessons from the study of NPC1. Steroids. 2002;67:947–951. doi: 10.1016/s0039-128x(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Vanier MT, Suzuki K. Niemann-Pick disease. Moser HW, editor. Amsterdam: Elsevier Science,; Handbook of Clinical NeurologyNeurodystrophies and Neurolipidosis. 1996:pp 132–162. [Google Scholar]
- Liscum L. Niemann-Pick type C mutations cause lipid traffic jam. Traffic. 2000;1:218–225. doi: 10.1034/j.1600-0854.2000.010304.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR. Cholesterol: stuck in traffic. Nat Cell Biol. 1999;1:E37–E38. doi: 10.1038/10030. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Αβ42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT., Jr The C-terminus of the β protein is critical in amyloidogenesis. Ann NY Acad Sci. 1993;695:144–148. doi: 10.1111/j.1749-6632.1993.tb23043.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC. Diet-induced hypercholesterolemia enhances brain Aβ accumulation in transgenic mice. Neuroreport. 2002;13:455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Αβ42 and Αβ40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat Cell Biol. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Aβ levels. J Biol Chem. 2002;277:48508–48513. doi: 10.1074/jbc.M209085200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Ikonomovic MD, Skoko J, Lefterov PI, Isanski BA, DeKosky ST, Lazo JS. 22R-Hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid β secretion. J Biol Chem. 2003;278:13244–13256. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by binding of Aβ-(1-40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997;272:22987–22990. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR. Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic. 2002;1:203–211. doi: 10.1034/j.1600-0854.2000.010302.x. [DOI] [PubMed] [Google Scholar]
- Jin LW, Hearn M, Ogburn CE, Dang N, Nochlin D, Ladiges WC, Martin GM. Transgenic mice over-expressing the C-99 fragment of βPP with an α-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol. 1998;153:1679–1686. doi: 10.1016/s0002-9440(10)65681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Xiang H, Hochman DW, Saya H, Fujiwara T, Schwartzkroin PA, Morrison RS. Evidence for p53-mediated modulation of neuronal viability. J Neurosci. 1996;16:6753–6765. doi: 10.1523/JNEUROSCI.16-21-06753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Doms RW, Lee VM. Detection of a novel intraneuronal pool of insoluble amyloid beta protein that accumulates with time in culture. J Cell Biol. 1998;141:1031–1039. doi: 10.1083/jcb.141.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klafki HW, Wiltfang J, Staufenbiel M. Electrophoretic separation of βA4 peptides (1-40) and (1-42). Anal Biochem. 1996;237:24–29. doi: 10.1006/abio.1996.0195. [DOI] [PubMed] [Google Scholar]
- Shie FS, LeBoeur RC, Jin LW. Early intraneuronal Aβ deposition in the hippocampus of APP transgenic mice. Neuroreport. 2003;14:123–129. doi: 10.1097/01.wnr.0000051151.87269.7d. [DOI] [PubMed] [Google Scholar]
- Bu B, Klunemann H, Suzuki K, Li J, Bird T, Jin LW, Vincent I. Niemann-Pick disease type C yields possible clue for why cerebellar neurons do not form neurofibrillary tangles. Neurobiol Dis. 2002;11:285–297. doi: 10.1006/nbdi.2002.0551. [DOI] [PubMed] [Google Scholar]
- Vuletic S, Jin LW, Marcovina SM, Peskind ER, Moller T, Albers JJ. Widespread distribution of phospholipid transfer protein (PLTP) in human central nervous system: evidence for PLTP synthesis by glia and neurons and for increased levels in Alzheimer’s disease. J Lipid Res. 2003;44:1113–1123. doi: 10.1194/jlr.M300046-JLR200. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Chang TY, Haass C, Ihara Y. Accumulation and aggregation of amyloid β-protein in late endosomes of Niemann-pick type C cells. J Biol Chem. 2001;276:4454–4460. doi: 10.1074/jbc.M009598200. [DOI] [PubMed] [Google Scholar]
- Lange Y, Ye J, Rigney M, Steck T. Cholesterol movement in Niemann-Pick type C cells and in cells treated with amphiphiles. J Biol Chem. 2000;275:17468–17475. doi: 10.1074/jbc.M000875200. [DOI] [PubMed] [Google Scholar]
- Wolf D, Quon D, Wang Y, Cordell B. Identification and characterization of C-terminal fragments of the β-amyloid precursor produced in cell culture. EMBO J. 1990;9:2079–2084. doi: 10.1002/j.1460-2075.1990.tb07375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ. Aggregation of secreted amyloid β-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- Sopher BL, Fukuchi K, Smith AC, Leppig KA, Furlong CE, Martin GM. Cytotoxicity mediated by conditional expression of a carboxyl-terminal derivative of the beta-amyloid precursor protein. Brain Res Mol Brain Res. 1994;26:207–217. doi: 10.1016/0169-328x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Jin LW, Hua DH, Shie FS, Maezawa I, Sopher B, Martin GM. Novel tricyclic pyrone compounds prevent intracellular APP C99-induced cell death. J Mol Neurosci. 2002;19:57–61. doi: 10.1007/s12031-002-0011-9. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Terakado K, Usami M, Yoshikawa K. Formation of amyloid-like fibrils in COS cells overexpressing part of the Alzheimer amyloid protein precursor. Nature. 1990;347:566–569. doi: 10.1038/347566a0. [DOI] [PubMed] [Google Scholar]
- Kammesheidt A, Boyce FM, Spanoyannis AF, Cummings BJ, Ortegon M, Cotman C, Vaught JL, Neve RL. Deposition of β/A4 immunoreactivity and neuronal pathology in transgenic mice expressing the carboxyl-terminal fragment of the Alzheimer amyloid precursor in the brain. Proc Natl Acad Sci USA. 1992;89:10857–10861. doi: 10.1073/pnas.89.22.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten B, Vance DE, Campenot RB, Vance JE. Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons. J Neurochem. 2002;83:1154–1163. doi: 10.1046/j.1471-4159.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R, Hartmann T. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci. 2002;22:1679–1689. doi: 10.1523/JNEUROSCI.22-05-01679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Barnett JL, Pieroni C, Nixon RA. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci. 1997;17:6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond J, Korsak RA, Morrow JW, Torok-Both G, Catlin DH. Dietary cholesterol and the origin of cholesterol in the brain of developing rats. J Nutr. 1991;121:1323–1330. doi: 10.1093/jn/121.9.1323. [DOI] [PubMed] [Google Scholar]
- Turley SD, Burns DK, Rosenfeld CR, Dietschy JM. Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J Lipid Res. 1996;37:1953–1961. [PubMed] [Google Scholar]
- Cruz JC, Chang TY. Fate of endogenously synthesized cholesterol in Niemann-Pick type C1 cells. J Biol Chem. 2000;275:41309–41316. doi: 10.1074/jbc.M008272200. [DOI] [PubMed] [Google Scholar]
- Karten B, Vance DE, Campenot RB, Vance JE. Trafficking of cholesterol from cell bodies to distal axons in Niemann Pick C1-deficient neurons. J Biol Chem. 2003;278:4168–4175. doi: 10.1074/jbc.M205406200. [DOI] [PubMed] [Google Scholar]
- German DC, Quintero EM, Liang C, Xie C, Dietschy JM. Degeneration of neurons and glia in the Niemann-Pick C mouse is unrelated to the low-density lipoprotein receptor. Neuroscience. 2001;105:999–1005. doi: 10.1016/s0306-4522(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikin AF, Annaert WG, Takei K, De Camilli P, Jahn R, Greengard P, Buxbaum JD. Alzheimer amyloid protein precursor is localized in nerve terminal preparations to Rab5-containing vesicular organelles distinct from those implicated in the synaptic vesicle pathway. J Biol Chem. 1996;271:31783–31786. doi: 10.1074/jbc.271.50.31783. [DOI] [PubMed] [Google Scholar]
- Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH. Trafficking of cell-surface beta-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci. 1997;17:140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Lang W. Alzheimer’s disease: amyloid plaques in the cerebellum. J Neurol Sci. 1989;93:277–287. doi: 10.1016/0022-510x(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Mathews PM, Cataldo AM, Kao BH, Rudnicki AG, Qin X, Yang JL, Jiang Y, Picciano M, Hulette C, Lippa CF, Bird TD, Nochlin D, Walter J, Haass C, Levesque L, Fraser PE, Andreadis A, Nixon RA. Brain expression of presenilins in sporadic and early-onset, familial Alzheimer’s disease. Mol Med. 2000;6:878–891. [PMC free article] [PubMed] [Google Scholar]
- Garver WS, Erickson RP, Wilson JM, Colton TL, Hossain GS, Kozloski MA, Heidenreich RA. Altered expression of caveolin-1 and increased cholesterol in detergent insoluble membrane fractions from liver in mice with Niemann-Pick disease type C. Biochim Biophys Acta. 1997;1361:272–280. doi: 10.1016/s0925-4439(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Morishima-Kawashima M, Waki H, Kobayashi K, Kuramochi T, Frosch MP, Ding K, Ito M, Kim TW, Tanzi RE, Oyama F, Tabira T, Ando S, Ihara Y. Mutant presenilin 2 transgenic mice. A large increase in the levels of Aβ 42 is presumably associated with the low density membrane domain that contains decreased levels of glycerophospholipids and sphingomyelin. J Biol Chem. 2000;275:27901–27908. doi: 10.1074/jbc.M004308200. [DOI] [PubMed] [Google Scholar]
- Vanier MT. Lipid changes in Niemann-Pick disease type C brain: personal experience and review of the literature. Neurochem Res. 1999;24:481–489. doi: 10.1023/a:1022575511354. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Ninomiya H, Ohsaki Y, Higaki K, Davies JP, Ioannou YA, Ohno K. Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells. Proc Natl Acad Sci USA. 2001;98:12391–12396. doi: 10.1073/pnas.221181998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276:24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- German DC, Liang CL, Song T, Yazdani U, Xie C, Dietschy JM. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–450. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome. Differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer RN, Bausch KM, Fracasso P, Hammond LJ, Wunderlich D, Wirak DO, Davis G, Brini CM, Buckholz TM, Konig G, Kamarck ME, Tamburini PP. Processing of the pre-β-amyloid protein by cathepsin D is enhanced by a familial Alzheimer’s disease mutation. Eur J Biochem. 1994;224:265–271. doi: 10.1111/j.1432-1033.1994.00265.x. [DOI] [PubMed] [Google Scholar]
- Evin G, Cappai R, Li QX, Culvenor JG, Small DH, Beyreuther K, Masters CL. Candidate γ-secretases in the generation of the carboxyl terminus of the Alzheimer’s disease βA4 amyloid: possible involvement of cathepsin D. Biochemistry. 1995;34:14185–14192. doi: 10.1021/bi00043a024. [DOI] [PubMed] [Google Scholar]
- Hoffman KB, Bi X, Pham JT, Lynch G. β-Amyloid increases cathepsin D levels in hippocampus. Neurosci Lett. 1998;250:75–78. doi: 10.1016/s0304-3940(98)00364-4. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Doms RW, Lee VM. Intracellular APP processing and Aβ production in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:787–794. doi: 10.1097/00005072-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Nam EE, Edgar M, Gouras GK. Alzheimer β-amyloid peptides: normal and abnormal localization. Histol Histopathol. 2002;17:239–246. doi: 10.14670/HH-17.239. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer MF, Soreghan B, Burdick D, Kosmoski J, Glabe CG. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci USA. 1992;89:7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science. 1989;245:417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Oster-Granite ML, McPhie DL, Greenan J, Neve RL. Age-dependent neuronal and synaptic degeneration in mice transgenic for the C terminus of the amyloid precursor protein. J Neurosci. 1996;16:6732–6741. doi: 10.1523/JNEUROSCI.16-21-06732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, Shapiro ML. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, McPhie DL, Arters JA, Greenan J, Oster-Granite ML, Neve RL. Impairments in learning and memory accompanied by neurodegeneration in mice transgenic for the carboxyl-terminus of the amyloid precursor protein. Brain Res Mol Brain Res. 1999;66:150–162. doi: 10.1016/s0169-328x(99)00014-5. [DOI] [PubMed] [Google Scholar]
- McPhie DL, Golde T, Eckman CB, Yager D, Brant JB, Neve RL. β-Secretase cleavage of the amyloid precursor protein mediates neuronal apoptosis caused by familial Alzheimer’s disease mutations. Brain Res Mol Brain Res. 2001;97:103–113. doi: 10.1016/s0169-328x(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer’s disease. J Mol Neurosci. 2001;17:137–145. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]