Abstract
A novel member of the human AMPK family, ARK5, was recently discovered to be a key molecule in mediating cancer cell migration activity in human pancreas cancer cell line PANC-1, and its activation was found to be induced by Akt-dependent phosphorylation at Ser 600. DNA array analysis with 241 paired cDNAs from 13 different types of tumors and corresponding normal tissues derived from cancer patients revealed ARK5 overexpression in the samples of colorectal cancer. ARK5 expression was measured and an in vitro invasion assay was performed in six human colorectal cancer cell lines, WiDr, HCT-15, DLD-1, SW620, LoVo, and SW480, and since high invasion activity was concordant with higher ARK5 expression, ARK5 expression was examined in relation to tumor progression and metastatic activity in clinical samples. In 56 clinical samples of primary colorectal cancers and their liver metastases, higher ARK5 expression was observed in the samples from more advanced cases, and much higher expression was observed in the liver metastases. In situ hybridization analysis showed ARK5 overexpression in tumor cells. Based on these findings, we propose that ARK5 overexpression is involved in tumor progression of colon cancer clinically.
Because of excessive proliferation, energy demands, and insufficient and structurally and functionally inappropriate angiogenesis, tumor tissues are often exposed to an insufficient blood supply, and in turn are usually exposed to both hypoxia and nutrient starvation in their microenvironment.1 The impact of the microenvironment on the progression of various tumors has been clearly recognized. The importance of the response of tumor cells to hypoxic conditions has long been studied,2–4 and the angiogenic ability of tumors was first recognized as a key factor in tumor biology about 30 years ago by Folkman.5 The molecular mechanism of tumor angiogenesis, and its control mechanisms have been thoroughly studied.6,7 Excessive tumor angiogenesis is often naively regarded as improving tumor’s blood supply, but tumor hypoxia is now known to be a good marker for poor prognosis in various cancers.8–10
When cells and tissues are exposed to hypoxia, they sustain their excessive growth and proliferation in this adverse environment by improving blood flow, cell cycle regulation, and energy metabolism.11,12 These reactions are called the hypoxic response, and hypoxia-inducible factor 1 (HIF-1) is known to be a key transcriptional factor in the response. HIF-1 transactivates a series of hypoxia response genes, including the genes encoding VEGF, erythropoietin, and glycolytic enzymes, in response to hypoxia, and for this reason the ability of tumor cells to produce angiogenic factors is often correlated with tumor’s ability to invade, metastasize, and progress.13–15 Based on these observations, it has been proposed that exposure to hypoxia might stimulate the ability of tumor cells to produce angiogenic factors and in turn tumor stimulate invasion and metastasis by the tumor16–18 and that under these conditions, increased glycolysis might temporarily compensate for the oxygen insufficiency.19,20 However, during the sustained severe hypoxia that typically occurs in pancreatic cancer, for example, increased glycolysis might be inadequate, because the glucose supply is also very limited under these conditions.
As a result of the insufficient blood supply for energy production by tumor cells, increased glycolysis alone might not be sufficient because of the limited glucose supply, as stated above. We discovered an interesting biological response by hepatoma and fibroblasts to glucose starvation in which the cells acquired strong tolerance to glucose deprivation during hypoxia, and pancreatic cancer cell lines were subsequently found to be constitutively tolerant to glucose deprivation.21,22 Based on these observations, we have proposed that the ability of cancer cells to tolerate glucose deprivation might be another aspect of the ability of tumor cells to overcome an insufficient blood supply, and Akt and AMPK have been found to be key molecules in this response, which is referred to as “austerity”.22
AMPK, a metabolite-sensing protein kinase family members, is activated by various cellular stresses which consume intracellular ATP, and plays a major role in protecting cell by converting energy metabolism from anabolic to catabolic by inhibiting and activating various molecules, including HMG-CoA reductase, acyl-CoA carboxylase, and glucose transporters.23–25 We recently identified a novel AMPK family member, ARK5, which is directly activated by Akt, and ARK5 has been found to induce tumor cell survival during nutrient starvation in an Akt-dependent manner.26 In addition, ARK5 overexpression has been found to markedly stimulate tumor cell invasion and metastasis by pancreatic cancer in both in vitro and in vivo models via the activation of MMPs and MT1-MMP. Akt is known to be a very important molecule in supporting cancer cell proliferation, survival, tumorigenesis, invasion and metastasis,21,22,26 and ARK5 is a key mediator of these actions of Akt.
In this study, the role of ARK5 in the progression, invasion, and metastasis of cancer was investigated by using human colon cancer cell lines (WiDr, DLD-1, HCT-15, SW620, LoVo, and SW480) and samples of clinical colorectal cancers and their metastases.
Materials and Methods
DNA Array
The DNA array (Cancer profiling array; BD Biosciences, Franklin Lakes, NJ) consisted of 241 paired cDNAs that had been reverse-transcribed and amplified from tissue sources by SMART technology (BD Biosciences) from 13 different tissue types. Each pair consisted of a tumor sample and a corresponding normal tissue sample was obtained from the same patient. Specific primers of ARK5 spanning a 258-bp nucleotide sequence of cDNA were used, and the 258-bp fragment was amplified by polymerase chain reaction (PCR) with the cycling conditions of 94°C for 4 minutes, followed by 35 cycles at 94°C for 1 minute, 58°C for 1 minute, 72°C for 2 minutes, and final extension at 72°C for 8 minutes. The nucleotide sequences of these primers were: forward, 5′-GAGTCCACTCTATGCATC-3′; reverse, 5′-GGCCACTATTGAGGACA-3′. The PCR product was 32P-dCTP (α-32P-dCTP: 6000 Ci/mmol, Amersham Biosciences Corp., Piscataway, NJ) labeled with RediprimeII (Amersham Biosciences Corp.) and used as a probe. Prehybridization was performed at 68°C for 1 hour in 40 ml of prehybridization solution containing heat-denatured salmon sperm DNA. The DNA array was then hybridized overnight at 68°C in the 32P-dCTP labeled probe mixed hybridization solution. ARK5 expression was detected with a BAS2000 system, and the amount of ubiquitin was used as an external control in each spot, as recommended by the supplier.
Cell Lines
Human colon cancer cell lines DLD-1, WiDr, HCT-15, SW620, LoVo, and SW480, human hepatoma cell line HepG2, and human pancreas cancer cell line PANC-1 were purchased from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Nissui Corp., Tokyo, Japan) supplemented with 10% fetal calf serum (σ-Aldrich Corp., St. Louis, MO), 2 mmol/L l-glutamine, and 25 mmol/L HEPES-NaOH (pH 7.3) at 37°C under an atmosphere of 5% CO2 in air.
Plasmids
The same expression vectors of ARK5 and dominant negative-ARK5 (DN-ARK5) and an antisense RNA expression vector of ARK5 (ARK5-AS) as previously reported26 were used. The expression vector of the dominant negative form of Akt1 (DN-Akt-1), the expression vector of the constitutive active form of Akt-1 (CA-Akt-1), and anti-FLAG antibody were purchased from Upstate Biotechnology (Lake Placid, NY).
Transfection
For transfection, cells were seeded into a 24-well plate at 5 × 104/well, and transfection was performed with TransFast transfection reagent (Promega Corp., Madison, WI) with some modifications. Transfection efficiency in the current study was assessed with a green fluorescence protein expression vector, and was about 80%.
In Vitro Invasion Assay
In vitro invasion assay was performed with a matrigel-coated invasion chamber (BD Biosciences). Cells (5 × 104/chamber) were seeded into each chamber, and the media in the inner and outer chamber were changed after 6 hours. Cells that had invaded were counted under a phase-contrast microscope after 48 hours.
Northern and Southern Blot Analysis
Total RNA was isolated from the six colon cancer cell lines by the AGPC method using ISOGEN (Nippon Gene Co. Ltd., Tokyo, Japan), and 10 μg total RNA was fractionated on a 1% agarose gel containing 6% formamide and then transferred to a nylon filter (Hybond-N; Amersham Biosciences Corp.). After UV cross-linking, the filter was prehybridized and hybridized as described previously.21 The 258-bp fragment of ARK5 cDNA as describes in the DNA array method was labeled with 32P-dCTP using RediprimeII (Amersham Biosciences Corp.). The filter was washed four times at 58°C in 2X saline sodium citrate containing 0.1% sodium dodecyl sulfate and 0.2% sodium pyrophosphate. The ARK5 mRNA was visualized by BAS2000 system. To evaluate the amount of RNA analyzed, the filter was rehybridized with 32P-dCTP-labeled cDNA probe for human β-actin.
Southern blot hybridization was performed by a standard method. Genomic DNA was isolated from the six colon cancer cell lines using SepaGene (Sanko Junyaku Co. Ltd., Tokyo, Japan), and 10 μg of the DNA was digested with EcoRI, fractionated on a 1% agarose gel, and transferred to a nylon filter (Hybond-N; Amersham Biosciences Corp.). The filter was prehybridized for 12 hours at 62°C. The 1187-bp fragment of NcoI digested ARK5 cDNA containing the region corresponding from 2985 to 4172 (Gene Bank ID: NM 014840) was labeled with 32P-dCTP using RediprimeII (Amersham Biosciences Corp.). To evaluate the amount of DNA analyzed, the filter was rehybridized with 32P-dCTP labeled cDNA for human PERK containing the region corresponding from 1711 to 1890 (Gene Bank ID: AF110146). Genomic DNA for ARK5 and PERK were visualized, and an intensity of the band was quantified using the BAS2000 system.
Tissue Sampling
A total of 71 clinical samples, consisting of 41 samples of primary colorectal cancers (Dukes’ B: 19 samples, C: 19 samples, and D: 3 samples), 15 samples of liver metastases, and 15 samples of normal colon mucosa were obtained from the surgical specimens of 56 cancer patients who had undergone surgical resection at National Cancer Center Hospital East (Chiba, Japan). The tissues were collected at the time of surgery and immediately stored in liquid nitrogen until used for RNA extraction. All tissue samples were collected after obtaining informed consent.
Quantitative-PCR (Q-PCR) and Reverse Transcription PCR (RT-PCR)
The specific primers for ARK5 detection have been described previously.26 Total RNA was isolated from the tissues by the AGPC method using ISOGEN (Nippon Gene Co. Ltd.). Q-PCR was performed with a Light-Cycler (Roche Diagnostics, Mannheim, Germany). The cDNA was prepared by using 100 ng of total RNA and a RNA PCR Kit (AMV) Ver. 2.1 (Takara Bio Inc., Kyoto, Japan) according to the manufacturer’s instructions. The specific PCR primers for detection were: ARK5 forward primer 5′-ATGCTAAGTACCCTCTGAATG-3′ and ARK5 reverse primer 5′-GCAACAAGCAGTCAGTCGATC-3′, Akt-1 forward primer 5′-AGCTATCTGTCATCTCTCTGGGGC-3′ and Akt-1 reverse primer 5′-GTCAAGTGCTACCGTGGAGAGATC-3′, and Akt-2 forward primer 5′-AGGTCATGGAGCACAGGTTCTTCC-3′ and Akt-2 reverse primer 5′-GTCAGGGGGTGTGATTGTGATGGA-3′. Q-PCR was performed with cycling conditions of 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 10 seconds, and extension at 72°C for 20 seconds. To quantify the data, ARK5 mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. Q-PCR of samples without reverse transcription was also performed to test for genomic DNA contamination in each sample.
Total RNA was isolated from cancer cells by the AGPC method and reverse-transcripted into cDNA with oligo-dT primers and AMV-reverse transcriptase (Takara Bio Inc.). RT-PCR was performed using the ARK5 primers. The cDNA was subjected to 30 cycles of PCR amplification on a PCR Thermal Cycler 480 (Takara Bio Inc.), and PCR products were fractionated by agarose electrophoresis, stained with ethidium bromide, and then visualized under UV light. GAPDH served as a control for RT-PCR.
RNA Probe Preparation
An ARK5-specific 258-bp nucleotide sequence was designed (forward: 5′-GAGTCCACTCTATGCATC-3′, reverse: 5′-GGCCACTATTGAGGACA-3′). A cDNA fragment spanning from 2022nd to 2279th nucleotide was cloned into pcDNA2.1 to prepare the RNA probe for in situ hybridization. RNA probes were prepared by transcription with T7 RNA polymerase using Biotin RNA Labeling Mix (Roche Diagnostics).
In Situ Hybridization
Tissue sections 6-μm thick were prepared from frozen samples of colorectal cancers, liver metastases, and normal colon mucosa. The sections were processed according to the standard protocol,27 and digested with 10 μg/ml protein K for 10 minutes at room temperature. Endogenous peroxidase was inactivated by incubating the tissue sections in 0.3% H2O2 for 20 minutes. Hybridization was performed in 200 μl of hybridization solution with biotinylated ARK5-antisense RNA probe (1 μg/ml) or ARK5-sense biotinylated RNA probe (1 μg/ml) for 2 hours at 37°C as a negative control. DAKO GenoPoint (Dako Cytomation Co. Ltd., Kyoto, Japan) and the streptavidin-horseradish peroxidase, tyramide and diaminobenzidine tetrahydrochloride (DAB) method were used for visualization according to the manufacturer’s instructions.
Statistical Analysis
The data are expressed as means ± SE or means ± SD. Student’s t-test was used to determine statistical significance, and the differences were considered significant if the P value was less than 0.05.
Results
DNA Array Analysis of ARK5 Expression
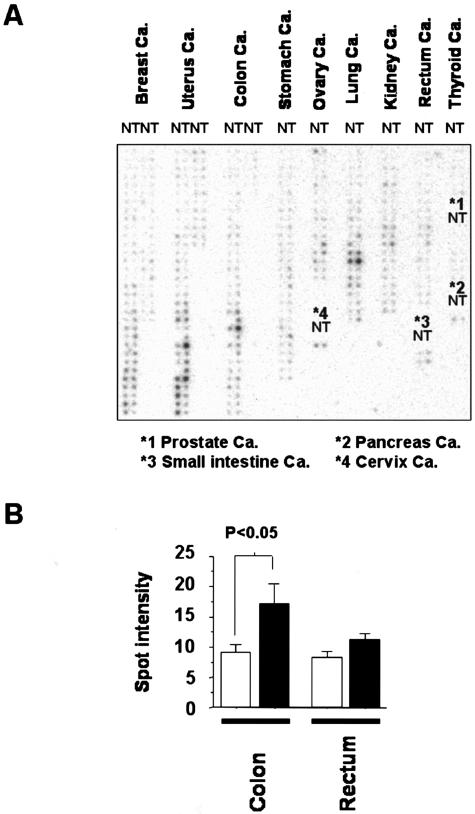
Firstly, ARK5 overexpression was examined among various human cancers to determine whether ARK5 expression is involved in certain type of human cancer. DNA array generated from 13 different tissue types was used to investigate ARK5 expression among a wide variety of human clinical tumors (Figure 1A). The amounts of ARK5 mRNA in 13 different tissues and cancers were quantified by densitometry and normalized to Ub mRNA, varied among the types of cancer. Higher levels of ARK5 mRNA were detected in colon and rectal cancers than in normal tissues. Breast, uterus, and ovary cancer also showed high levels of ARK5 expression in both the tumors and the corresponding normal tissues (Figure 1A). As shown in Figure 1B, the ARK5 mRNA levels were 2.1-fold higher in colon cancer and 1.4-fold higher in rectal cancer than in corresponding normal tissue. The majority of colorectal cancers expressed higher ARK5 mRNA levels than the normal tissue, although the degree of the increase varied greatly.
Figure 1.
Expression of ARK5 mRNA in various cancers (cancer: Ca.) and corresponding normal tissues. A: DNA array analysis of ARK5 expression in 13 different types of cancers (T) and corresponding normal tissues (N). B: Densitometric analysis of ARK5 mRNA expression in colon or rectal cancers (filled bars) and corresponding normal tissues (open bars).
Correlation between ARK5 Expression and the Invasion Activity of Human Colorectal Cancer Cell Lines
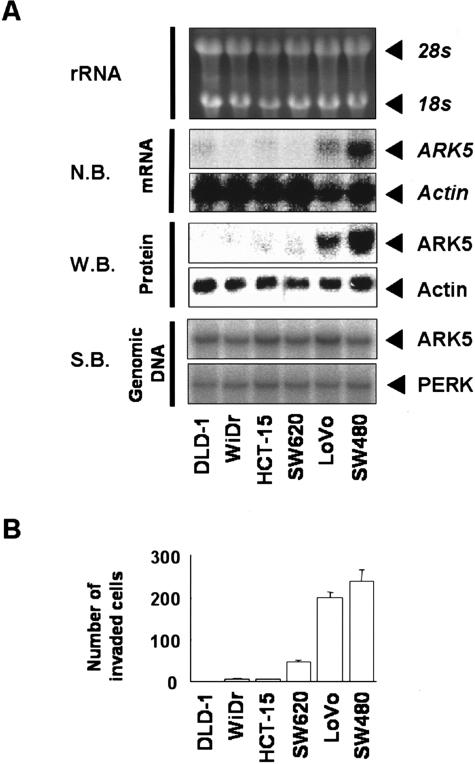
DNA array analysis is convenient to screen large number of cancers for their expression of a given gene but the specificity is not sufficient. To confirm ARK5 gene expression in colon cancers, six human colon cancer cell lines were used. As shown in Figure 2A, LoVo and SW480 cell lines showed high ARK5 expression at both the mRNA and protein level. These data clearly indicate that some human colon cancers actually express ARK5 gene.
Figure 2.
A: Total RNA, protein, and genomic DNA were extracted from each cell line, and Northern blot (N.B.) or Western blot analysis (W.B.) was performed with probe or antibody for ARK5 or β-actin. Southern blot analysis (S.B.) was also performed with probe for ARK5 or PERK. B: Strong association between ARK5 expression and tumor cell invasion activity. Each cell line was seeded on a matrigel-coated invasion chamber (5 × 104 cells/chamber), and cells that had translocated to the lower chamber were counted after 48 hours. Results are shown as means of three experiments, and the bars represent SE values.
To determine whether ARK5 gene amplified in LoVo and SW480 cells, Southern blot hybridization was performed. Contrary to the overexpression of ARK5 mRNA in LoVo and SW480 cells, ARK5 gene was not amplified in any colon cancer cell lines (Figure 2A). The mean and variance value of the intensity of each band were 0.94 ± 0.26 (SD) and the range was 0.70 in SW480 cells to 1.41 in LoVo cells, and no difference was detected with statistically significance. Similar result was also observed in Southern blot analysis for PERK gene.
SW620 and LoVo cells are derived from lymph node metastases, and SW480 cells are derived from the primary lesion of a colon cancer patient who had later lymph node metastasis. Because ARK5 was highly expressed in two of three cell lines, we hypothesized that ARK5 expression might be associated with colon cancer metastasis as noticed in pancreas cancer (Suzuki at al, unpublished). Therefore, in vitro invasion assay of the above six cell lines was carried out.
Interestingly, LoVo and SW480 cells were extremely potent in their in vitro invasion activities (Figure 2B). Thus, a good correlation was found between expression of ARK5 by human colon cancer cell lines and their cell invasion activity.
Tumor Cell Invasion Is Stimulated by the Akt/ARK5 System
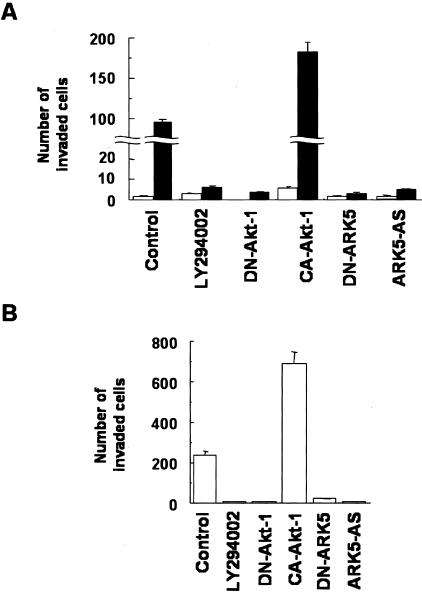
To determine whether there is a causal relationship between ARK5 overexpression and cell invasion activity, we prepared the ARK5 overexpressing cell line DLD-1 (D/ARK) and compared its invasion activity with that of the parent DLD-1 cell line, which exhibited very low expression of ARK5 (Figure 3A). DLD-1 cells displayed very low invasion activity, whereas the D/ARK cells showed high activity (Figure 3A). The increased activity was further accelerated by transient expression of CA-Akt-1 in D/ARK cells but not in DLD-1 cells, and completely suppressed by transient expression of DN-Akt-1, DN-ARK5, or ARK5-AS, or by treatment with PI-3K inhibitor LY294002 (Figure 3A).
Figure 3.
Tumor cell invasion mediated by the Akt/ARK5 pathway in human colorectal cancer cell lines. A: In vitro invasion activity of DLD-1 (open bars) and D/ARK (filled bars) cell lines exposed to or unexposed (control) to 20 μmol/L LY294002, or 1 μg of the expression vector of dominant negative-Akt-1 (DN-Akt-1), constitutive active-Akt-1 (CA-Akt-1), dominant negative-ARK5 (DN-ARK5), or ARK5-antisense (ARK5-AS) was used. Results are shown as means of three experiments and the bars represent SE values. B: In vitro invasion assay of SW480 cells. Either 20 μmol/L LY294002 or 1 μg of the expression vector of each DN-Akt-1, CA-Akt-1, DN-ARK5, or ARK5/AS was used. Results are also shown as means of three experiments.
As shown in Figure 3B, SW480 cells showed high expression of ARK5 and high invasion activity. Their invasion activity was further increased by transient expression of CA-Akt-1, but completely suppressed by DN-Akt-1 and LY294002 (Figure 3B).
Expression of ARK5 mRNA in Fresh Clinical Samples of Colorectal Cancer and Their Liver Metastases
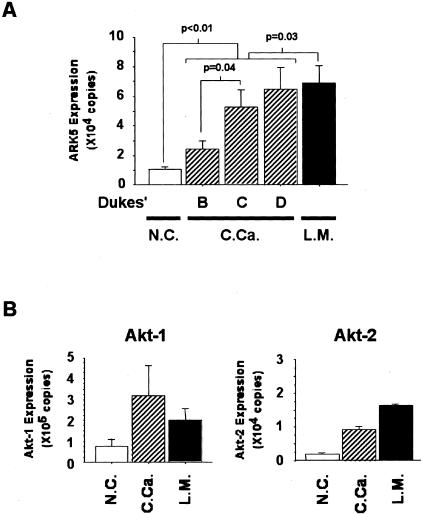
Surgically resected colorectal cancers and their liver metastases were investigated to determine whether there was an association between ARK5 expression and invasion and metastasis ability. Higher expression of ARK5 was found in colorectal cancer than in normal mucosa in most of the cases (P < 0.01) (Figure 4A), and Q-PCR showed much higher expression in liver metastases of colorectal cancer, with a statistically significant difference (P = 0.03). No clear stage-dependent increases in ARK5 mRNA expression were detected, but there was a significant difference between Dukes’ B and C/D (P = 0.04) (Figure 4A).
Figure 4.
A: Quantitative analysis of ARK5 mRNA expression in primary colorectal cancers and liver metastases. Total RNA (100 ng) extracted from 15 samples of non-tumor colon mucosa (normal colon mucosa, N.C.), 41 primary tumors (colorectal cancer, C.Ca.) consisting of Dukes’ B: 19 samples, C: 19 samples, and D: 3 samples, and 15 liver metastases of colorectal cancer (L.M.) were reverse-transcribed, and Q-PCR was performed. Each value was normalized to the amount of GAPDH. B: Expression of Akt-1 and Akt-2 mRNA was also measured by Q-PCR in the same samples of colorectal cancers and their liver metastases.
Since ARK5 is the direct target of Akt and mediates signals from Akt, its expression was examined in surgical materials. Expression of Akt-1 and Akt-2 was estimated by performing Q-PCR with Akt-1- and Akt-2-specific primers in the same clinical samples. Higher Akt-1 expression was detected in the clinical samples of colorectal cancer, but expression was not much higher in the liver metastases (Figure 4B). Akt-2 overexpression was also observed in the clinical samples of colorectal cancers, but Akt-2 expression was much higher in their liver metastases (Figure 4B).
In Situ Hybridization of ARK5 mRNA in Clinical Samples
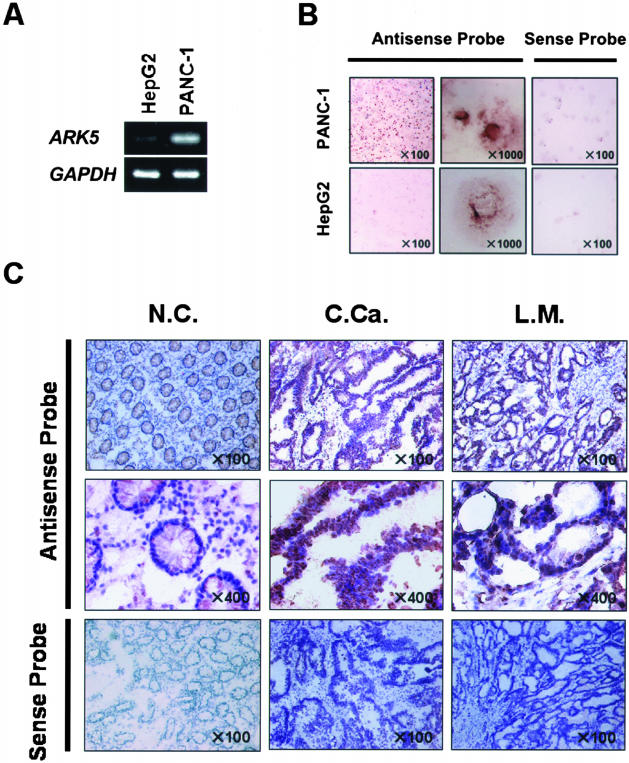
Because the clinical samples contained both tumor and stromal cells, the cellular distribution of ARK5 mRNA was examined by in situ hybridization. We chose HepG2 cells and PANC-1 cells, which had been confirmed to be a low expresser and high expresser by RT-PCR (Figure 5A), respectively, to verify the specificity of in situ hybridization detection of ARK5 mRNA.
Figure 5.
Distribution of ARK5 mRNA expression in fresh clinical samples. A: ARK5 and GAPDH expression were examined by RT-PCR in PANC-1 and HepG2 cells. B: In situ hybridization of AR5 mRNA in PANC-1 and HepG2 cells. In situ hybridization was performed by using the antisense and sense probe. C: Tissue sections of non-tumor tissue (normal colon mucosa, N.C.), primary tumor tissue (colon cancer, C.Ca.), and liver metastatic tissue (liver metastases of colorectal cancer, L.M.) were hybridized with the antisense probe for ARK5, and the sections were counterstained with hematoxylin. Nuclear stained cells as brown (diaminobenzidine tetrahydrochloride, DAB) are ARK5 mRNA-positive. As a negative control, in situ hybridization was also performed using the sense probe in the same tissue sections.
As shown in Figure 5B, a strong signal was detected in PANC-1 cells only when antisense RNA was used as a probe, and the signal was mainly in the cytoplasm and the perinuclear region, clearly demonstrating the specificity of in situ hybridization detection of ARK5 mRNA.
In situ hybridization was then performed on 6-μm-thick frozen sections of colorectal cancers, their liver metastases, and normal colon tissue. The results clearly showed high expression within the tumor cells of the colorectal cancers and much higher expression in their liver metastases (Figure 5C).
Discussion
Colorectal cancer is still one of the leading causes of cancer death in developed countries, and liver metastasis is an especially serious problem as the main cause of death in the later stages. Cell invasion is well known to be an essential element for tumor metastasis, and Akt has been found to play a key role in tumor cell invasion.28–30 Akt is often overexpressed in colorectal cancer cells and gene amplification is sometimes observed. Since Akt is thought to be a pivotal factor in colorectal carcinogenesis and progression,31–34 it is very important to understand the molecular basis of involvement of the Akt pathway in invasion and metastasis by colorectal cancers.
We recently identified ARK5 as a key tumor cell survival factor that mediates Akt signaling,26 and it has also been found to mediate Akt-dependent tumor cell invasion and metastasis in vitro (Suzuki et al, unpublished). Some cancer cells with high ARK5 expression show high invasion activity. To investigate whether cancer cells with ARK5 oxerexpression increase in vivo invasion activity, we recently performed in vivo liver metastasis assay using SCID mice that had been transplanted with human pancreatic cancer cell line PANC-1 or P/ARK, PANC-1 cells that had been stably transfected with ARK5 full-length expression vector, and the results showed a much higher incidence of liver metastasis by the P/ARK cells than by the PANC-1 cells.35
Since colon cancers are known to express Akt, and overexpression and gene amplification of Akt are sometimes observed in them,36–38 we speculated that ARK5 might also be important in tumor invasion and metastasis by human colon cancer, and the results of the present study clearly demonstrated that ARK5 is a key factor mediating Akt-dependent colon cancer cell invasion in vitro. Based on the results of both an in vitro and an in vivo analysis in an animal model, we hypothesized that ARK5 expression is associated with tumor progression of clinical colorectal cancer. The results of the present study clearly showed higher expression of ARK5 in colorectal cancer than in normal colon mucosa, and it was especially higher in their liver metastases.
What is the biological significance of ARK5 overexpression? As previously mentioned, ARK5 mediates the tumor invasion signal through Akt, and we therefore performed Q-PCR to estimate the expression of Akt-1 and Akt-2 as the upstream of ARK5 in the same clinical samples. As shown in Figure 4B, higher expression of both Akt-1 and Akt-2 mRNA was clearly observed in the colorectal cancers and their liver metastases than in normal colon mucosa, and Akt-1 expression was predominated over Akt-2 of expression in terms of the total number of copies expressed. Recent reports have implicated Akt in various human malignancies, and Akt-2 is the predominant isoform involved in colon carcinogenesis.39 Indeed, as in the expression pattern of ARK5, Akt-2 overexpression was observed in colorectal cancers, and much higher expression was observed in their liver metastases. More recently, we found that ARK5 mediates tumor cell invasion activity induced by the activation of Akt signaling pathway by IGF-I in human pancreatic and colorectal cancer cells. One of the mechanisms for the stimulation of invasion by ARK5 was found to be the induction of MT1-MMP production at translational level (Suzuki et al, unpublished). Therefore, it is quite reasonable to see the co-expression of ARK5 and Akt in colorectal cancers, especially at their advanced stage in the present study. Of course, tumor invasion and metastasis are complex biological processes, ARK5 and Akt co-expression might be one of the determinants of progression of colorectal cancer.
We also found that ARK5 overexpression significantly suppresses caspase 8 activation triggered by glucose starvation in human hepatoma cell line HepG2.40 It has been reported that some liver metastases of colorectal cancer express both Fas and Fas-ligand, which cause hepatocyte apoptosis,41,42 and similar observations have also been made in some liver metastases of pancreatic cancer.43 Moreover, we recently found that death receptor-induced cell death can be suppressed by increasing ARK5 expression.40 All of these observations taken together suggest that ARK5 overexpression might suppress cell death caused by various stimuli, leading to another aspect of tumor progression.
What is the molecular mechanism for ARK5 overexpression? One possible mechanism of the overexpression is gene amplification. Indeed, Akt gene is known to be amplified in some colorectal cancers, in which Akt is overexpressed and constitutively activated.31,44 We have examined a possibility of ARK5 gene amplification by taking six human colorectal cancer cell lines and found not to be amplified. There might be a possibility of gene amplification in clinical cases and this possibility must be examined in future. Another possible mechanism for ARK5 overexpression is transcriptional activation. We recently found that at least in some colorectal cancer cell line, ARK5 gene transcription is regulated by cofactor required for Sp1 transcriptional activation proteins (CRSPs) through Sp-1 site,45,46 and one of CRSPs was found to be regulated by a metabolic stress (Suzuki et al, unpublished). Detailed mechanisms remains to be determined, but these findings suggested that ARK5 gene might be regulated both genetic alterations and microenvironment of colorectal cancers through transcriptional activation.
The present study strongly suggests that Akt-ARK5 pathway plays important roles in progression of colorectal cancer. ARK5 may be a key molecule in understanding the biology of invasive and metastatic colon cancers and for developing a new strategy of chemotherapy.
Footnotes
Address reprint requests to Hiroyasu Esumi, M.D., Investigative Treatment Division, National Cancer Center Research Institute East, 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan. E-mail: hesumi@east.ncc.go.jp.
Supported in part by a Grant for the 2nd-term Comprehensive 10-year Strategy of Cancer Control from the Ministry of Health, Welfare and Labour, a grant from the Program for Promotion of Fundamental Studies in Health Sciences of Organization for Pharmaceutical Safety and Research, and a grant for Research on Cancer from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
G.K., A.S., and S.M. are Awardees of Research Resident Fellowship from the Foundations for Promotion of Cancer Research.
References
- Esumi H, Izuishi K, Kato K, Hashimoto K, Kurashima Y, Kishimoto A, Ogura T, Ozawa T. Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5′-AMP-activated protein kinase-dependent manner. J Biol Chem. 2002;277:32791–32798. doi: 10.1074/jbc.M112270200. [DOI] [PubMed] [Google Scholar]
- Koutcher JA, Alfieri AA, Devitt ML, Rhee JG, Kornblith AB, Mahmood U, Merchant TE, Cowburn D. Quantitative changes in tumor metabolism, partial pressure of oxygen, and radiobiological oxygenation status postradiation. Cancer Res. 1992;52:4620–4627. [PubMed] [Google Scholar]
- Prabhakar NR, Shenoy BC, Simonson MS, Cherniack NS. Cell selective induction and transcriptional activation of immediate early genes by hypoxia. Brain Res. 1995;697:266–270. doi: 10.1016/0006-8993(95)00994-2. [DOI] [PubMed] [Google Scholar]
- Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–5994. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19:331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–1893. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Choi KS, Bae MK, Jeong JW, Moon HE, Kim KW. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest. 2001;108:39–40. doi: 10.1172/JCI13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, de Wilde PC, Bussink J, Raleigh JA, van der Kogel AJ. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–7074. [PubMed] [Google Scholar]
- Kaluz S, Kaluzova M, Chrastina A, Olive PL, Pastorekova S, Pastorek J, Lerman MI, Stanbridge EJ. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3′-kinase. Cancer Res. 2002;62:4469–4477. [PubMed] [Google Scholar]
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;58:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A, Llanos S, Lu X. Hypoxia induces p53 through a pathway distinct from most DNA-damaging and stress-inducing agents. Carcinogenesis. 2003;24:1177–1182. doi: 10.1093/carcin/bgg044. [DOI] [PubMed] [Google Scholar]
- Lai JC, White BK, Buerstatte CR, Haddad GG, Novotny EJ, Jr, Behar KL. Chronic hypoxia in development selectively alters the activities of key enzymes of glucose oxidative metabolism in brain regions. Neurochem Res. 2003;28:933–940. doi: 10.1023/a:1023235712524. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1α in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem. 2003;278:40296–40304. doi: 10.1074/jbc.M302560200. [DOI] [PubMed] [Google Scholar]
- Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1α (HIF-1α): role of cytoplasmic trapping of HIF-2α. Mol Cell Biol. 2003;23:4959–4971. doi: 10.1128/MCB.23.14.4959-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56–64. doi: 10.1097/00006676-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Kruger EA, Blagosklonny MV, Dixon SC, Figg WD. UCN-01, a protein kinase C inhibitor, inhibits endothelial cell proliferation and angiogenic hypoxic response. Invasion Metastasis. 1998–99;18:209–218. doi: 10.1159/000024514. [DOI] [PubMed] [Google Scholar]
- Michelson S, Leith JT. Host response in tumor growth and progression. Invasion Metastasis. 1996;16:235–246. [PubMed] [Google Scholar]
- Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- Yim S, Choi SM, Choi Y, Lee N, Chung J, Park H. Insulin and hypoxia share common target genes but not the hypoxia-inducible factor-1α. J Biol Chem. 2003;278:38260–38268. doi: 10.1074/jbc.M306016200. [DOI] [PubMed] [Google Scholar]
- Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res. 2000;60:6201–6207. [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative α2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Ryomoto T, Ishikawa K. Role of cardiac ATP-sensitive K+ channels induced by HMG-CoA reductase inhibitor in ischemic rabbit hearts. Hypertens Res. 2001;24:573–577. doi: 10.1291/hypres.24.573. [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kusakai G, Kishimoto A, Lu J, Ogura T, Lavin MF, Esumi H. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J Biol Chem. 2003;278:48–53. doi: 10.1074/jbc.M206025200. [DOI] [PubMed] [Google Scholar]
- Lu XL, Qian KD, Tang XQ, Zhu YL, Du Q. Detection of H. pylori DNA in gastric epithelial cells by in situ hybridization. World J Gastroenterol. 2002;8:305–307. doi: 10.3748/wjg.v8.i2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23:5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- Liu W, Liu Y, Lowe WL., Jr The role of phosphatidylinositol 3-kinase and the mitogen-activated protein kinases in insulin-like growth factor-I-mediated effects in vascular endothelial cells. Endocrinology. 2001;142:1710–1719. doi: 10.1210/endo.142.5.8136. [DOI] [PubMed] [Google Scholar]
- Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Hernandez A, Hellmich MR, Gatalica Z, Evers BM. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. J Biol Chem. 2002;277:36602–36610. doi: 10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Kelly B, Semenza GL. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330–2334. [PubMed] [Google Scholar]
- Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. 2001;61:5275–5283. [PubMed] [Google Scholar]
- Kusakai, G, Suzuki A, Ogural T, Esumi H: Association of ARK5 with tumor invasion and metastasis. J Exp Clin Cancer Res 2004, in press [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL-sensitive colon carcinoma cell line SW480 using a genetic approach. J Biol Chem. 2001;276:37879–37886. doi: 10.1074/jbc.M103516200. [DOI] [PubMed] [Google Scholar]
- Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kusakai G, Kishimoto A, Lu J, Ogura T, Esumi H. ARK5 suppresses the cell death induced by nutrient starvation and death receptors via inhibition of caspase 8 activation, but not by chemotherapeutic agents or UV irradiation. Oncogene. 2003;22:6177–6182. doi: 10.1038/sj.onc.1206899. [DOI] [PubMed] [Google Scholar]
- Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoong KF, Afford SC, Randhawa S, Hubscher SG, Adams DH. Fas/Fas ligand interaction in human colorectal hepatic metastases: a mechanism of hepatocyte destruction to facilitate local tumor invasion. Am J Pathol. 1999;154:693–703. doi: 10.1016/S0002-9440(10)65316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Ninomiya I, Fushida S, Fujimura T, Nishimura G, Shimizu K, Miwa K. Human pancreatic cancer cells express non-functional Fas receptors and counterattack lymphocytes by expressing Fas ligand; a potential mechanism for immune escape. Int J Oncol. 2001;18:33–39. [PubMed] [Google Scholar]
- Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- Ryu S, Tijan R. Purification of transcription cofactor complex CRSP. Proc Natl Acad Sci USA. 1999;96:7137–7142. doi: 10.1073/pnas.96.13.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taatjes DJ, Naar AM, Andel F, 3rd, Nogales E, Tjian R. Structure, function, and activator-induced conformations of the CRSP coactivator. Science. 2002;295:1058–1062. doi: 10.1126/science.1065249. [DOI] [PubMed] [Google Scholar]